 Clinical Supersaturation Use
Clinical Supersaturation Use
A Direct Measurement
Physicians can and do use supersaturation to guide to treatment.
They should.
Only supersaturation can drive crystal formation and growth.
Patients, too, can use supersaturation – as a gauge of their success in managing fluids and diet. From even simple household experiments they can intuite its effect on crystallization.
Need for An Interpretive Maxim
But myriads of organic molecules which delay crystal formation and growth, molecules in urine and probably kidney tissue itself complicate and diffuse the natural power of supersaturation over crystallization. They confer on urine the very power to maintain a supersaturation over days or even weeks that allows us to measure supersaturation in a 24 hour collection.
Even so, the urine supersaturation of an active kidney stone former, whatever it may be, is too high with respect to the crystals in kidney stones forming. We need to lower it. This principal maxim cuts through the complications organic molecules produce and guides clinical supersaturation use for kidney stone prevention.
The Three Supersaturations
Calcium kidney stones
In these patients, urine organic molecules most weaken links between supersaturation and crystal formation. As a consequence, clinical supersaturation use depends on the principal maxim.
Uric Acid kidney stones
By contrast supersaturation links so strongly to uric acid kidney kidney stones we need no maxims. Reducing supersaturation below 1 prevents the stones. Nor does anyone doubt that urine pH controls uric acid supersaturation. No one will attempt a formal trial of potassium citrate – which raises urine pH – for uric acid kidney kidney stones. We lack the equipoise to permit a placebo arm.
Cystine kidney stones
This organic crystal forms when urine is supersaturated with cystine because transport defects in the kidneys lead to excessive excretion. Though not easy to measure commercially available urine cystine supersaturation gives reliable results, even despite thiol binding drugs. Clinicians can use this clinical supersaturation assay for patient management.
We have no magic potion to abolish cystine supersaturation. But even so, no one doubts that supersaturation causes cystine kidney stones, and that prevention depends upon lowering supersaturation.
The Components of Supersaturation
Laboratories Calculate Supersaturations
Although one can measure it directly, such tiresome and expensive methods cannot support large scale kidney stone prevention. Commercial vendors calculate supersaturations using updated versions of a program written originally by Birdwell Finlayson whose picture I feature in this article.
What Measurements are Needed?
His program uses 13 measurements (all but pH are amounts in 24 hours): Volume, calcium, oxalate, phosphate, citrate, magnesium, pH, uric acid, sodium, potassium, chloride, sulfate, and ammonium ion.
Vendors usually include urine urea to estimate protein intake, and creatinine to estimate completeness and consistency of collections.
Which Urine Measurements Most Affect Which Supersaturations?
For calcium kidney kidney stones: Volume, calcium, oxalate, citrate, and pH.
For uric acid kidney kidney stones: Volume, pH, and total uric acid excretion. Of these, pH and volume predominate.
For cystine stones: Volume and cystine; pH exerts a small effect when above 7.
Diurnal Variations
As we eat and sleep and work periodically over 24 hours, urine composition and supersaturations track the consequences of our behaviors. So supersaturations vary with how we lead our lives from hour to hour. How can we summarize so much variation in a convenient way for clinical supersaturation use?
Why Care?
Although most practical for clinical supersaturation measurements, the 24 hour urine collection averages these peaks and valleys of everyday life. As a result they minimize the worst of things. Multiple spot urines can track variations better, but cannot support patient care. They cost too much and burden patients with multiple containers. As a compromise, I advocate 24 hour collections but want to know where peaks and valleys usually lie.
Among Whom?
I have chosen data from calcium oxalate and calcium phosphate kidney stone formers studied in our clinical research center. All ate the same diet during measurements that extended throughout a full day and overnight. Reasonably well matched controls served as a contrast.
Although their kidney stones contained no uric acid, I include urine uric acid excretion and uric acid supersaturation by way of completeness.
