Perhaps the most common abnormality among calcium stone formers, idiopathic hypercalciuria (IH) causes calcium kidney stones and can lead to bone mineral loss and fracturing bone disease. Proper treatment requires a high calcium intake, a low sodium intake, moderation of very high protein intakes, avoidance of refined sugar loads, and – not rarely – use of diuretic drugs which can lower urine calcium losses, prevent stones, and protect bones.
Why The Bathers?
Bone seems, to me, a bather in a bathtub. Calcium flows in from faucets – the GI tract – and out down the drain – the kidneys – as they regulate serum calcium – the height of the water in the tub. I realize the bather does not take up or lose water, but if you ponder the image awhile you may see in it what I see.
The Large Bathers (1884-87) of Renoir and The Large Bathers (1900-1906) of
What is Idiopathic Hypercalciuria?
What is Hypercalciuria?
As hypertension is defined by blood pressures that associate with stroke, heart failure, and heart attack, hypercalciuria is defined by urine calcium excretions that associate with stones.

Increasing urine calcium losses associate with increasing risk of stones in two cohorts of women – red – and one of men – blue. Urine calcium is along the horizontal axis in six bins. The average relative risk of forming stones is marked by the tops of the bars. A value of 1 means no higher than among people with urine calcium below 100 mg/day – the reference population.
The lower 95th percentile of risk is at the bottoms of the bars. When the bottom of a solid bar lies above one, which is the case for all bars from 200-249 mg/d on, increased risk is very likely present. So the threshold of hypercalciuria is 200 mg/d both sexes.
Diet was not controlled, so we do not need special diets to diagnose hypercalciuria using this criterion.
As the urine calcium rises, risk – top of the bar – rises in smooth progression.
What Does ‘Idiopathic’ Mean?
The overwhelming majority of hypercalciuric stone formers have none of the many diseases that can raise urine calcium excretion. Their urine calcium exceeds 200 mg/d for no obvious reason – idiopathic, arising of itself, without overt cause.
Normal Calcium Excretion
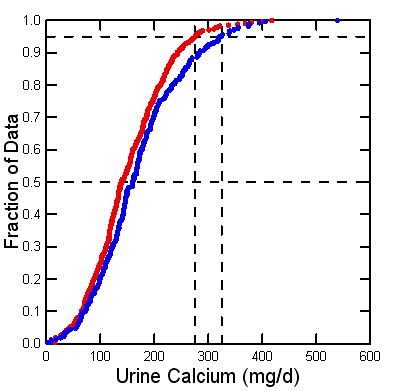
Since 1900 scientists have collected 24 hour urine samples from people in clinical research units, perfect collections, and measured urine calcium. I collected all such values I could from published papers – a tiring exercise. Here is my yield of values from normal adult men (blue) and women (red).
The threshold of clinical hypercalciuria, 200 mg/day, resides at about the 75th percentile: 25% of normal people are above it. But stone formers are perhaps 7-10% or less of the human population. So hypercalciuria raises stone risk, but not everyone gets the stones.
Likewise, stone disease is familial, but IH alone does not fully explain why. Presumably other inherited factors matter.
Decades ago we used the 95th percentiles of these two distributions, at about 275 and 325 mg/d of calcium for women and men, to define ‘hypercalciuria‘. No doubt such high values confer risk of stone, but they are too high for clinical use. They remain useful in research to define people with extremely high urine calcium values.
Hypercalciuria Raises Supersaturation and May Promote Plaque
Supersaturation produces and enlarges crystals and therefore stones. We now have superb evidence that rising supersaturation associates with rising stone risk. Calcium oxalate and calcium phosphate supersaturations rise smoothly with urine calcium, leaving no doubt that urine calcium raises risk of calcium stones via increasing supersaturation.
Many calcium stones form on plaque, tissue deposits of calcium phosphate crystals in human renal papillae. Plaque abundance rises with urine calcium excretion, and a plausible theory, vas washdown, links them.
Idiopathic hypercalciuria is Hereditary
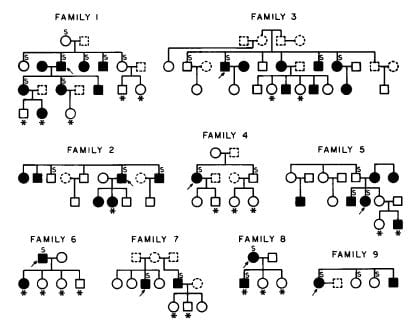 Family Studies
Family Studies
I am not sure if we were the first, but here is our evidence from 1979.
The arrows point to the stone formers whose families we studied. Filled symbols are men (square) and women (circles) with IH, asterisks mark children, open symbols did not have IH, and dashed people are deceased. About 50% of immediate blood relatives had IH, in successive generations. Others have also found IH heritable.
IH might look like a simple dominant trait from one abnormal gene, but it results from a number of genes. Incidentally, urine calcium is not the only stone forming trait that appears genetic. Urine citrate appears to be, as well.
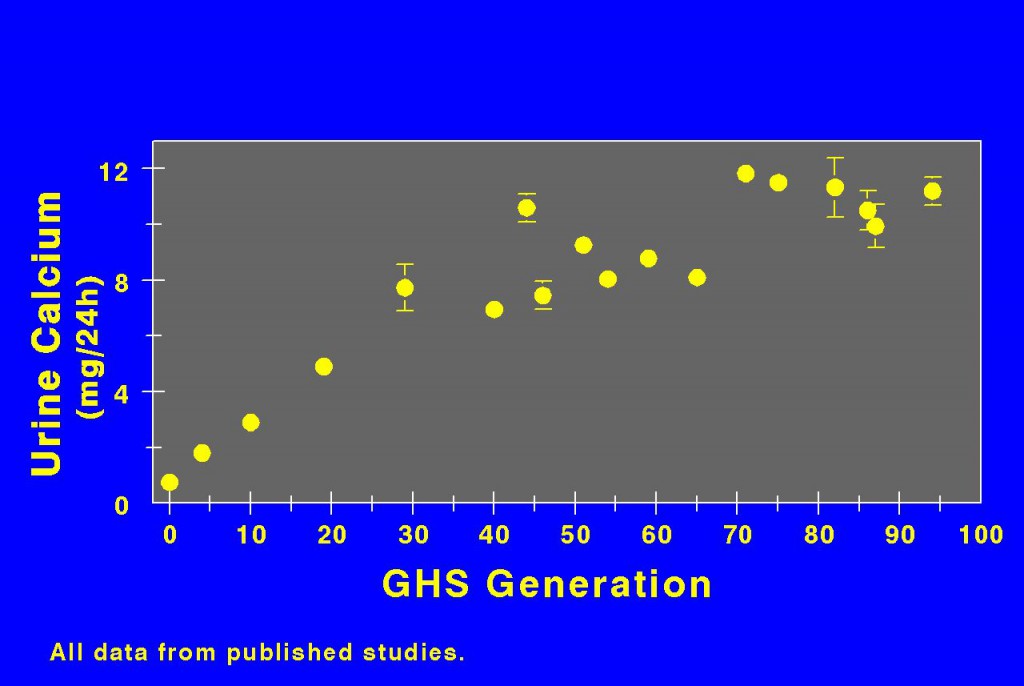
Animals
Dr. David Bushinsky bred rats with the highest calcium excretions. Urine calcium rose for the first 40 generations, and thereafter seems at a near plateau. So the trait is breedable.
These animals form calcium stones and develop a more severe bone disease than normal rats if diet calcium is not ample. So they well mimic human IH.
We humans did not breed ourselves for IH. Something about the trait must have conferred a benefit during evolutionary time.
Children
We had the opportunity to collect 24 hour urine samples from large numbers of boys and girls who were brothers and sisters of children with kidney stones, and also from children in families where none of the children, their parents, or other relatives were known to form stones.
Urine calcium excretions of siblings with more than two stones (left panel of the figure) are highest – farthest to the right. Next highest – second from the right – were siblings with 1 – 2 stones. Siblings with no stones were even 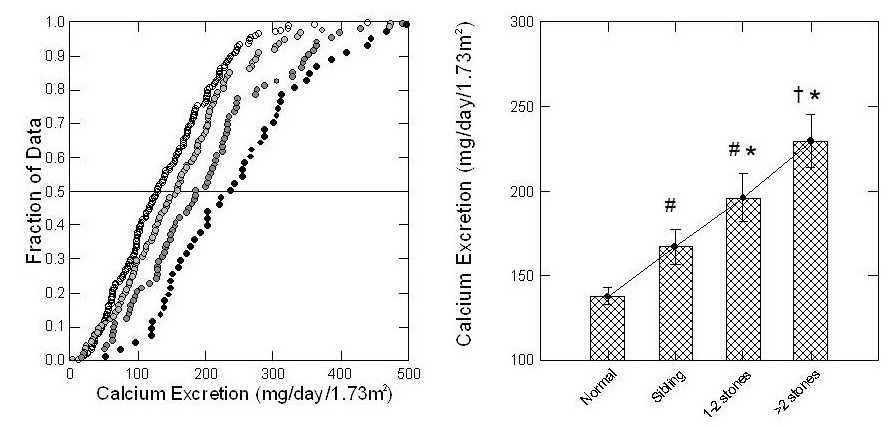 lower, third from the far right.
lower, third from the far right.
Children from families with no kidney stone history were lowest – most leftward – and almost none had above 200 mg/day of urine calcium loss.
The four bars in the right hand graph say the very same thing. Mean values of urine calcium, shown by the top of each bar, rose progressively with stones.
This is expected if IH is genetic and causes calcium stones.
Hypercalciuria with Hematuria
Hypercalciuria in children not rarely causes hematuria found on routine screening. Loin pain with hematuria is a common syndrome ascribed to crystal passage. IH can raise urine supersaturation and higher supersaturations promote crystals. Hematuria can be familial because it is due to IH and crystals or stones. In adults, unlike children, hematuria can be from malignancy so proper evaluation, even in stone formers, requires imaging and considerable care.
Bone Disease
There Is Bone Disease in Stone Formers
Epidemiology of Fractures
This figure, from people living in Rochester, Minnesota, shows the cumulative incidence of vertebral fractures among those who had a symptomatic stone (irregular line) and the expected fracture rate 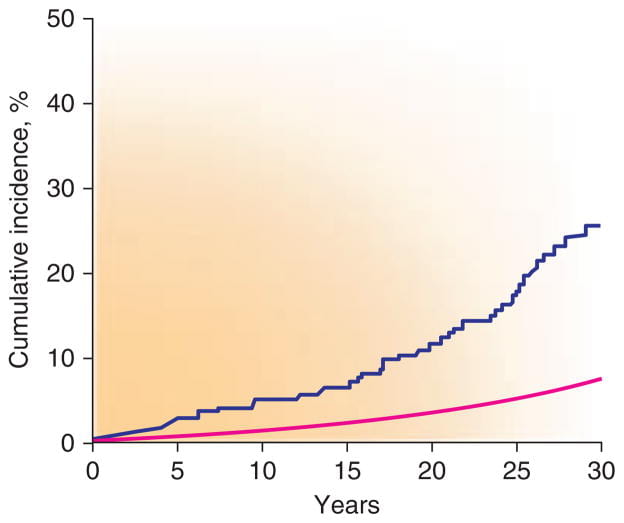 based on the entire population (the smooth line) between 1950 and 1974. The excess of fractures was not observed for hip or forearm.
based on the entire population (the smooth line) between 1950 and 1974. The excess of fractures was not observed for hip or forearm.
Bone Mineral Density
Reduced bone mineral density is a general finding in stone formers. 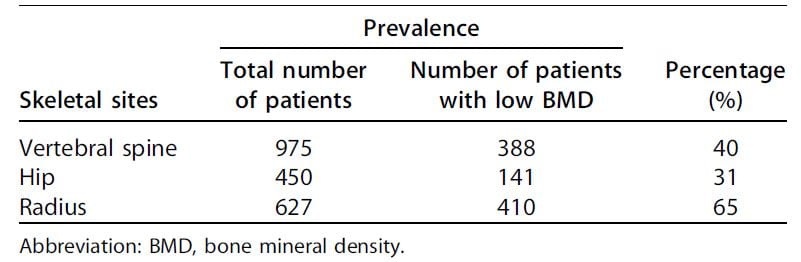
Among 2,052 patients assembled from 20 separate studies, between 31% and 65% had some reduction of bone mineral density (Table). Although not remarkable for fractures in the Rochester study, the radius was most affected.
The authors of this review did not conclude that IH caused the low bone density of stone formers. I infer it played an important role, however, because IH can promote bone mineral loss (detailed in the next section) and thiazide diuretics – well known to lower urine calcium in IH – appear to reduce bone disease.
Prospective Bone Mineral Observations
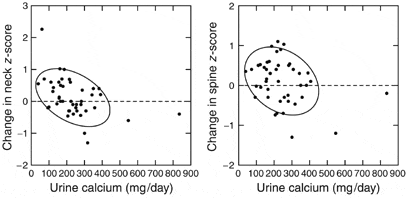
Another reason I make this inference is that the magnitude of urine calcium loss predicts future loss of bone mineral.