Urine Volume
Physicians try to raise it. Patients need somehow to find ways of drinking enough and not go mad. Beverages themselves pose hazards and opportunities. 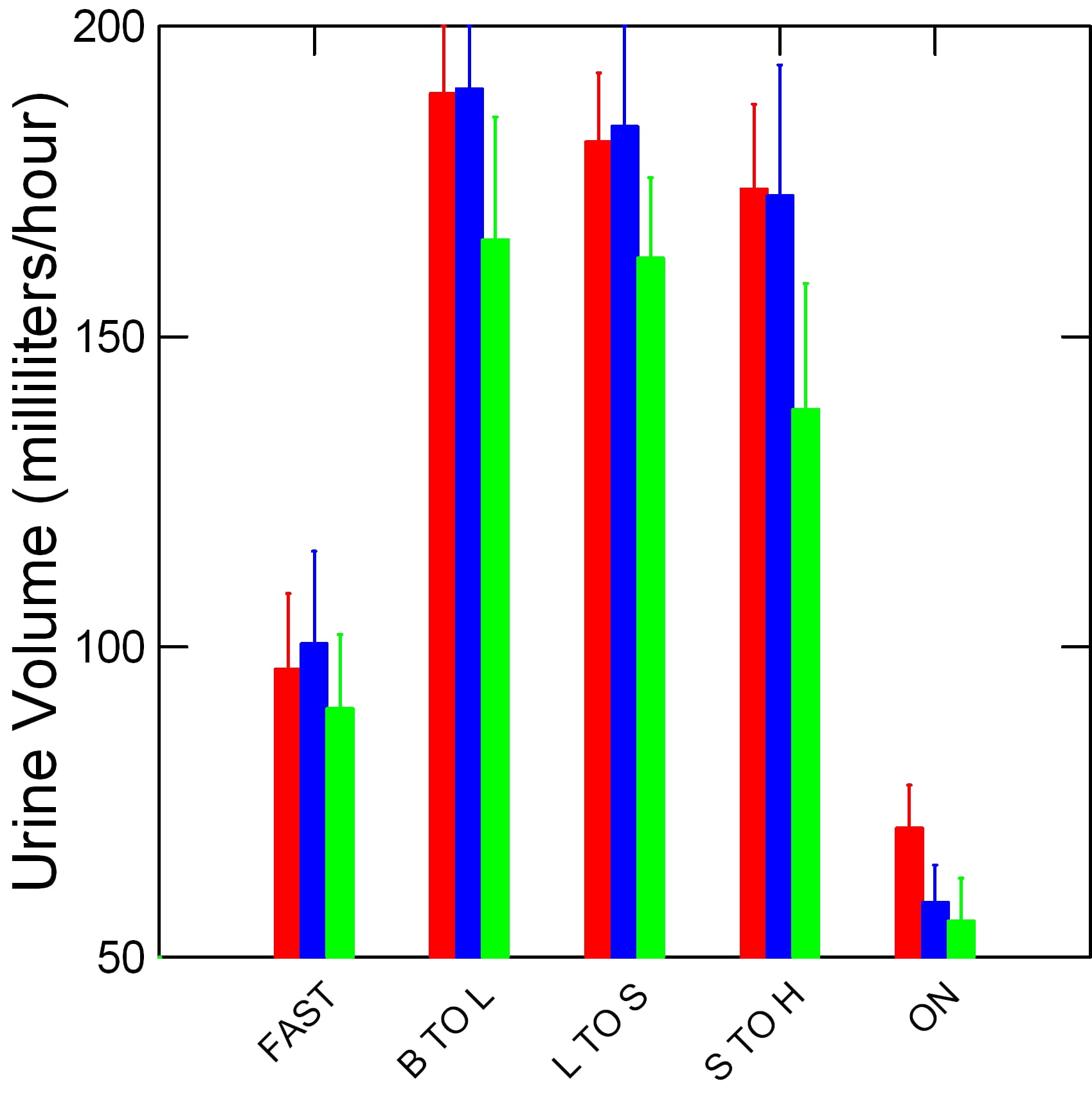 Even the sceptical and faintly absurd guidelines released by the American College of Medicine support an increase of urine volume as a stone prevention.
Even the sceptical and faintly absurd guidelines released by the American College of Medicine support an increase of urine volume as a stone prevention.
But the total urine volume we estimate from 24 hour urine collections hides a great danger.
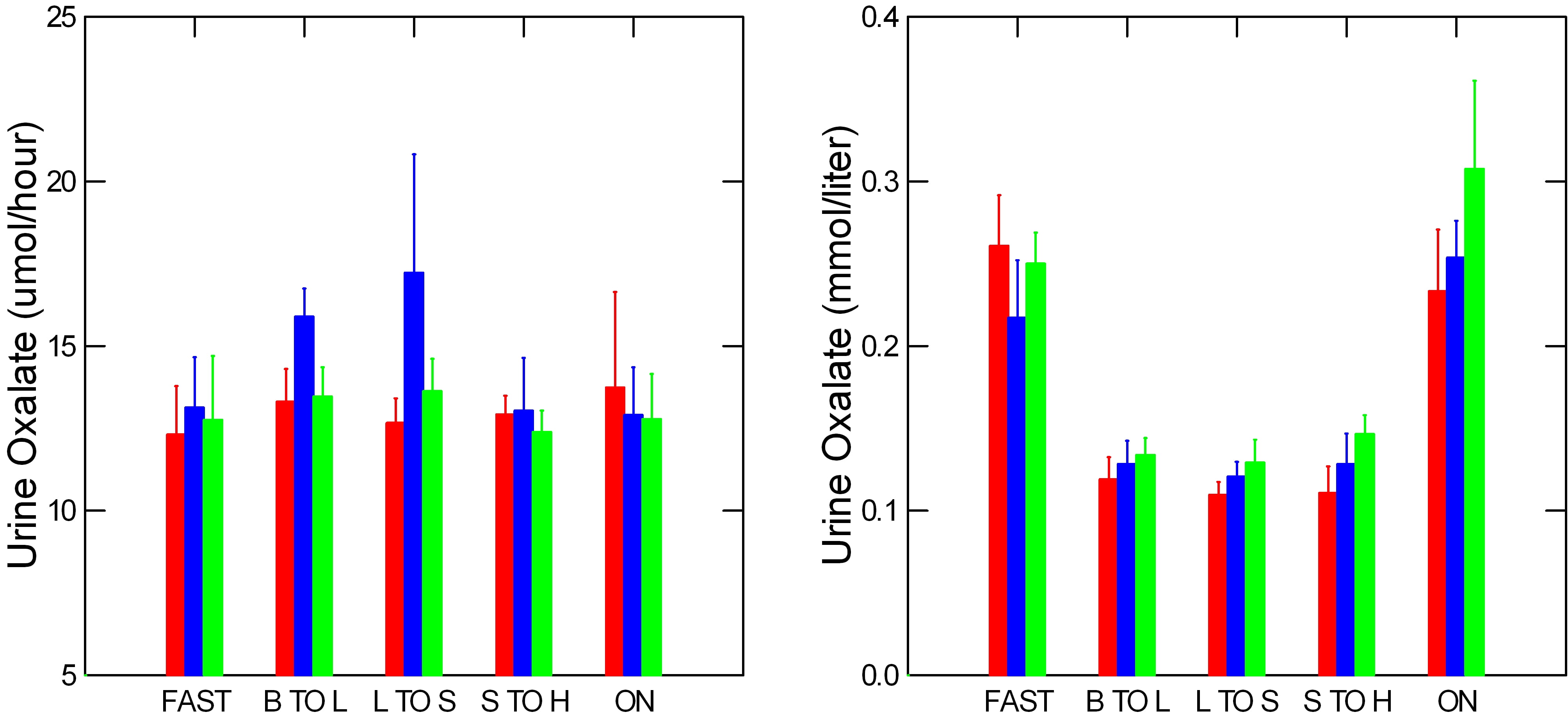
First, a word about the graph. The bars display urine volume in milliliters per hour; 30 ml is a shot glass – one ounce. B, L, and S are breakfast, lunch, and supper; H is home. ON is overnight. Red is normal people. Green and blue are calcium oxalate and calcium phosphate kidney stone formers. The lines are standard errors.
Fluids by day sustain high and stable urine volumes. But overnight, while only the moon rages, volume falls to conserve water within the body. Our patients conserved even more efficiently than our normals.