We measured bone mineral density in a number of stone formers with IH, collected 24 hour urine samples, and then re-measured bone mineral density three years later.
When change in bone mineral by three years (vertical axis) is plotted against the urine calcium loss at time 0, (horizontal axis), the trend – highlighted by 68% containment ellipses – points downward: More urine calcium loss at the beginning, more bone loss by three years. A majority of people with urine calcium above 200 mg/d lost bone mineral over three years, whereas those with values below 200 mg/d tended to gain bone mineral.
How Does IH Raise Urine Calcium?
The Extra Calcium Can Come From Diet
In the balance studies from which I derived normal calcium excretions, scientists fed subjects a fixed diet and measured all food calcium eaten and all calcium lost in the stool. The difference between calcium eaten and calcium lost in the stool is net calcium absorbed into the blood.
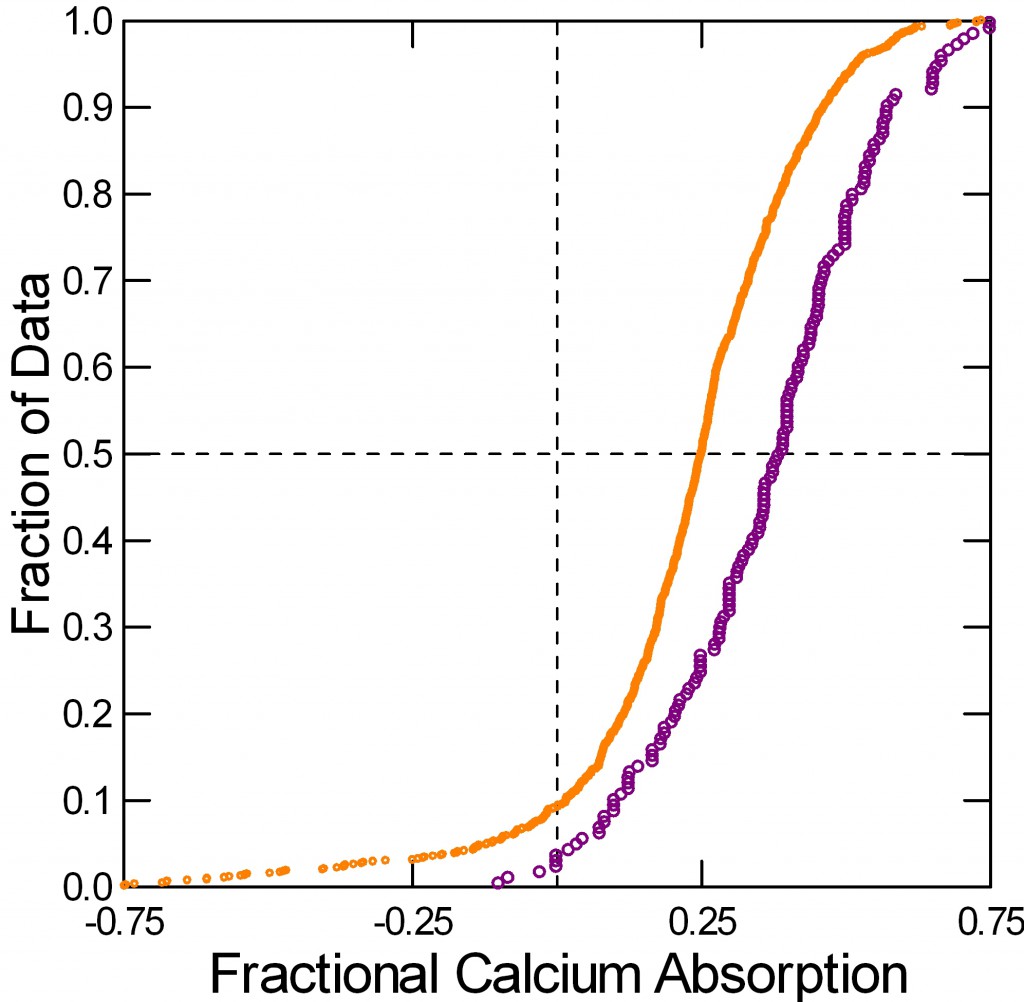
Typically measurements are made in 6 day blocks after a few days to equilibrate with the diet, so subjects participate for perhaps 8 – 10 days. I have aggregated the calcium absorption measurements that match the urine calcium excretions I already showed you.
Normal men and women (orange) absorb about 18% of diet calcium. I combined the sexes because they have almost identical values. Women and men with IH – the blue curve – absorb much more calcium, about 30%.
You might ask how calcium absorption can be negative – points to the left of the vertical 0 absorption line. It is because salivary glands, pancreas, liver via the bile, and perhaps the ileum secrete calcium from blood back into the bowel lumen. When diet calcium is less than this ‘endogenous secretion’, stool calcium loss exceeds what is eaten.
An early theory held that IH arose from over absorption of diet calcium: High absorption, more calcium comes into the blood, the kidneys lose it – done. This theory led to decades of low calcium diet as a treatment for stones. No one knew such diets might cause fractures.
The Extra Calcium Can Come From Bone
A Glucose Load Causes Bone Mineral Loss
Years ago Dr Jack Lemann did this informative study. He measured urine calcium excretion (vertical axis) then gave glucose or sucrose (table sugar) to normal people, calcium stone formers, and relatives of calcium stone formers.
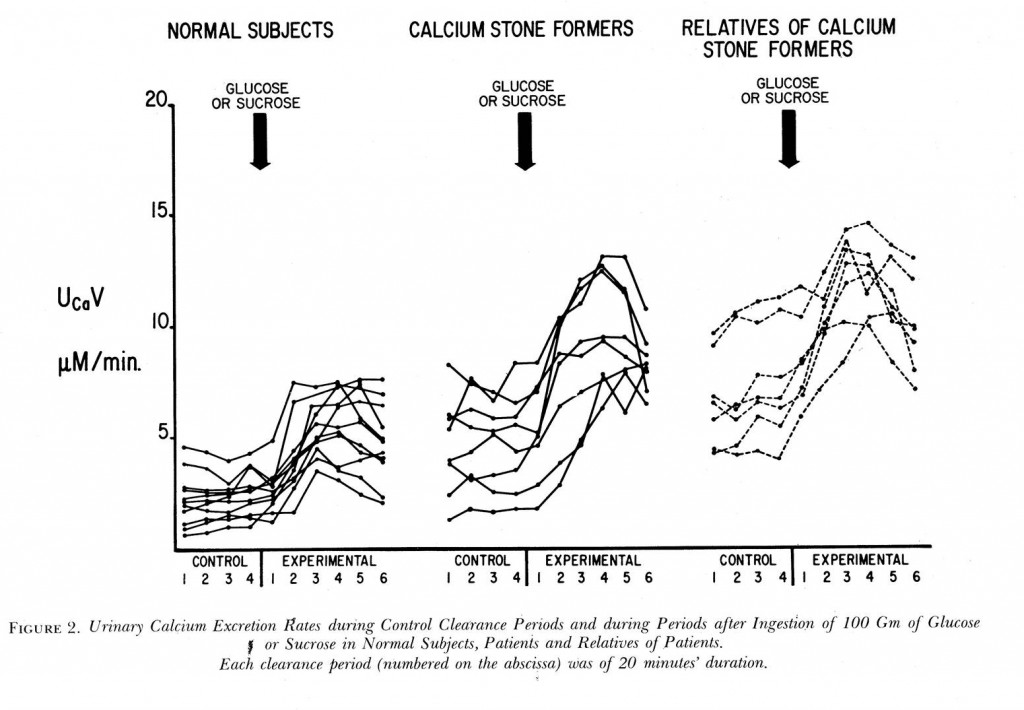 Compare the control (left of the big arrows) calcium excretions of the normal subjects to the stone formers: 5 of the stone formers have control values above all but the highest normals. The relatives of stone formers are even higher – and this is fasting, before the sugar load!
Compare the control (left of the big arrows) calcium excretions of the normal subjects to the stone formers: 5 of the stone formers have control values above all but the highest normals. The relatives of stone formers are even higher – and this is fasting, before the sugar load!
Each period was 20 minutes, so this experiment went on for 2 hours. The higher urine calcium with sugar must come from bone – there was no calcium in the sugar drink. It came from bone in normal people and in those with IH but the latter lost far more calcium than the former. Though fasting they had higher urine calcium losses.
In a separate experiment, Lemann proved that the kidneys themselves caused calcium loss from sugar by reducing their conservation of the calcium they had filtered out of blood.
Low Calcium Diet Causes Bone Mineral Loss
We persuaded nine normal people and 27 stone formers with IH to eat a very low calcium diet – 2 mg/kg body weight – for 9 days, and on days 7-9 we collected 24 hour urine samples and measured 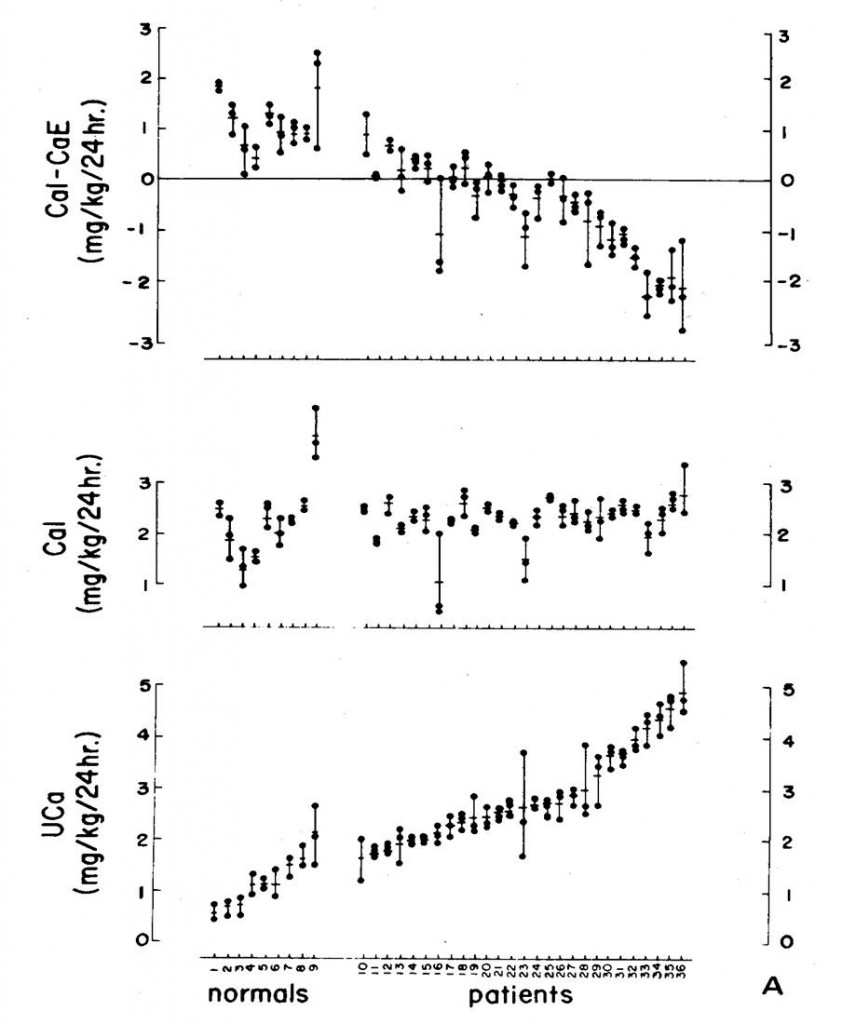 calcium losses.
calcium losses.
The diet went well; most people ate what we asked (middle panel). The normals (the 9 people to the left on the plot) lost in their urine less than 2 mg/kg of calcium daily – lower panel, to the left, so the difference each day between what they ate and lost was positive (upper panel, all normal points were above 0).
The patients were different. Many lost more calcium in their urine than they ate, and did so most of the time. This was bone mineral lost in the urine.
On such a low intake surely everyone was losing bone mineral because the fraction of diet calcium that is absorbed into the blood is far below 100%. I just showed you that it is about 18% in normal people and 30% for people with IH.
But those with IH were more flagrant than the normals. Because their urine contained more calcium than they ate we could prove bone mineral was lost.