Since supersaturations vary inversely with urine volume wise physicians and wise patients should best aim high. Push urine volumes as high as possible. And, perhaps, fear moonlight.
Urine Calcium
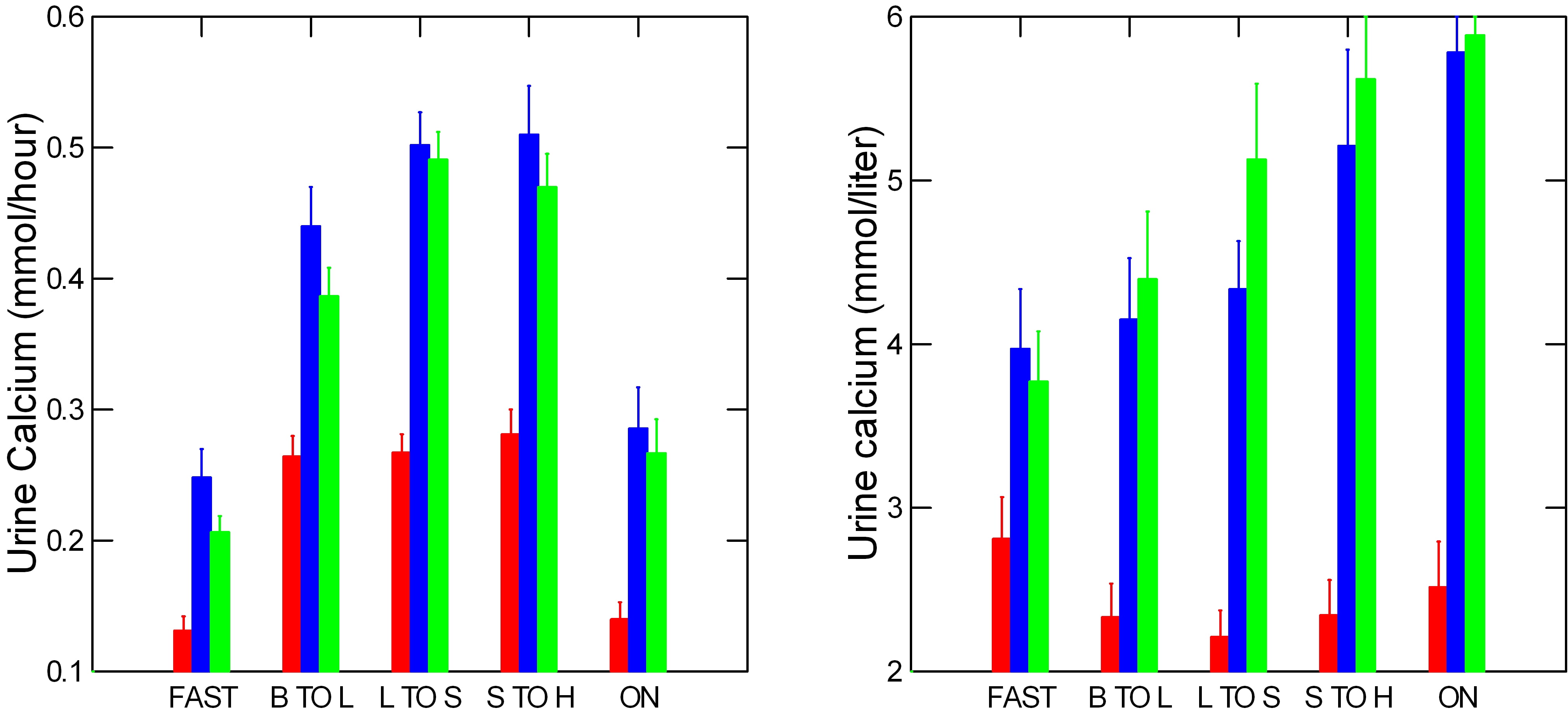 Like urine volume, urine calcium excretion rises with meals and falls overnight (Left panel). Values are millimoles/hour, one millimole of calcium is 40 mg.
Like urine volume, urine calcium excretion rises with meals and falls overnight (Left panel). Values are millimoles/hour, one millimole of calcium is 40 mg.