In IH Urine Calcium Usually Exceeds Net Calcium Absorbed
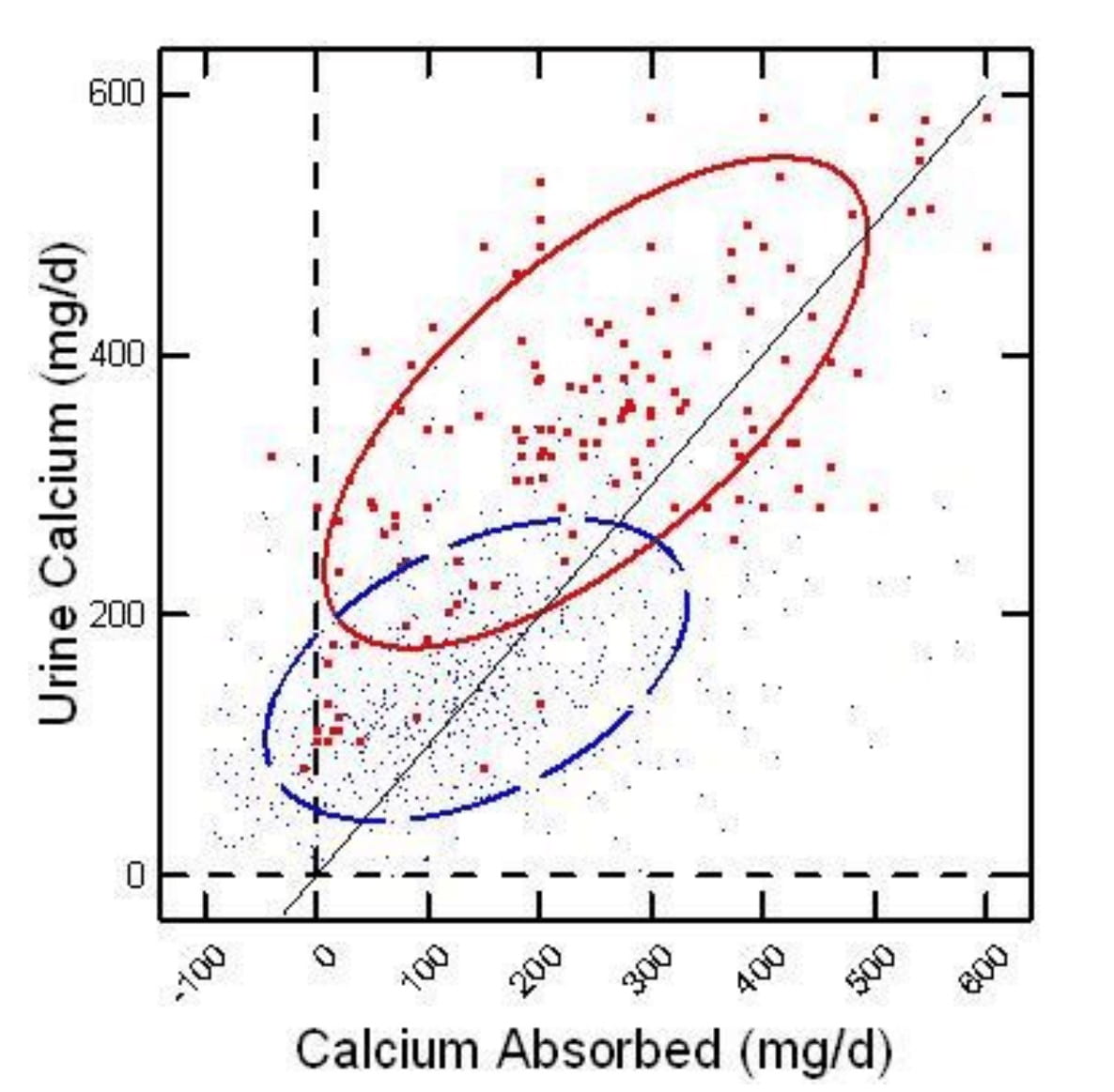
On the horizontal axis of this figure, calcium absorption is the difference between calcium eaten and lost in the stool. Urine calcium is on the vertical axis. People with IH are red large dots, and normal people are blue microdots.
Each point compares calcium absorbed in a day to calcium lost in the urine. If urine calcium is higher than calcium absorbed (points to the left of the diagonal line of identity), bone mineral is being lost in the urine. Those to the right the opposite – bone is gaining mineral.
At a net calcium absorption of 150 mg/d or more, a majority of the normal points lie to the right of the diagonal line – urine calcium is less than calcium absorbed. Bone mineral is stable or increasing.
Idiopathic hypercalciuria points all lie left of the diagonal line, negative bone mineral balance, until net absorption rises over 300 mg/d. It takes a huge amount of calcium absorption to overcome the tendency of IH people to lose bone mineral.
Bone Calcium Retention vs. Diet Calcium
Perhaps a more practical way to envision these balance data is to plot calcium retention – net calcium absorbed minus urine calcium excreted – against diet calcium intake.
At diet calcium intakes above 500 mg/day, the average retention (the jiggly blue line) for normals passes through 0, meaning that their bone mineral stores will, an average, be stable. Higher calcium intakes make the normal average rise so that by 1,000 mg/day 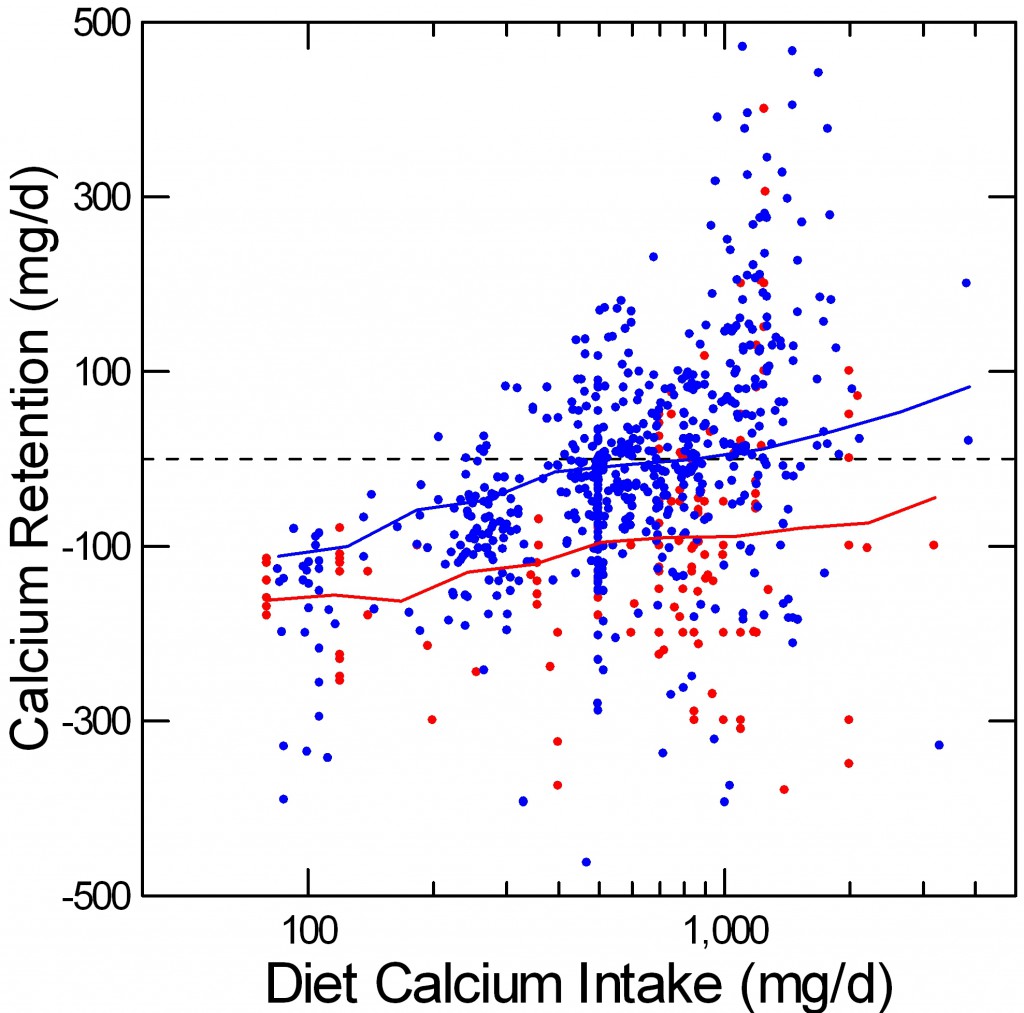 a majority of normal points are above 0.
a majority of normal points are above 0.
Among the IH subjects (red), retention rises with diet calcium intake, but the average – red line – never passes through 0. Some points do lie above 0, meaning that not all IH subjects will share the general high risk of bone mineral loss, just as some normal points lie below 0 even at high calcium intakes. But on average, at all reasonable calcium intakes, IH appears to hamper bone mineral retention.
What Have We Learned?
Low calcium diet is not ideal for normal people and a disaster for those with IH. Given IH, even a liberal calcium intake will not achieve stable bone mineral balance for the average person.
These balance data lay latent in papers published from 1900 through until even recent times. Using a different and sophisticated way to assess bone mineral balance, Lieberman and his colleagues showed as early as 1965 that IH reduced bone mineral stability. Yet low calcium diets remained a common treatment for stone disease for more than a decade thereafter.
IH Kidneys Release Excess Calcium
Filtered Calcium
Calcium gets into the nephrons of the kidneys by filtration from blood. If you do not know about filtration, use this link to learn about it.
Each of the 2 million nephron units we possess in our two kidneys has a glomerular filter that filters water, sodium, calcium, phosphate, oxalate, and thousands of other small molecules and ions out of blood into the long tubules that process the filtrate into urine.
Reclaimed Calcium
The process we care about here is reclaiming filtered calcium back into the blood. Normal people excrete about 2% or less of filtered calcium, those with IH excrete 4% to 5% or more.
Here are a few numbers. We filter about 150 liters/d. The filtrate contains about 40 mg/l of calcium: 40×150 = 6,000 mg/d of calcium. Of that 2% is 120 mg/d, 4% is 240 mg/d, 5% is 300 mg/d. So the differences in percent excreted account for the range of calcium between normals and stone formers.
Where Along the Tubule?
Review the Proximal and Distal Tubules
Each kidney tubule resembles a woman’s hair – long as a long hair, and that thin. Down the center of the hair is its lumen through which the filtrate passes to become urine, and where calcium is reclaimed.
Go back to the filtration article and check out the tubule picture. Pay special attention to the proximal tubule. In the proximal tubule calcium is reclaimed in parallel with sodium. In the distal tubule – on the picture in the link – calcium can be reclaimed independent of sodium.
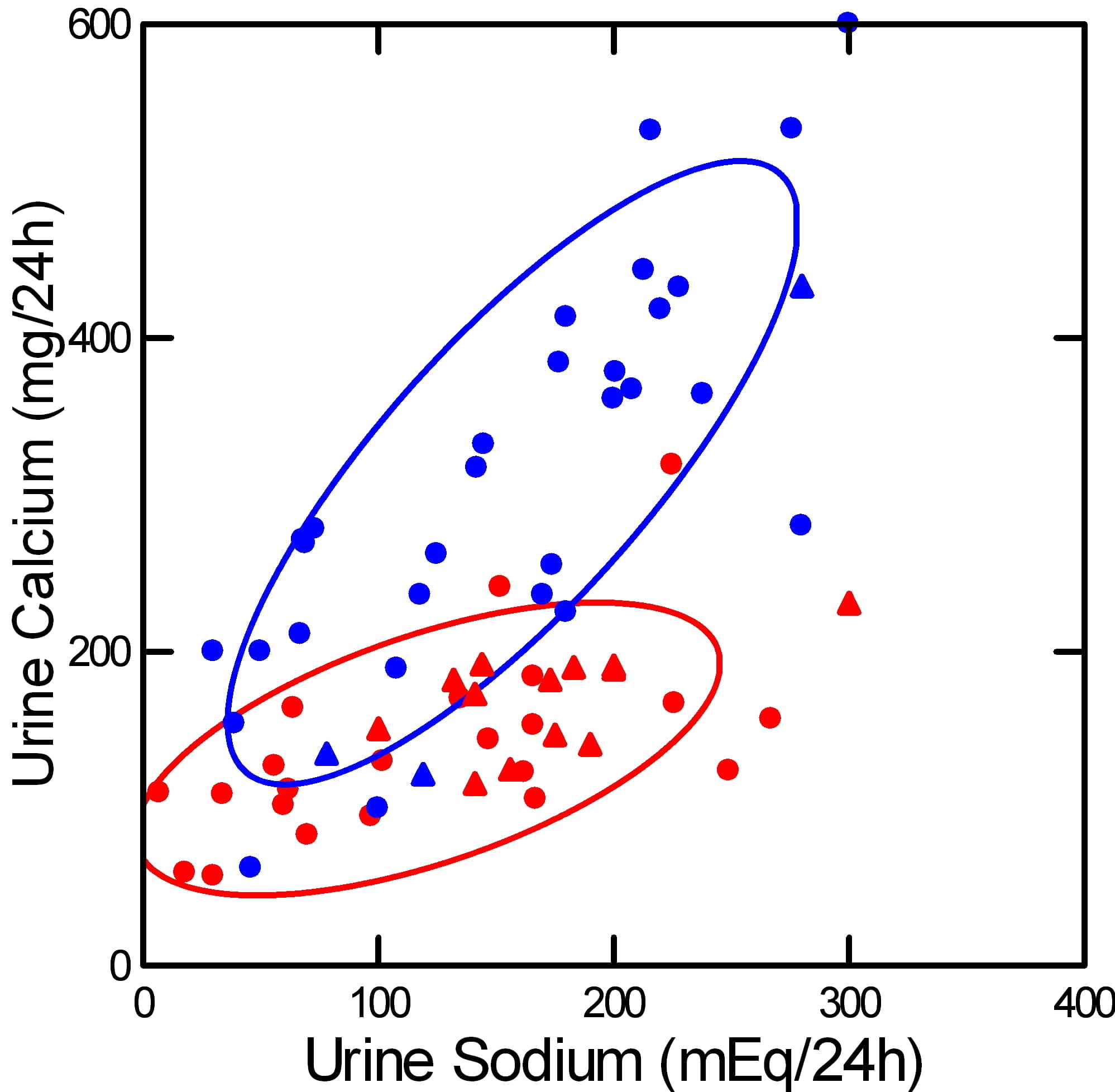
Urine Calcium Follows Urine Sodium
This picture illustrates the basis for recommending a low sodium diet to lower urine calcium in IH.
It shows how urine calcium (vertical axis) rises as urine sodium (horizontal axis) rises. The rise is far steeper among stone formers with IH (blue) than in normal people (red). Circles show experiments – diet sodium was deliberately altered. Triangles show observations – diet sodium and urine calcium varied on their own.
Urine Calcium and Sodium are Linked in the Proximal Tubule
As you eat more sodium, urine sodium goes up so output balances intake. One way the kidney accomplishes this balance is that filtration rises with higher sodium intake. Another is that reclamation of water and sodium in the proximal tubule (the part nearest the glomerular filter) goes down – more sodium and water flow downstream in the nephron. Calcium goes with it, the two are linked by the way that part of the nephron works.
For this reason, the steeper slope of urine calcium vs. urine sodium in IH must arise from abnormalities further downstream from the proximal tubule. We cannot presently identify where or how this happens.
What Can We Do With What we Know?
We can shut down filtration and increase reclamation of sodium in the proximal tubule. Both will reduce urine calcium by reducing delivery of calcium downstream. Lowering diet sodium does both, reduces filtration and increases proximal tubule sodium reclamation. The latter is usually more prominent than the former.
Thiazide diuretics do the same. They increase reclamation in the proximal tubule.
Once you understand this, you understand why reducing diet sodium and taking thiazide are two ways to do one thing. So the more you limit diet sodium the less you need thiazide, or at least the less dosage you need. On the other hand, if you take thiazide and eat a lot of sodium, the sodium will undo the effect of the drug.
What Happens to Bone?
Diet Calcium Must Be High
All this gives some insight into why IH appears to reduce bone mineral.
When we eat, the kidneys release calcium into the urine, normals and IH alike. But IH patients release a lot more calcium, depending on their sodium intake. If the diet has adequate calcium in it, bone can get its share even if more than normal is lost in the urine. If the diet is not so adequate, less than 1,000 mg/d, bone may not get its share even in normal people. Given IH, diet calcium must be quite high, at least 1,000 to 1,200 mg. But that cannot be sufficient as I have shown you. Even at such high calcium intakes, bone balance in IH is usually negative.
Diet Sodium Must be Low
The only present remedy for renal calcium wasting in IH is to lower delivery out of the proximal tubule. Low diet sodium, thiazide, ot both can do it. We presently have no other means that have proven effective.
The Combination of High Diet Calcium and Low Diet Sodium Can Preserve Bone Mineral
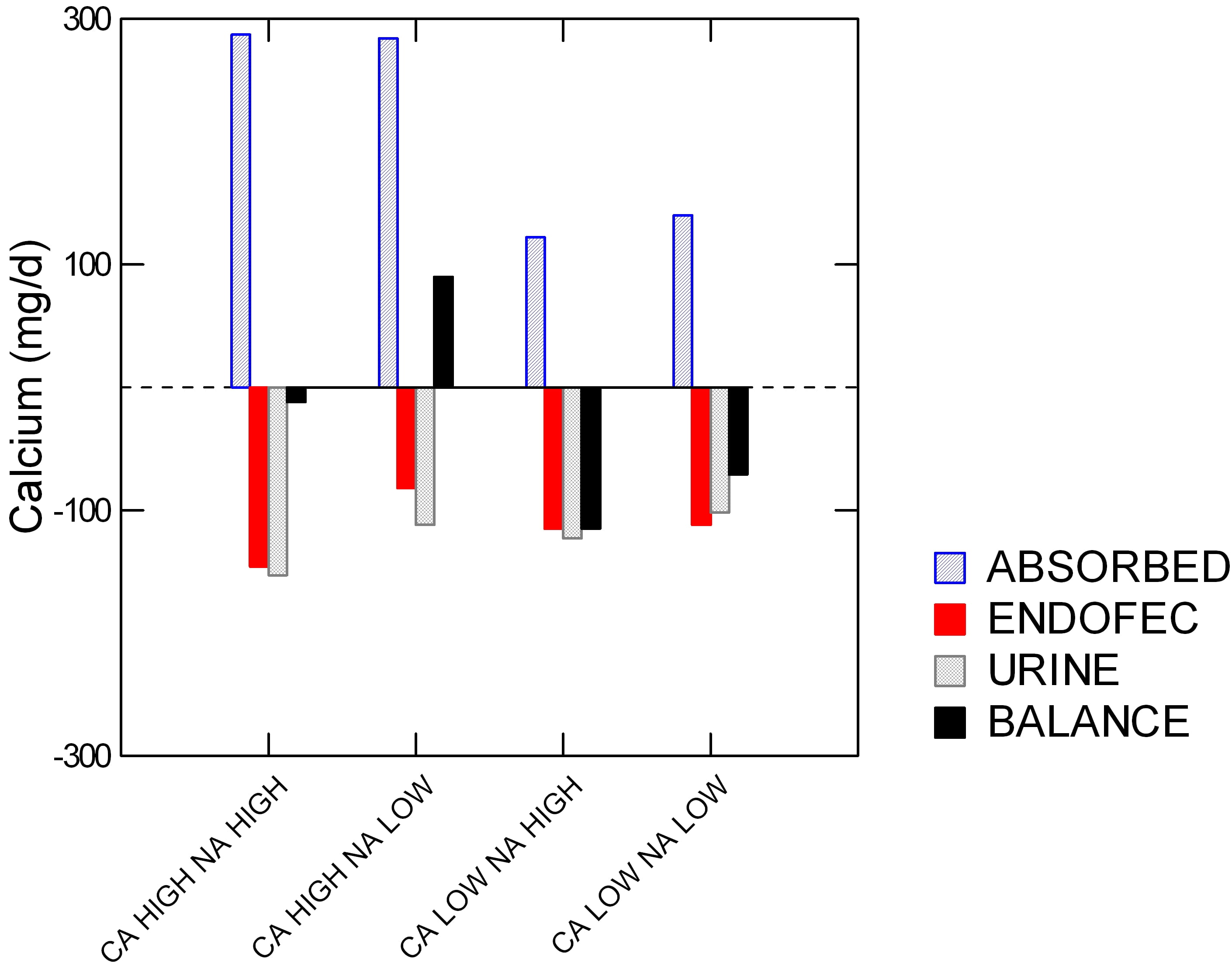 The best proof of this is one study showing that in perimenopausal women the combination of low diet sodium and high diet calcium can promote bone mineral gain.
The best proof of this is one study showing that in perimenopausal women the combination of low diet sodium and high diet calcium can promote bone mineral gain.
The women each ate all four of the diets shown along the horizontal axis: high and low calcium (Ca) and sodium (Na). Specifically, the sodium levels were 1600 and 4400 mg/day, and low and high calcium (518 and 1284 mg/day.
On the vertical axis is calcium in mg/d. The colors say if ‘calcium’ on the vertical axis is calcium absorbed (blue), secreted by the GI tract (red), lost in urine (gray), and bone balance (black).
Low calcium diets were hopeless. High calcium diets with high sodium led to high absorbed calcium (blue) but also high urine and GI endogenous secretion losses (‘ENDOFEC’): red and gray bars point downward. Reducing diet sodium lowered the urine loss (gray bar was less down) and also – surprise – less GI calcium secretion (red bar is less down).
The net result is good for bone. This one combination drove bone mineral balance positive (Black bar above 0).
Before we leave this powerful demonstration, look back on urine calcium (gray bars). The high calcium low sodium diet gave the very same urine calcium as the low calcium high sodium diet. In other words, the women could raise their diet calcium from 500 to nearly 1300 mg/day and yet by lowering diet sodium to 1600 mg/day keep urine calcium unchanged.
What Makes Calcium Go In or Out of Bone?
Blood is saturated with respect to the initial phases of bone mineral, so called early hydroxyapatite forms. Likewise bone has considerable circulation, so that the outer layers of bone can be in physicochemical equilibrium with the blood. In isolated bone reduction of calcium phosphate supersaturation leads to physical dissolution of bone mineral.
It seems not unreasonable that tiny reductions in blood calcium phosphate saturation can occur when kidneys release calcium into the urine at a rate that exceeds diet calcium absorption. The loss of bone mineral from simple sugar ingestion may well be an example of this effect. Of course bone is regulated by myriads of hormone signallers, but short term mineral balance could be affected by physical forces. This is an area that deserves research.
I should say that in presenting this conjecture about bone it is just that. Furthermore I doubt it is sufficient as an explanation. But it shows at least one plausible connection that can be demonstrated in isolated bone, and perhaps in humans.
In another article, as yet unwritten, I will take up the larger issues of bone and kidney in IH, and show the deeper science that is now available.
What Should We Do?
Kidney Stone Prevention
Without doubt, reduced diet sodium and refined sugar are valuable in all people with IH. Although I did not demonstrate it here, a high diet protein load raises urine calcium and is best brought into the normal range of 0.8 – 1 gm protein/kg body weight/d. Protein intake is calculated from urine urea excretion as the protein catabolic rate (PCR) and best quality kidney stone testing vendors present it on reports.
Bone Disease
Provide Adequate Diet Calcium
Diet calcium must be adequate, 1,000 to 1,200 mg/d. Without concomitant control of diet sodium this alone would raise urine calcium and kidney stone risk. But when combined with low sodium it will not. Multiple proofs of this statement exist. I just showed you one in the lovely four way bone experiment.
Measure Bone Mineral Density
US insurance practices exclude bone evaluation in large swathes of stone forming populations. But bone mineral scans are not very expensive compared to the eventual costs of fractures. A useful medical buying guide places the bone mineral density scan cost to uninsured people at about $200.00, and mentions that in May prices can be lower because it is national osteoporosis month. The price usually includes a simple medical interpretation.
The Kidney Stone Diet
The proper diet for prevention of the common calcium stone arises almost totally from the requirements to treat IH: reduced diet sodium, refined sugar, and protein, and adequate calcium for bone. The last of these, high diet calcium plays another role in stone prevention by lowering urine oxalate. That is fully described in other articles.
As well as stone prevention and the protection of bone, this diet is thought beneficial for reduced risk of hypertension and vascular disease, to which stone formers seem unduly prone.
Because it accords with general diet recommendations for the entire US population, I can recommend its use without hesitancy for stone formers and, incidentally, all the rest of us, too.


Hi Dr. Coe,
Along with everyone else here – thanks for your valuable insights. I am hoping you can shed some light on my situation.
I am in my 60’s, in good health, physically fit, proper weight and I lead an active lifestyle. I’ve had a number of calcium oxalate stones in the past 5 years – some have apparently passed and some were surgically removed. I was diagnosed with hypercalciuria and put on a dietetic a month ago. I was told that my kidneys are leaking calcium, and that diet alone would not address my problem. Here some key values from recent labs:
Urine Calcium: 350
Urine Oxelate: 34
SS CAP: 2.10
Urine pH: 6.5
Na 24: 64
Ca 24/Kg: 4.6
Ca 24/Cr 24: 232
The dietetic has the bad side effect of waking me up to use the bathroom every 60 minutes at night. I am on the minimum dose of chlorthalidone, so reducing dose is probably not the answer. I have the discipline to maintain a specific diet, and would be happy to do so as a long term fix, but I am uncertain if this would work for me. I would like to know if the lab numbers offered above indicate a case where diet would have no significant effect, and therefore living with the diuretic is the way to go.
I’ll add that my Doc is running the required potassium test today (it’s been a month) and will also look at parathyroid hormone, so maybe we’ll discover something there.
Again, I look forward to your thoughts on my case.
Thanks!
Hi Billy, Your urine calcium is very high despite a very low urine sodium of 64 mEq/d and chlorthalidone as well. I presume your serum calcium is perfectly normal, and your PTH is not suppressed below normal. Such a high calcium with so low a diet sodium and CTD is very unusual and points to alternatives to common idiopathic hypercalciuria. Your physician is right to measure PTH, she/he might want to measure 1,25d as well. Regards, Fred Coe
Thanks for the response Dr. Coe!
First off, sorry for my consistent misspelling of “diuretic”!
I just got my latest blood panel labs back and here are key results relevant to the conversation so far…
PTH 31 pg/ml
Sodium 138 mmol/L
Potassium 3.8 mmol/L
Calcium 9.4 mg/dL
The only item in the full panel not tagged as “normal” is glucose at 107 ml/dL, but I wasn’t fasting (wasn’t told to!) and as far as I can tell, 107 is ok if the patient is not fasting. Otherwise everything is a-ok.