But unlike urine volume, kidney stone formers lose far more calcium in urine than normal people (red bars) do. They do this because of genetic – idiopathic – hypercalciuria (IH). Their higher urine calcium loss persists into the night.
As a consequence and not surprisingly, urine calcium concentrations (right panel) among patients exceed the normals. They differ most overnight.
Think about this. While our stone formers slept, they conserved water like normals, but lost far more calcium into their urine. Calcium concentrations peaked. Even their evenings were risky. In truth, patients throughout the day occupied the siege perilous, a place unsafe except for those somehow specially immune to crystal formation.
Urine Citrate
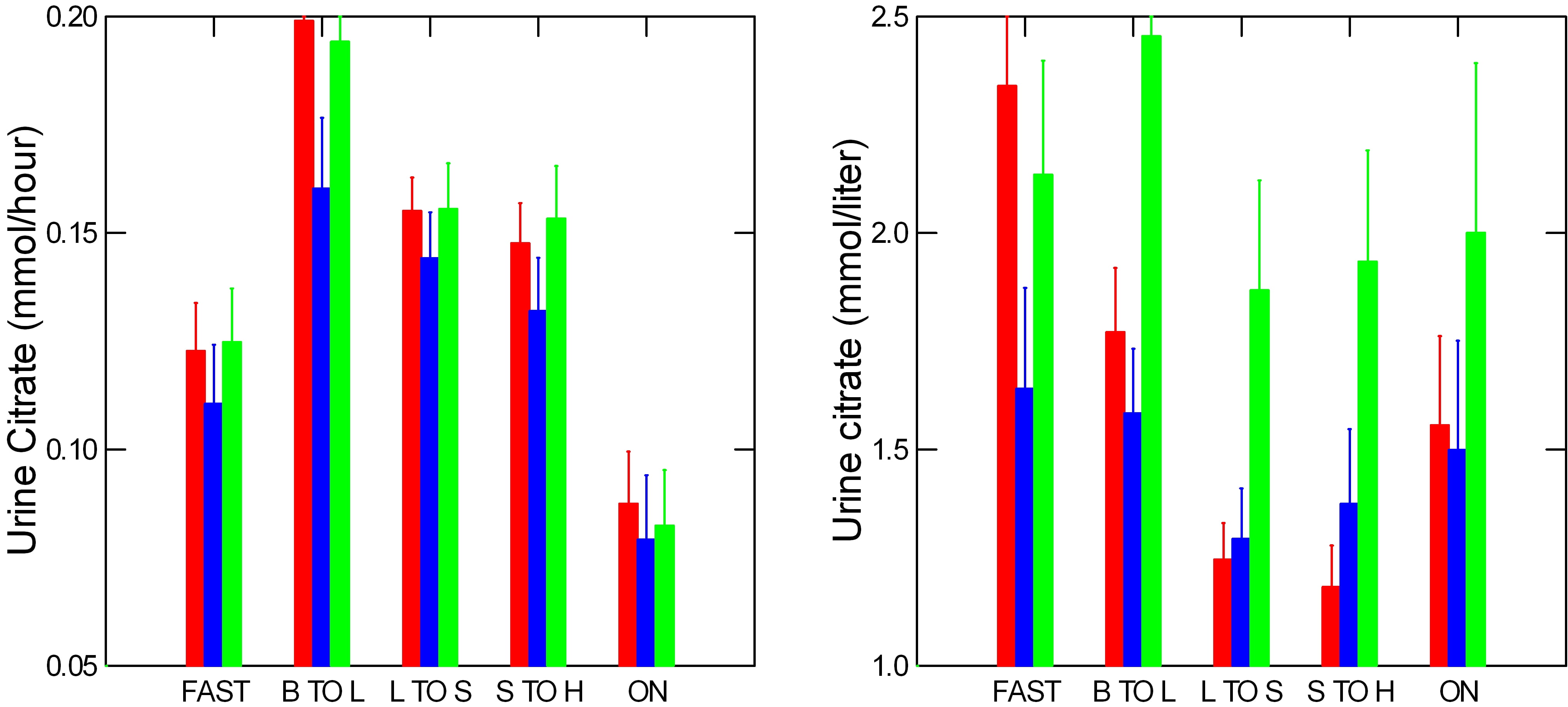 The grand inhibitor, binder of calcium, regulator of the Ostwald limit of metastability. Surely, citrate deserves pride of place next to calcium.