The only clarification that I’d make is that I wasn’t taking any diuretic at the time of the 24-hour urine test, so the results in my initial post were from before I was taking a diuretic. Not sure if that matters to what you are thinking.
Your comment “Such a high calcium with so low a diet sodium and CTD is very unusual and points to alternatives to common idiopathic hypercalciuria” I took to mean that idiopathic hypercalciuria is not a likely an accurate diagnosis for me, is that correct? If so, are there likely avenues for me to look into with my Doc?
Hi, I should perhaps say better that such high values do occur in IH but are very uncommon. That your PTH is not suppressed removes one category of unusual hypercalciuria. I have to assume fasting serum calcium is absolutely normal – be sure it is. Perhaps your 1.25 vitamin D is very high, a known driver of very high urine calcium levels. If it is IH with very high urine calcium despite moderate diet sodium, the only reasonable approach is to limit diet sodium as much as possible – usually 1500 mg is ideal, or even lower – and use thiazide type diuretics in that order. Perhaps your person physician has already thought much the same. Fred
Thanks again Dr. Coe.
I looked back in my recent labs and my serum calcium was 9.4 mg/dL. I am not finding any evidence of any vitamin D results. I will request this when I speak to my Doc this week.
Regarding diet, I’d like to try to bring my sodium down further to see if that attenuates the urine calcium level. Also, to what degree does alcohol have on urine calcium? I typically have 1-2 glasses of wine with dinner. I can cut that out if it’s likely to have an effect.
I’d prefer do explore all aspects of my diet first, rather than taking a diuretic, given the side effects of frequent visits to the bathroom at night, not to mention the need to always factor in bathroom accessibility every time I go out somewhere during the day.
Hi Billy, I hope diet can suffice. But diuretics do not increase urine volume, so they do not affect voiding habits. It is high fluid intake that does that. Fred
For IH patients who have allergic reactions (hives) to sulpha drugs and penicillin, is it safe to take thiazides?
Hi Barbara, Most people who have sulfa allergies tolerate thiazides, but your physician will want to take some precautions. Fred
Hello Dr. Coe-
Pleased to find this article and “meet” you! I have recently been diagnosed with idiopathic IH. Two bone scans and two 24-hour urine test to make sure. I’m taking a thiazide which has shown to help:
Ca/Cr ratio 424 to 294
Ca 24 hr urine 534 to 180
After four months of seeing what the thiazide would do on its own, I want to help increase my bone density by adding AlgeaCal; they guarantee a better bone scan in 6 months of using their product. (I’m 40 but already showing osteopenia and osteoporosis in certain areas!!)
-HERE IS MY SITUATION: the support team needed my calcium intake from my multivitamin; it’s 300mg. AlgaeCal has 720mg. They suggest I go to 150mg or less in my multivitamin so I do not ingest too much calcium. Given my idiopathic IH, and needing to be on a high calcium diet, do you think keeping my 300mg multivitamin, 720mgAlgaeCal, and the “average” Ca intake of 500mg would be too much? I assume, even if the thiazide works, that I’ll still pee out excess Calcium. I did not question them yet; it was just yesterday that I’m starting AlgaeCal. What do you think?
Thank you for your time,
S. Lee
Hi S. Lee, Calcium is an atom, and any of the sources you mention are usable. The goal is 800 to 1000 mg of calcium a day and if it is from supplements they are best taken with meals, not in between. Thiazide has done well for you, and will do even better if you keep your diet sodium low – 1500 mg or less. As for the bone disease, if there is already regional osteoporosis your physicians may want to add a bone specific medication to prevent more losses. That may in turn help lower urine calcium, and stone risk. Regards, Fred Coe
Hi Dr. Coe! I found your page in April 2018 and faithfullyfollow your dietary advice, and have good 24hr urine test results. In the 11 years of urine testing (even before I found this diet) I did not have hypercalciuria. My urine calcium is always below 100. I am a vegan, and I do use plant milks (coconut and pea) to get my calcium. One has 460mg per cup, the other has 440mg per cup. I drink one with breakfast, one with dinner. However, today I just totaled up what a typical days calcium intake looks like, and I am way higher than I ever could have imagined. About 1330mg, and I am a female under 50, so should be aiming for 1000mg. I also eat less than 1500mg of sodium per day because I cut out most processed foods 3 years ago.. My question is…if my urine sodium is low, and my urine calcium is also low despite the higher-than-necessary calcium intake, am I causing myself problems, stone-wise? Should I scale back the amount of fortified plant milks I’m drinking? I had no idea my fruit and veg contained that much calcium until I totaled it up. I also don’t want to go too low with calcium because I don’t want to raise my urine oxalate levels, which are currently below 25. Thank you for any help! My nephrologist doesn’t really get into diet with me.
Hi Amy, It sounds like you are doing very well, eating a lot of calcium and with little in the urine. Why change anything? You do not mention stones, but with a low urine calcium and oxalate that is not surprising. My only advice is to get a bone mineral density test to be sure bones are indeed doing well. Regards, Fred Coe
Hi again, Dr. Coe-
Thank you so much for responding! I have had stones for years…they tend to be 80-90% calcium oxalate, and 10-20% calcium phosphate. I found your page in April 2018 when I found out I had 7 new stones…which was a shock to me. My Litholink results have been good, both on my “old” diet and the kidney stone prevention diet, but I am guessing that before 2018’s diet change, I had high supersaturations because I drank very little fluid. Urine volume was the only thing ever flagged on my pre-Litholink testing. Now my risk factor is my high pH, but I also have high citrate levels, plus the low urine calcium and oxalate levels, which we already talked about. I do have low bone density, found on dexa scans starting in 2010. I am small, and my birth mother was also small and was diagnosed with osteoporosis also in her 30s. Anyway, thank you for responding about my high calcium intake. I have my annual Litholink coming up next week, so am anxious to see if urine calcium is still low, now that I’m aware I was accidentally taking in too much. I really appreciate all your hard work, Dr. Coe! This website, and Jill’s facebook group have changed my life for the better in so many ways…thank you, thank you, thank you!
Amy
Thank you Dr. Coe for your lengthy article on I.H. I diagnosed myself with this condition after reading a transcript of my medical record after moving. There were many clues over many years, but no one Doctor had put it all together. I am taking 50 mg of Chlorthalidone, 2000 IU Vitamin D, 500 mg Calcium supplement with a recommended +1500 mg Calcium from dietary sources. I was told to watch my sodium and animal protein.
I currently eat vegetarian every other day and meat the other days. After reading your article, I began calculating the sodium and calcium for a few days in everything I consumed.
The diuretic last June 2020 had my 24 hour Calcium level to normal levels. June 2021 my Calcium levels are back to double normal, as they were when first diagnosed.
I did noticed that your article does not distinguish between animal and vegetable protein. I am currently eating veggie meat substitutes every other day. Also my dietary calcium comes milk and cheese, which both from animals.
Is 100 gm daily the amount of protein I should be targeting? What source or does it matter?
I am watching my sodium intact moving forward. This is so complicated to change your diet for this condition.
Hi Brian, I am not sure the protein source matters. Vegetable protein, however, comes with potassium alkali that are protective via increased urine citrate, a benefit in that alkali loads benefit bone. You are right that IH is complex – we have spent decades studying it. The lowest diet sodium possible is the most critical base for everything else. Normal protein intake should be about 1 gm/kg/day. Regards, Fred Coe
Thank you. The information concerning sodium intake was very informative. I have cut down my sodium intake just buy looking at all the nutrition labels and after a few weeks, I believe it has had some significant effect. I will know after my next 24 hr urine test. This website is invaluable as information about IH is hard to come by. I was very surprised with many of the sodium levels in the foods I was eating. I seldom added salt anyway, but that was not enough.
Hi Brian, Good. Let’s hope the followup values show improvements in stone risk. If they do not, do not despair, patience and slow changes usually get you to where you want to be at. Regards, Fred Coe
Hi Dr Coe
I am beholden to you and the wonderful Jill Harris who 4 years ago helped me learn the diet by which I follow closely. Despite all the numbers looking good—I have persistent urine calcium of 330. I am on Vitamin D 2,000IU, chorthalidone 25mg daily and KCL 20 meq three times per day. Since adding the diuretic, my urine calcium hasn’t budged. It is frustrating! I am 62 and have osteoporosis in my radius (hips and back look OK so far). Should we increase my diuretic dose? My Vitamin D level is 28. PTH is fine. Should we switch to hydrochlorothiazide? or is there another diuretic to try that might by more helpful? I love Dr. Borofosky, but he seems satisfied with low 300’s because the rest looks OK. thank you for your time.
Hi Barb, I gather you have lowered your diet sodium and therefore your urine sodium is at an optimal level of 65 mEq/d or less, and you have reduced your diet refined sugar as well. Given that, I cannot say much more about chlorthalidone vs other drugs. IN general CTD will be about as good as indapamide, and both are better than hydrochlorothiazide in my experience. So be sure about the urine sodium and diet sugar as I suspect they are making CTD ineffective. Regards, Fred Coe
Hello Dr. Coe, I learned a lot from your website, thanks for sharing your knowledge. I had autoimmune disease and was treated with prednisone for some time then tapered down. In July 2021 my bone density scan showed osteoporosis, my serum calcium level was 8.4, Vitamin D was 41, PTH was elevated to 124, 24H urine calcium output was high at 395mg. Then I was prescribed with 25mg chlorthalidone QD as well as taking 1200mg calcium supplements, in September my serum calcium level was 9.1, PTH was lowered to 47 and 24H urine calcium output was down to 297mg. All seemed good, but after taking 25mg chlorthalidone I had to frequently urinate in the night (4-5 times even though the volume is not a lot) and did not get enough sleep every day. Do you think can I take a lower chlorthalidone dose like 12.5mg? And do I need to take chlorthalidone for life or at what time point can I stop? Thank you very much!
Hi Tim, It sounds like the drug has benefitted you and I would continue it. CTD does not raise urine volume after the first few days, and it sounds like your night time urination is not from excessive volumes of urine but from frequent emptying of a less than full bladder. This latter usually reflects prostate disease, in men, and perhaps your urologist might have a solution. Regards, Fred Coe
Hi Dr. Coe:
I have heard pro’s and con’s on HCTZ.
some says it works other say to use chorthalidone or indapamide.
Is it because HCTZ has more side effects than the chorthalidone or indapamide?
Thank you.
Hi Dianne, The former two have a longer life so are active over nearly 24 hours. The last has a 6 hour 1/2 life. I prefer one of the first two but all three have proven effective in stone prevention. Be sure to alter your diet, not only use pills. Regards, Fred Coe
Hi Dr Coe, thank you for the wonderful resource that this website is! Can IH lead to secondary hyperparathyroidism? I have a patient with severe hypercalciuria (519, 360: on 2 24hr collections), iPTH 90, serum calcium 8.6, 8.9. 25hydroxyVitamin D 31.
I am doing a trial of HCTZ 25mg daily to see if the calciuria improves with HCTZ- if yes, I may be able to call it IH. Otherwise, it may be normocalcemic primary hyperparathyroidism (?with low calcium intake causing low-normal serum calcium?). I would appreciate your insight and experience!
Thank you
Hi Dr, Interesting problem of hypocalcemia, high PTH, very high urine calcium, and normal 25D. Primary hyperparathyroidism is not present, as you surmise. Given the low serum calcium, tubule calcium reabsorption is quite reduced (I assume normal albumin and patient is not diabetic with hyperfiltration, and there is no kidney disease). The picture suggests a tubule defect or perhaps very marked IH and low calcium diet. Perhaps I would add low diet sodium and high diet calcium to the thiazide and see if the PTH comes down. I assume no use of Lasix. Regards, Fred
Dear Dr. Coe,
I am a 62-year-old 5’2″ petite woman who was diagnosed with severe osteoporosis 2 years ago. My physician said that I have IH due to my 24-hour urine calcium score of 319 on 8/27/21, calcium/creatine was 309, my blood calcium was flagged high at 10.5, and PHT was normal at 33.
On 10/21/21 my urine calcium was 359, calcium/creatine ratio 354, sodium normal in urine 129.
I started Hydrochlorathizide 12.5mg on 11/21. On 1/26/22, 24-hour urine was 316, calcium/creatine ratio 330, blood calcium, 9.8, PTH normal at 39.
On 3/29/22, blood calcium flagged high at 10.6, PTH normal range but now 69, creatine in random urine 28 and protein flagged at less than 4.
My hydrochlorothiazide was increased to 25 mg in March 2022. All along I follow low calcium, low sodium diet to the best of my ability. I take a total of 740 mg calcium supplement of Algae Cal Plus Bone formula. The rest to total 1000 is food. (I try not to go over, but it happens.
Now in August 2022, my 24-hour urine is 332, calcium/creatine ratio 354, and creatinine is normal .96, PTH 43, vit d 60.
I do not take any osteoporosis drugs because the doctor said my urine levels need to get under control first.
It’s really not better than before medications, actually worse than my original score on 8/27/21. I do not have Cushings or PTH issues. I am always constipated, have heart calcifications from a CT scan in 2020 and benign bilateral adrenal nodules, no kidney stones, high anxiety, and really, really worried. I do not have IH in my family that I know of. My mom is 91, in perfect health besides osteoporosis at 50, no fractures, she said all the women on her side have it. (sister, mother, grandmother, and aunts all had it)
I would like to find out if I REALLY have IH. If so, why is my urine not getting better and blood calcium always on the high end? What other tests could I request that cause high urine calcium and higher than normal blood serum, please? What are your thoughts about all of this, please? Lastly, do you have a recommendation for a specialist in Massachusetts, Boston area?
I thank you so much for your time and greatly appreciate all of your knowledge.
Sincerely, Maureen G
Hi Maureen, The presence of high blood and urine calcium while NOT taking the hydrochlorothiazide, with normal serum PTH make a good case for primary hyperparathyroidism. This disease causes bone disease. I would stop the diuretic and after 2 weeks get more fasting morning bloods for calcium and PTH to confirm high serum calcium and normal (or high) PTH. That is enough to point one to this disease which is almost always cured by non invasive surgery. I have been working with a very fine physician (Dr Jessica Tangren) at MGH who may not be an expert in this problem but certainly can point you to one. The link is to her email. Regards, Fred Coe
Dear Dr. Coe,
I greatly appreciate your response and thank you so much. I am a bit confused though, lol! I have records dating back from a few years until now, before taking any thiazide meds, as well as, after taking the meds starting 10/21, showing my blood serum calcium in the 10’s. It’s either on the very end of the scale or flagged for being a bit over, with the highest of 10.7. However, a few times it has also been in the 9’s. both before and after taking the meds, but only maybe 3 times at most. My PTH is “always” in the normal range.
It wasn’t until 10/21, that I did my 1st 24-hour urine and found out about having hypercalciuria. My doctors keep saying that I DO NOT have a parathyroid issue because it’s always in the normal range. They keep saying it’s idiopathic.
My questions:
Is “primary” parathyroidism different from hyperparathyroidism?
Also, if I have high serum calcium(end of the scale or flagged) and high urine calcium BUT a normal PTH serum I could have primary hyperparathyroidism?
That seems to be my case regardless of taking a thiazide or not, except for those few times when my serum calcium was in the 9’s. Actually, what is a normal number for my age, please?
I do know one thing, I have never been told that this test requires fasting, the order always says non-fasting. I will definitely fast and stop the thiazide for 2 weeks before the next test.
I appreciate you and your knowledge more than you know! Thank you, thank you, thank you!!!!
Sincerely,
Maureen
Hi Maureen, Yours is a common story for me. Primary hyperparathyroidism often presents with borderline high serum calcium values, and PTH is usually in the normal range. I recommend (again) multiple morning fasting bloods for calcium, phosphate (low in PHPT) and PTH (you might add 1,25 vitamin D on one occasion as it runs high in PHPT). IF the general average calcium is above normal and urine calcium is above normal and PTH is not abnormally LOW, PHPT is the usual problem and surgically curable. The very nature of PHPT creates the high serum calcium and variable PTH – so long as PTH is not abnormally low. You must be off thiazide for at least 2 weeks before attempting these bloods, and fasting means nothing to drink or eat prior to blood draw. I have many patients who lingered for months or even years with on and off serum calcium elevations before we committed to curative surgery – all had the disease. Perhaps you physicians will agree with me if they read the two articles. It is their responsibility, as I am an outsider and speaking here just as an unofficial conduit of medical information. Regards, Fred Coe
Hi Dr. Coe,
Thank you so much for your work. Please, I am hoping you can shed some light on my situation too.
I am 50 years old, in good health, proper weight (63kg) and I lead an active lifestyle (mother, wife, teacher), living in Montreal Canada. I’ve had a number of stones in the past 20 years – five of them have passed ( the one analysed in 2003 was Oxalate – CA 40% and PHOS.ca Hydroxy 60%) with pain and no medication and some are still waiting. The largest ones that I have now are 5.5 mm on one kidney and a 5mm on the other plus many smoller ones.
I was diagnosed with hypercalciuria 448mg/L, PTH 5.9, hypophosphatemia 0.78 and the conclusion of a thyroid ultrasound: ‘A mixed primarily cystic lesion is noted inferior to the left lobe of the thyroid with increased flow within the solid peripheral wall component. In view of the clinical history, this is suggestive of functional parathyroid cystic adenoma. Further evaluation with parathyroid scintigraphic scan is suggested. Bilateral millimetric TI-RADS 2 thyroid nodules of no significance.” The nefrolog that directed me to have the tests decided on the second apointment to send me to have the operation. I really want to try everything else before and leave the surgery as last choice.
I was told that I’m leaking calcium, and that diet alone would not address my problem. Please read some key values from recent lab results and guide me throught this labirint.
May 2021 March 2022 Val Ref
PTH: – 5.9 1.2 – 8.4
Ca: – 2.45 2.15 – 2.62
Phosphore: 0.79 0.78 0.80 – 1.45
HbA1c 0.052 0.04 – 0.06
G.B: 3.59 3.6 4 – 11
Urine Ca: 8.09 11.2 (448mg/L) 2.5 – 7.5 (=30
Urine pH: 6.5 7.0
Ca Endocr: 2.43 2.45 2.15 – 2.62
Bone density : – normal
They are many more but they are in normal limits.
I can maintain a specific diet (I do not drink sodas and I cook with no salt and I realy, realy rarely eat procesed food. Thanks to Jill Harris I stopped eating the top oxalate foods.), and would be happy to do so as I know what exactly can help me. I would like to know if the lab numbers offered above indicate a case where diet and treatment (?) would help or the next step is surgery.
I look forward to your thoughts on my case.
Thank you very much!
Hi Mihaela, Your serum calcium appears normal, so I have much doubt about primary hyperparathyroidism. To be sure, I would suggest at least three fasting, morning serum samples for calcium and PTH. I predict all three will have normal serum calcium and PTH. You do indeed have marked high urine calcium and stones with low serum phosphate. Your care might be enhanced by genetic testing for a NaPi2c gene variant of significant consequence that could explain the low phosphate and high urine calcium. A serum 1,25D would be expected as well. If this is what you have, then oral phosphate supplement could much reduce the urine calcium. Perhaps your physicians might wish to consider this possibility. Regards, Fred Coe
Hi Dr. Coe,
Please, I am hoping you can shed some light on my situation too.
I am 50 years old, in good health, proper weight (63kg) and I lead an active lifestyle (mother, wife, teacher), living in Montreal Canada. I’ve had a number of stones in the past 20 years – five of them have passed ( the one analysed in 2003 was Oxalate – CA 40% and PHOS.ca Hydroxy 60%) with pain and no medication and some are still waiting. The largest ones that I have now are 5.5 mm on one kidney and a 5mm on the other plus many smoller ones.
I was diagnosed with hypercalciuria 448mg/L, PTH 5.9, hypophosphatemia 0.78 and the conclusion of a thyroid ultrasound: ‘A mixed primarily cystic lesion is noted inferior to the left lobe of the thyroid with increased flow within the solid peripheral wall component. In view of the clinical history, this is suggestive of functional parathyroid cystic adenoma. Further evaluation with parathyroid scintigraphic scan is suggested. Bilateral millimetric TI-RADS 2 thyroid nodules of no significance.” The nefrolog that directed me to have the tests decided on the second apointment to send me to have the operation. I really want to try everything else before and leave the surgery as last choice.
I was told that I’m leaking calcium, and that diet alone would not address my problem. Please read some key values from recent lab results and guide me throught this labirint.
May 2021 March 2022 Val Ref
PTH: – 5.9 1.2 – 8.4
Ca: – 2.45 2.15 – 2.62
Phosphore: 0.79 0.78 0.80 – 1.45
HbA1c 0.052 0.04 – 0.06
G.B: 3.59 3.6 4 – 11
Urine Ca: 8.09 11.2 (448mg/L) 2.5 – 7.5 (=30
Urine pH: 6.5 7.0
Ca Endocr: 2.43 2.45 2.15 – 2.62
Bone density : – normal
They are many more but they are in normal limits.
I can maintain a specific diet (I do not drink sodas and I cook with no salt and I realy, realy rarely eat procesed food. Thanks to Jill Harris I stopped eating the top oxalate foods.), and would be happy to do so as I know what exactly can help me. I would like to know if the lab numbers offered above indicate a case where diet and treatment (?) would help or the next step is surgery.
I look forward to your thoughts on my case.
Thank you very much!
Hi, I believe this is a duplicate and that I answered the question. Fred
Dear Dr Coe!
Thank you so much for being so generous with your knowledge! I haven’t had any kidney stones in a while, but just found out that I excrete lots of Ca in my urine, and I am getting dangerously close to an osteoporosis diagnosis.
I am a 57 year caucasian female (grew up in Sweden), 115 lbs, 5’5” very physical active. No family history of kidney stones or hypercalciuria.
Dexa 2014 show osteopenia in spine, not in hips. Dexa 2022 show close to cutoff for osteoporosis in spine, and midrange osteopenia in hips. Menopause in 2015 at age 50.
I am on HRT, (estrogen patch/progesterone), since 2020. VitD,25-hydroxy is usually 21 to 29ng/ml. I take vitD 1000IU when I remember. Diet with lots of fruits/vegetables, full fat dairy, and some meat. On a normal day my intake is around 1000-1200mg of Ca, no supplementation. Staying away from high oxalate food. Trying to lower sodium.
Kidney stones in oct 2016 (ER visit), Dec 2016, Nov 2020, and Apr 2021. CAT scan 2016 showed biggest stone 3mm and the captured stone was calcium oxalate. Abdominal X-ray in 2018 and 2021 show 1mm stone which is no longer there in this year’s x-ray. I believe those kidney stones formed during 2016 when I was making smoothies with lots of spinach, almond milk and chia seeds and taking calcium supplement. I just didn’t know the danger.
Saw urologist in 2016 after first stone, see results below. He recommended fluids, less oxalate, and did not mention hypercalciuria or made me aware of osteoporosis risks even though my Ca was high in the 24hr test.
Visited new urologist this summer and have two new 24 hr test results. I drink 3 cups+ of drip coffee everyday and have done so for years. I dropped my consumption to 1 cup a day for a month and then stopped completely for 2 weeks before my latest 24 urine collection.
Here is some of my data:
2016: Volume 2 L, Ca 320mg, pH 6.2, sodium 51.8meq, oxalate 14.1mg, PTH 41.7. Fasting serum Ca 9.5mg/dl
2022 august: Volume 3 l, Ca 449, pH 5.9, sodium 76 ,oxalate 22, brushite 1.14. Fasting serum Ca 9.1 (in May 22)
2022 September: Volume 3 l, Ca 490, pH 6.9, sodium 101, oxalate 25, brushite 4.75
PTH was checked once in 2016. My calcium excretion didn’t change after stopping coffee, but my pH level and brushite levels increased. Clearly I have excreted too much calcium since 2016, and I wish I had known then. As a side note, I have had very nervy and tingly hands and feet for the last 8 years, and my body aches a lot at night. Limbs often get numb, and after exercise it feels like my bones ache the whole night, sometimes also without exercise. This is not muscle soreness, I know what that feels like. I have seen doctors for this, one of them a neurologist. Since I am thin and in shape and feel fine during the day most of the time, they don’t take me seriously. So I have started to ignore it but it doesn’t get better. Could it be Calcium related?
Since I don’t seem to actively make kidney stones any more, should I still go on medication? Since I can’t know if my bone loss is due to estrogen loss (5 years in menopause before staring HRT) or hypercalciuria, or something else, I am worried about my diet and also about intense exercise. I don’t even know if my bone density is stable or if I am constantly loosing bone mass. Would thiazides be recommended and would they help for bone loss or should I first ask for a parathyroid test? Imaging of my kidney?
I am going back to my coffee habit, but will stick to 1, sometimes 2 cups a day.
What type of doctor do you recommend I see, stay with my urologist (who is now on maternity leave), go to Sutter’s kidney stone department, or find an endocrinologist? Any suggestions for the San Jose/Palo Alto Ca area? Should I set up video appointment with someone in your department?
Thank you again!
Hi Anna, Here are your results (in order of year): Sodium 51, 76 101; here are the corresponding calcium excretions: 320, 440, 490. If you make a graph, the two correlate. I suspect you have genetic hypercalciuria, which exhibits this kind of sodium response, and have bone disease in part because of the excessive kidney calcium losses. Thiazide is bone friendly and often used to stabilize bone. Since your values are fallen rather far, your physicians might wish to consider a bisphosphonate. Sutter is certainly a fine group. If your physicians believe telehealth with me would help, I can certainly do that. Regards, Fred Coe
Thank you so much for your quick response. I have been thinking hard about your response and my situation after your comment. Maybe a bit too much!!! I have started to track my food intake, and my urologist have given me one more chance of a 24 hr urine test. I am lowering my sodium now, trying to stay below 1200mg/day. I realize that my attempts earlier wasn’t serious enough. I know I probably can’t fix my issues through diet alone but I want to try since I don’t seem to create new kidney stones. The thiazides scare me because I am already on the low end of blood pressure, 109/67 last time it was measured, and a low resting heart rate of about 43. I easily get head rushes and dizzy.