The grand inhibitor, binder of calcium, regulator of the Ostwald limit of metastability. Surely, citrate deserves pride of place next to calcium.
Like calcium, citrate excretion rises promptly with meals and stays up all day. But kidney stone formers and normals scarcely differ. So urine citrate concentrations fall by afternoon in both, just as, in patients, calcium concentration most rises.
At night, citrate concentration rises, but much less than calcium. For example, compare overnight calcium molarity of about 6 to citrate – less than 2. Because it’s overnight concentration so much exceeds that of citrate, much of the calcium is free to combine with oxalate or phosphate and form kidney stones.
Urine Oxalate
A word about the graph. To avoid extra zeros, I graph the very small urine oxalate excretion rates and concentrations in umol/hour, millionths of a mole instead of the thousandths of a mole for calcium and citrate.
A long standing myth held that changes in oxalate concentration raised supersaturation more than equal changes in calcium concentration. Like all myths this one was easily proven false. So we can view oxalate excretion and concentration with the same cold eye as for calcium, and citrate.
Many have said that urine oxalate excretion by stone formers exceeds normal, but apart from a few scattered high blue bars (calcium phosphate kidney stone formers), our three groups excreted much the same amounts. Meals hardly influenced urine oxalate excretion, perhaps because the diet contained 1,200 mg of calcium.
The oxalate concentration pattern resembles that of calcium in having a nighttime peak. But steady urine oxalate excretion and rising urine volumes pushed oxalate concentration down in mid day.
Urine Phosphate
Just as oxalate partners calcium in the calcium oxalate kidney stones, so does phosphate partner calcium in calcium phosphate 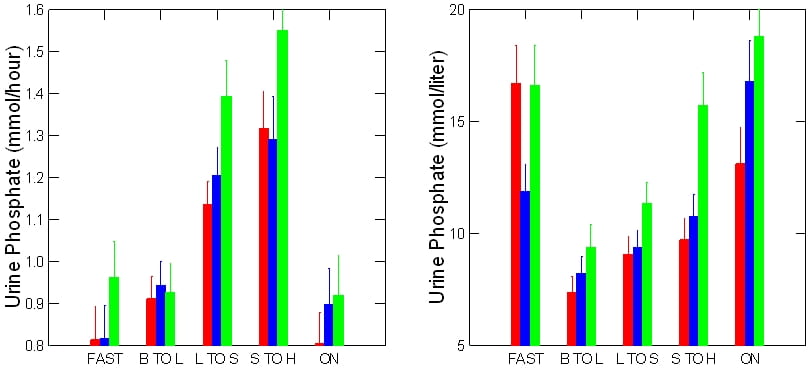 kidney stones.
kidney stones.
The pattern of phosphate excretion matches neither oxalate or calcium or citrate. Excretion rises throughout the day then falls abruptly by night. As a result its high fasting concentration falls with feeding and attendant fluids, then rises progressively throughout the day into the night.
Need I say, like oxalate and calcium concentrations, high nocturnal of phosphate concentrations can promote kidney stone formation.
Just as during a few meal periods calcium phosphate kidney stone formers excreted more oxalate than others (blue bars in the prior figure), so did calcium oxalate kidney stone formers (green bars). To these small variations I attach little meaning.