My GP has told me I can have another Dexa scan in a year, so I am hoping I won’t cause to much damage by waiting one more year before deciding on medication and instead focus on serious strength training (with supervision), continue my HRT, some careful supplementation, as well as a better natural diet for bone health. I really need to gain weight but it is difficult for me while still eating healthy.
I assume I have had the hypercalciuria all my life? Or is this something you can develop? I have never been on any medication except birth control and the HRT. My 87 year old mom is healthy and broke her first bone (in the wrist) last summer after a nasty fall and my dad never broke any bones and he lived till 91. None of them ever had a Dexa scan or any tests for hypercalciuria unfortunately. Neither have my two older brothers but there has been a few fractures between them, but always due to falling from bikes, while skiing, or slipping on ice.
Thanks again, I am so grateful that I have your website to learn from!
Hi Anna, If you do indeed have idiopathic hypercalciuria it was present at birth. Although 1/2 of first degree relatives usually have it, many do not form stones. Bone disease arises from so many causes – low calcium diet, menopause. I do not know your medical situation, so please do not undertake long term bone oriented treatment simply from my note. Your physicians are responsible to you for your care. If they believe reduced sodium + normal diet calcium – 1000 to 1,200 mg/d from foods will help fine. If they want bone oriented medication, I urge you to follow their advice. Regards, Fred Coe
Hi Dr. Coe
Thank you so much for your sharing the knowledge and tremendous help here – so grateful!
I’d like to gain your perspective on my recent kidney stone and urologist’s Chlorithalidone prescription:
– I’m a 40 male, no other underlying medical conditions.
– I had observed stone crytals since 2011 and finally a 5mm kidney stone got surgerically removed by Ureteroscopy in June 2022.
– Following that, my urologist did a 24-hr urine test and found out that my urine calcium is high at 10.2 mmol/d (normal range 2.5-7.5). Other than this, all other blood and urine markers are within the normal range, although sodium is borderline high at 146 mmol/L (normal range 136-146).
– A few other key markers: PTH 3.0 pmol/L (normal range: 1.6-6.9); Calcium Ionized 1.29 mmol/L (normal range 1.15-1.35); Oxalate 388 umol/d (normal range: 135-460); Cretinine 14.4 mmol/d (normal range 6.3-22.7)
– My urologist prescribed Chlorithelidone 50 mg per day for lifetime and he said this will prevent stones in the future
My questions:
– Is my condition actually the Idiopathic hypercalciuria?
– In 2021-2022, I have been taking Vitamin D3 supplementation of 3000 UI per day.. I shopped recently. Could that be bad for me?
– Taking the Chlorithelidone for lifetime is a shocking news to me – what should I do? Should I just follow the instruction and take it for lifetime? Any follow-up tests that I should consider?
Thanks so much!
Hi Will Y, It would appear that you do have idiopathic hypercalciuria, and that treatment is long term. Perhaps with a lesser vitamin D supplement rate the urine calcium may fall, but I doubt it will become normal – you do not mention your serum 25D level. But chlorthalidone, though appropriate, need not be sole treatment, or perhaps even needed as diet can play a large role. Here is an article I like about treatment in situations like this one. Here is my recent book that has all the details. Regards, Fred Coe
Dear Professor Coe,
Thanks so much for your quick reply – I will definitely read your new book! I can’t thank you enough for all what you are doing here to help people!!!
You mentioned diet change as a treatment vs. my urologist’s lifetime chlorthalidone treatment – how do I decide what to do with the options in front of me? How do I know diet treatment is actually working?
Thanks a lot!
Hi Will, The article points out low sodium and avoidance of refined sugar, high diet calcium, and of course 24 hour urine testing to be sure the sodium is low enough to prevent the calcium from raising urine calcium and stone risk. If there is bone disease you may need more, so be sure you get a routine DEXA scan to check it. The book expands on the site with regard to bone disease. Regards, Fred Coe
I was recently diagnosed with nephrocalcinosis of both kidneys after experiencing ongoing flank pain. I have since had 2 ultrasounds and a CT scan all of which confirm the nephrocalcinosis, but didn’t show any stones. I am a 42 year old female who has never had kidney stones and who doesn’t have any other health conditions.
I’ve completed two Litholink tests and will include the relevant number:
8/14/2022: Vol-2.71; Ca-252; Ox-25; Cit-547; SSCaP-1; Na-109; PCR-1.2; ph-6.211
12/09/2022: Vol-3.61; Ca-293; Ox-599; Cit-599; SSCAP-1.17; Na-95; PCR-1.1; pH-6.607
I’m confused as to why my sodium levels dropped, but my calcium increased. I am also confused as to why I continue to have flank pain without stones. My last kidney bloodwork was really good (creatinine.73 and GFR 106) so it appears that my kidney function isn’t affected, yet, but the calcifications worry me. I have seen a nephrologist, but they just wanted me to take thiazide despite my having really low blood pressure and didn’t offer other suggestions besides drinking lemon water.
I have read most of your articles and they are excellent. I would just like to hear your suggestions about my situation.
Thank you very much!
Hi Sara, I saw this today and believe I answered – there were perhaps two entries of the same question? – but the software does not let me see all my answers in good order. Your urine calcium is high, and a probable cause of the kidney crystals. Your urine pH is on the high side. So everything points to calcium phosphate deposits arising from the calcium and pH. The high citrate is against renal tubular acidosis. I think your physicians might want to have your entire situation reviewed at a convenient stone center where perhaps something more might be found for you. If there is nothing available in your region, we can visit via telehealth. Regards, Fred Coe
Hi Dr. Coe.
I could really use some guidance! I am being seen by my nephrologist, who diagnosed me with idiopathic Hypercalciuria. He put me on thiazides, but the were causing me diverticulitis (5 attacks within a 3 mo. period). I went off, the attacks went away. My 24 hr urine is as follows:
PH 6.6
Potassium 32
Total urine volume 2.87
Calcium 485
Oxalate. 48
Uric Acid 545
Sodium 182
Phosphorus 938
Calcium oxalate. 2.68
Brushite. 4.14
PTH 32
Serum phosphorus 3.0
Suspected problem Hypercalciuric nephroliasis, hyperoxaliric nephroliasis.
My sister has the same issue of high calcium urinary output. 4 of 5 siblings get cal ox stones, often. I lowered my sodium and now drinking 96+ Oz water daily. My diet is very good. No meat for 3 months and urine calcium is still thru the roof. Is there any wat to lower this besides thiazides? As I understand it, I must increase dietary calcium?
Hi Michelle, That is a lot of urine calcium, and I suspect your sister may be similar – as well as the other 3 sibs. All this points to possible gene defects that may have more specific treatments. This is an emerging area in stone disease and as yet not very widely spoken about. Possibly you have a defect in your renal sodium phosphate cotransporter (Npi 2c) which your physicians might want to consider, and it may run through your whole family. That is pure guesswork. If this were true oral phosphate supplements of fluconazole both are not unreasonable. Warm regards, Fred Coe
Thank you so much for you response. I did have a gene test, and all came back normal. Including CPY24A1 and SLC34A1. I’m at a loss. Any other suggestions? Again, thank you so much for your time. Yes, 4 of 5 siblings are affected by this.
Thank you so much for you response. I did have a gene test, and all came back normal. Including CPY24A1 and SLC34A1. I’m at a loss. Any other suggestions? Again, thank you so much for your time.
Hi Michelle, Was SLC34A3 also considered? Were there possibly ‘variations of unknown significance’ in that gene. Some variants are in flux as to their significance. I am afraid at this distance I cannot do more. That would require I work as a consultant for your physicians and I cannot be sure if that would or would not materially add. Regards, Fred Coe
Dr. Coe,
If you raised your 24 hour Urine Citrate considerably and made no other changes would that increase or decrease 24 hour Urine Calcium or is there no correlation between the two? Also same question for Calcium Phosphate Supersaturation, does increased Citrate increase or decrease this SS?
Hi Diane, Alkali loads that raise urine citrate reduce urine calcium excretion. CaP SS is more complex. If urine pH rises more than calcium falls and citrate rises, risk goes up, and the converse. Of course my use of the word ‘more’ is sans context, so let me offer one. SS CaP is the resultant of calculations that include calcium, pH, citrate and phosphate. The last is usually trivial because its effect depends on pH, so it is the trio of calcium, pH and citrate. Their interactions are very involved so we use a computer program to calculate the SS CaP. Regards, Fred Coe
Hi Dr. Coe,
I have idiopathic hypercalciuria as the likely cause of osteoporosis of the spine (>3.0). With 12.5 HCTZ, I’ve reduced urine calcium loss from >300 to below 200 (average) but have had poor to marginal response to both oral and IV bisphosphonates – so I am now trying 12.5 HCTZ with 10 potassium citrate BID in the hope of further reducing calcium loss and perhaps improving bone outcomes. My K is doing fine but I’m now being evaluated for possible glucose issues (fasting glucose and A1C) after a slightly high (145) non fasting glucose. After 4+ years on 12.5 HCTZ, my A1C was 5.7 but my glucose has generally been normal for the 5 yrs of 12.5 HCTZj, with a couple of low values. I don’t fit any prediabetic profile otherwise. Do you have any advice or thoughts regarding this issue?
Many thanks for all you share! Your websites are the source of a great deal of helpful information in a field that, for patients, sometimes feels like a “Wild West” of medicine that gets much less attention (and funding?) than the public health impact would justify.
Mary
I feel like my story is very similar to Maureen G’s. I am a 61-year-old 5’5″ woman who was diagnosed with severe osteoporosis 3 years ago. Previously, health problems of irritable bowel, depression and anxiety and high IOP. Currently on 25 mg of sertraline and Timolol eye drops. I had an echocardiogram that showed mitral valve prolapse and some heart calcifications. I’ve had breast cysts and fibroadenomas in the past but mammograms and ultrasounds have been normal for at least 10 years.
I made an appointment with the endocrinologist my PCP recommended but she didn’t have openings for six months and I wanted to get started, so I found another who could see me earlier. I insisted on tests because my mother died of multiple myeloma at age 74. Otherwise, no extreme osteoporosis in the family. My father did have some kidney problems and was on a low sodium diet for years, but he lived to 97. He had a TAVR procedure and a pacemaker and I guess the stress led to kidney failure.
In February 2021:
Creatinine .73
Calcium 9.8.
Parathyroid normal at 43.
In April, 24-hour urine was
Creatinine .87
Calcium 383
Calcium/creatinine ratio 441
My endocrinologist prescribed 12.5 mg of HCTZ and prescribed Tymlos for two years.
I also went to a second endocrinologist (the one my PCP knew) for a second opinion. She said the Tymlos wouldn’t help much due to the IH, which my first endo disagreed with, so I stayed on it. She also recommended a third endo who was known as the best in my area for osteoporosis, on par with ones in Manhattan. The first available appointment was in six months so I made it.
Meanwhile, Endo #1 increased my dose of HCTZ from 12.5 to 25 to 37, but there was no significant difference in IH.
In the fall of 2021 my PCP said my urine test showed crystals. I had an ultrasound of kidneys and bladder but no abnormalities or stones were found.
In December 2022 I went to Endo #3 the osteo specialist. He did a DEXA which showed no significant improvement in the year and a half I’d been on Tymlos. He suggested going off but I stayed on it because I’d had a tooth extracted and was going for an implant.
Repeated 24-hour urine test in July 2022:
Calcium 380
C/c ratio 464
Repeated 24 hour urine test in Dec. 2022:
Calcium 372 calcium
C/c ratio 375
Creatinine .81.
My bloodwork in Dec. 2022 showed potassium of 3.1. The HCTZ was reduced to 25 mg and I went on 12mg potassium. Jan. 6 bloodwork showed 3.3 potassium, 97 chloride and 10.9 calcium. Potassium increased to 20 mg a day. Still no improvement, so increased to 50, and endo switched me from HCTZ to Triamt/HCTZ 37.5. In May 2023 my Potassium was 3.5 and calcium 10.6.
In May 2023 I consulted a fourth endo in NYC. This one said my hypercalciuria should be monitored, low sodium diet and after the two years of Tymlos, to switch to Fosamax for a year and then Evinity.
However, this doctor was several miles away, and the idea of going once a month for Evinity injections would be inconvenient.
I went back to Endo #3, who said the connection of IH to osteoporosis has never been conclusive, and the thiazides can cause more problems than benefits, as I experienced. He recommended Prolia, which I am currently on.
My physician told me my bloodwork two weeks ago showed normal calcium and potassium.
At this point I don’t know how to handle this or which doctor to listen to. Do I need to travel to the Mayo Clinic or some super superspecialist? I will gladly do so but am getting the impression that no one knows what to do.
I would so appreciate your help. And thank you so much for all you do!
ES
Hi Ela, Let’s see if I can distill the problem a bit. You have obvious loss of bone mineral and very high urine calcium loss. No stones. You are now on another anabolic agent that can promote increase of bone mineral – and bone – but when it ends you will have to begin a bisphosphonate to maintain your mineral. The underlying causes are unclear because rapidly progressive bone loss can cause high urine calcium loss and look just like idiopathic hypercalciuria. Your problem was detected when you were 57 and presumably began some years before which is reasonably times for postmenopausal bone loss – in your care rather severe. I am in no position to render a medical diagnosis or course of treatment, knowing only the snitch of data you have disclosed but to me – once again I am far away and cannot truly evaluate your condition – things seem most compatible with primary and rather rapidly advancing osteoporosis and therefore several of your consultants seem to me on the right track with anabolic agents and consequent bisphosphonate stabilization. A few blood calcium levels were high as if in primary hyperparathyroidism but you were taking thiazide. kMy only addition to your physicians would be several morning fasting blood calcium measurements to assure the values are 10 mg/dl or less. The normal serum PTH does not exclude PHPT. Regards, Fred Coe
Thank you, Dr. Coe. I am just seeing this now, as I thought I’d get a notification when you responded (my mistake). If there’s any other information I can include, let me know and I’d be happy to do so. I can ask about the blood calcium. I’m not having problems with another tooth and fear this is causing bone loss in my jawbone. I honestly wish I had hyperparathyroidism so they could remove the glands and hopefully make this stop.
Hi, As I said I would be cause about HPT: stop the diuretic, wait several weeks, then get blood drawn for calcium, phosphate, PTH and 1,25D. On at least one more occasion I would get a repeat of that but not bother with a second 1,25D. If the blood calcium is even at the upper end of normal, I would stay off the diuretic and get more bloods until it was clearly normal or high. High can be just a bit above ‘normal’, and despite the ‘official normal range’ values above 10.1 are very suspicious. The bloods must be fasting and in the morning. Of course this is all from an outsider, and it is your physicians who are responsible for your safety and care. Perhaps they may find what I say reasonable, perhaps not. I am not there, and they are. Regards, Fred Coe
Dr. Coe,
Do the drugs for osteoporosis the bisphosphonates increase the risk for kidney stones at all? In your experience which ones are the best for patients with Calcium Phosphate Stones? Thank you.
Hi Diane, Bisphosphonates are not a cause of stones and may lower urine calcium – beneficial against stones. All bisphosphonates work in the same way and differ only in terms of dosage and timing. Calcium phosphate stones are a special problem and need special handling.The common causes are high urine calcium and pH and low urine citrate. Regards, Fred Coe
Hi Dr. Coe,
First, thank you for this site and the resources it provides. There’s surprisingly not a lot of easily accessible information (or information I find completely trustworthy) when it comes to kidney stones and prevention. This has been invaluable for me to help make preventive changes in my lifestyle.
I have visited a nephrologist twice, and I do think he is quite intelligent and truly good with bedside manner, but after reading your site it has me questioning if I should have pushed harder for medication to help lower my calcium output.
Two separate 24 hour tests revealed the following (I worked to reduce my sodium intake between two tests (from 219 to 106), but ultimately it made no difference to calcium excretion:
2/9/23 (initial)
Volume = 4.12L
SS CaOx = 2.68
Calcium = 390 mg/day
Oxalate = 25 mg/day
Cit = 863
SS CaP = 1.73
pH = 6.744
SS UA = 0.08
UA = 0.766
Na = 219
K = 87
Mg = 92
P = 1.181
Nh4 = 40
Cl = 217
Sul = 37
UUN = 11.49
PCR = 1.1
9/16/23 (second test after working to lower sodium intake)
Volume = 4.92
SS CaOx = 2.47
Calcium = 401 mg/day
Oxalate = 23 mg/day
Cit = 698
SS CaP = 1.21
pH = 6.438
SS UA = 0.14
UA = 0.763
Na = 106
K = 76
Mg = 125
P = 1.196
Nh4 = 40
Cl = 111
Sul = 24
UUN = 9.41
PCR = 0.9
My doctor thinks that with the volume of water I am taking in daily he is not all that concerned by the calcium excretion and doesn’t think it’s worth the side effects of medication. What are your thoughts on medication as a prevention? I am concerned that water volume alone won’t solve that I have high calcium, and meanwhile it concerns me to drink more calcium (although your data is quite compelling that I should).
Appreciate any insight,
Chris
Hi Chris, On the one hand your physician is perfectly rational as SS CaOx is not very high. On the other hand you have constant SS CaP above 1 and brushite – the crystal for which the SS is given – can seed CaOx crystals and stones. This is not to say that trial data imply you need medication, but your urine calcium is indeed high and did not fall with urine sodium as is usually the case: 390/400 219/109 for Ca and Na. That latter is unusual. Whereas I might not jump to drugs I might wonder why urine calcium is like that and consider genetic causes. At very least I would consider measuring serum PTH, 1,25D, calcium and phosphate in one fasting sample of blood. Regards, Fred Coe
Hi Dr. Coe,
Thank you for your response. I recently had serum tests for all but 1,25D and had the following numbers.
PTH = 17
Ca = 9.8
Phosphorus = 3.9
In the past I had been tested for 1,25D and my readings were consistently low at 17.9 and 22.3, but that has been admittedly 3-4 years ago.
My doctor also suggested genetics and had his suspicions about what, but it was very complicated.
Thank you,
Chris
Hi Chris, the combination of low PTH and low 1,25D and high urine calcium and normal calcium and phosphate is unusual. I would get a new fasting morning blood for the entire collection once again: Ca P PTH 1,25D 25D; I would have the PTH done on plasma, not serum, as the latter can sometimes give low readings. Your urine calcium is not only high it does not seem responsive to urine (diet) sodium, which is itself unusual. I am suspicious of lab variation more than genes here. In saying this, your situation is complex and I do not know your medical situation. Your physicians are in charge here and do indeed know all the details. Frankly I suspect the 1,25D values, but if they are low within a new suite of measurements perhaps a genetic cause is not so unreasonable. Regards, Fred Coe
Dr. Coe – thank you so much for your website and thoughtful responses to questions. My question relates to bone loss and IH.
I was diagnosed with severe osteoporosis (spine Z score of -3) last year at age 47 and went to see an endocrinologist, who sent me for several blood and urine tests. The first 24 hour urine test came back high at 371, blood calcium 10.0, and normal PTH 28. My doctor suspected that I was over supplementing with vitamin D and calcium and sent me for a re-test a month later after reducing supplements.
The next 24 hour urine test was improved but still high (271), serum calcium 9.2 and PTH 27. ,
The doctor suspects IH but wants me to retest again after a month of monitoring my diet to ensure I don’t exceed 1200 mg/day of calcium intake and that I maintain a low sodium diet (although my normal diet is low sodium). I have not had a urine sodium test but my serum sodium is low normal (135). I am perimenopausal.
You mentioned above that rapidly progressive bone loss can cause high urine calcium and look just like IH. My question for you is: how to tell the difference between the two and would that impact the recommended treatment? My doctor wants to start me on Tymlos but cannot do so until my urine calcium is normal. She suggests that I should start a thiazide if the next 24-hour urine test is high. If the high urine calcium is caused by bone loss rather than IH, would thiazides help?
Thank you again for your extremely valuable website.
Hi Jess, Very complex. You have a variable and high urine calcium, one borderline high and one normal serum calcium, and marked bone disease. Tymlos is related to parathyroid hormone and acts in broadly the same manner as an anabolic agent that can increase bone mass. One cannot tell IH from rapidly progressing bone disease in clinical practice. Given you are not a stone former I am not sure the distinction matters for your care. The drug is rational. Low diet sodium 1500 mg/d or less does improve bone mineral balance and is worthwhile. Thiazide can improve bone balance. Possibly urine calcium may fall as bone mineral increases from the drug but even that is not proof of cause. I am afraid you fall into a less than charmed circle but your physician seems on the right track. Regards, Fred Coe
Thank you, Dr. Coe, for your wonderful posts and beautiful writing!
My question is whether there is an alternative to thiazides (in addition to ensuring low dietary sodium, of course) for treating IH that would not lower blood pressure. My BP is usually lowish, and I was unable to tolerate even 12.5mg HCTZ once daily, which resulted in overwhelming fatigue, with (Consumer Reports top-rated home monitor) BP readings averaging systolic mid- to upper 70s and mid-to-upper 50s diastolic. I stopped HCTZ to see if my BP improved, and it did, so that it was still low-ish but better: Systolic usually around mid 80s; diastolic upper 50s to low 60s.
My endocrinologist and urologist both suggested that thiazides are really the only medication option, and they ruled out other typical causes for the (repeatedly found with Litholink tests) hypercalciuria. I had several small stones (bilateral, don’t know what type) that apparently passed without my noticing and that had been found after a routine annual urinalysis.
Given my osteoporosis and osteopenia (female, age 57), I have been taking Fosamax for about 10 months and plan to stay on that for a while. I get plenty of dietary calcium, get about 1500 IUs supplemental vitamin D (though now I’m not sure that’s a great idea?), moderate protein intake, usually low sugar intake, and have been keeping my dietary sodium low, which probably helps keep my BP low, I guess.
Anything I’m missing that you might recommend? Many thanks!
Hi Explorer, The low blood pressure is probably a health benefit if a nuisance for calcium. Here is an article that you and your physicians might want to peruse. You might have one of the gene abnormalities it describes. Either way oral phosphate was used for a long time in stone prevention and does lower urine calcium and raise bone mineral balance. It has gone out of favor in my generation, and that is probably the fault of my research colleagues and myself. Regards, Fred Coe
Hi Explorer, The low blood pressure is probably a health benefit if a nuisance for calcium. Here is an article that you and your physicians might want to peruse. You might have one of the gene abnormalities it describes. Either way oral phosphate was used for a long time in stone prevention and does lower urine calcium and raise bone mineral balance. It has gone out of favor in my generation, and that is probably the fault of my research colleagues and myself. Regards, Fred Coe
Hello Dr. Coe,
I am a 68 yr old female who has Osteoporosis (-3.2 – -3.7 range of scores in both hips and spine). I started Fosamax 2 years ago. I am also prescribed 12.5 mg Hydrochlorothiazde, and 12.5 mg Armour Thyroid.
My first calcium oxalate kidney stone (the only one ever tested) event was about 8 years ago. I have always been able to pass stones on my own (some more painful than others) but much reduced the past 5 years. I’ve also made dietary changes to my diet via Jill Stone’s recommendations. I am not perfect but I feel that I have greatly improved in my fluid consumption, lowering oxalates, lowering sodium, increasing calcium, and watching sugar. Last year my nephrologist told me to lower my calcium intake, because my calcium urine was high, and I have, even though it seemed counterintuitive to what I’ve learned. I’ve been keeping my dietary calcium intake, this past year, to around 600 mg/day. According to the results, it hasn’t made a difference. I had an at-home stone event a month ago, four yrs since a previous one. My annual appt with my nephrologist is in a few weeks. My endocrinologist and nephrologist aren’t working in sync with each other and are only concerned with what is in front of them. I worry about both my bones and kidney issues. I have recently done the 24 hr urine collection and bloodwork, though, do not have bloodwork results back yet. These are my Litholink Panel results:
Urine Volume preserved- 2820
Calcium oxalate saturation-3.46 Low
Calcium Urine 213 High
Oxalate urine- 26
Citrine urine- 859
Calcium phosphate saturation- .81
Ph 24 hr urine- 6.74 High
Uric acid saturation- .06
Uric acid urine- 402
Sodium urine- 124
Potassium urine- 52
Magnesium urine- 119
Phosphorus urine- 405 Low
Ammonium urine- 32
Chloride urine- 130
Sulfate urine- 6.01
Protein catabolic rate- 0.8
Creatinine urine- 991
Creatine Kg body weight- 16.9
Calcium Kg body weight- 3.6
Calcium creatinine ratio- 215
Although my physician has never stated I have Hypercalciuria, I am at a loss as to what needs to change and I don’t feel my once a year 15 min long appointments are addressing my issues. With all the dietary changes I have made, certain numbers continue to get worse not better. At least oxalates are no longer an issue.
If I were to make a guess, I think maybe I need to be prescribed Chlorthalidone 25 mg, increase my dietary calcium back to 1,200 mg/day or more, ‘further’ decrease sodium and sugar (it is low already, but I can work at decreasing them more), and maintain or increase fluids. Is there more I could do? No one in my entire extended family has ever had kidney stones or osteoporosis. I also think I might benefit from seeing a good Functional physician? I would love to know your opinion.
Hi Kathy, indeed your urine calcium is high (mg/gm creatinine is very high at 215!), sodium also high given your modest muscle (lean body mass) of 991. I would lower my diet sodium to about 1500 mg/d (be sure your physician keeps track of your serum sodium given the thiazide) and see if that does not lower urine sodium. Given your time of life, be sure your fasting morning serum calcium is really normal (<10.1 mg/dl) as primary hyperparathyroidism is more common for you than in general. I might also suggest serum phosphate be measured (not on all panels) as you might have a gene variant for phosphate handling – just a thought. Diete calcium from food should be about 1000 mg/d but only with much lower diet sodium. These are just general statements, as I do not know the details of your situation. Best, Fred Coe