Urine pH
As you might expect, the urine pH of the calcium phosphate kidney stone formers (blue) is higher than that of the calcium oxalate kidney stone formers 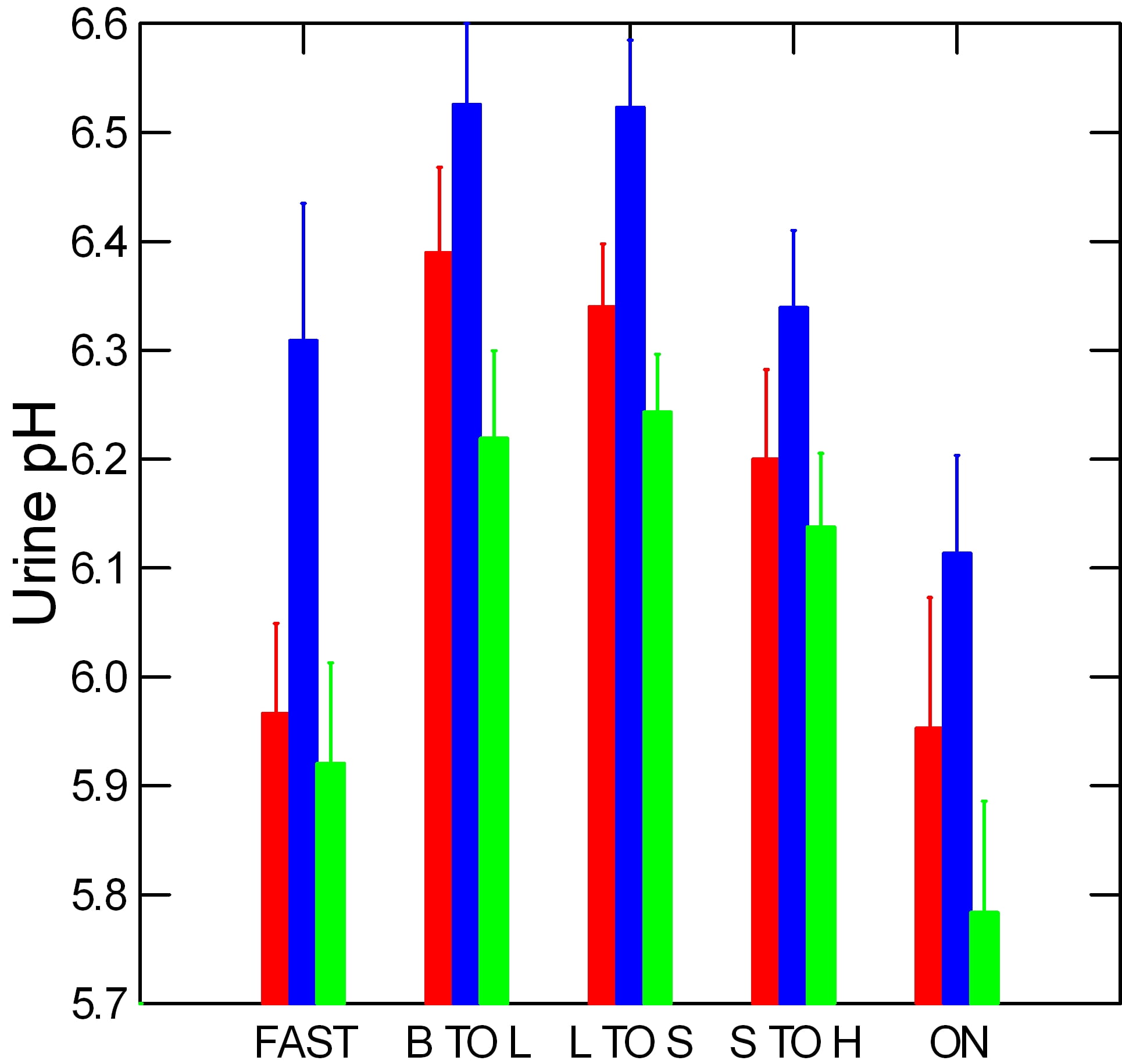 (green) and in fact higher than that of urine from normal people, too. High pH raises calcium phosphate supersaturations because it increases the fraction of urine phosphate that can combine with calcium.
(green) and in fact higher than that of urine from normal people, too. High pH raises calcium phosphate supersaturations because it increases the fraction of urine phosphate that can combine with calcium.
Overnight, calcium oxalate kidney stone formers have a very low pH, so calcium phosphate crystals cannot form. Calcium phosphate kidney stone formers have a much higher pH, so calcium phosphate crystals could form. pH of normal people lies between them.
Urine Uric Acid
Like most urine constituents, uric acid excretion rises with meals, but not as much as urine volume does so concentrations tend to fall during the day. Overnight, excretion falls.
Concentrations are much like those of oxalate with a distinct dip in the middle of the day and marked peaks overnight. 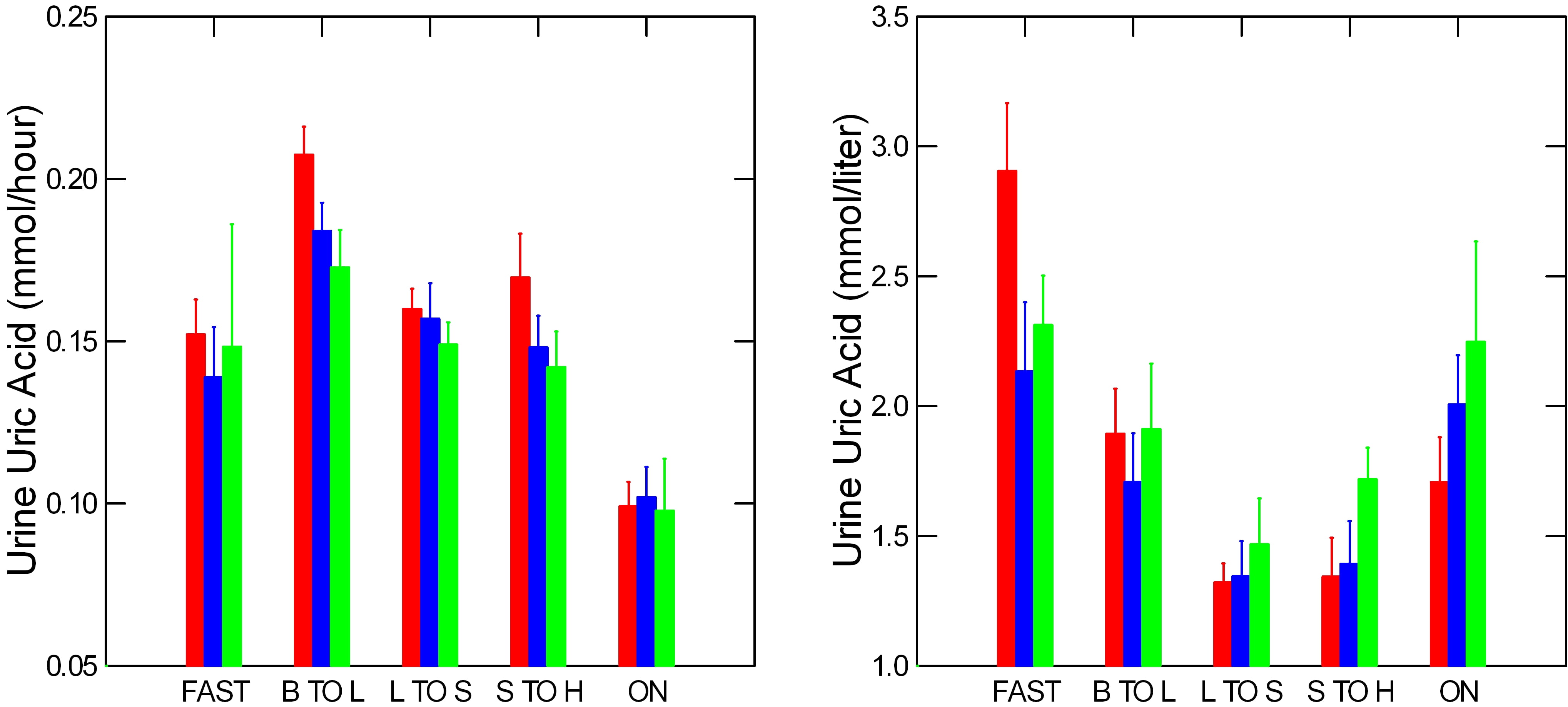 There is little variation between the patient groups and between kidney stone formers and normal people.
There is little variation between the patient groups and between kidney stone formers and normal people.
Note, these are calcium kidney stone formers, so the uric acid excretions are not related to uric acid kidney stones.
Urine Supersaturation
Like minor comics prepare an audience for the star, like a concert orchestra plays something by itself to accustom the 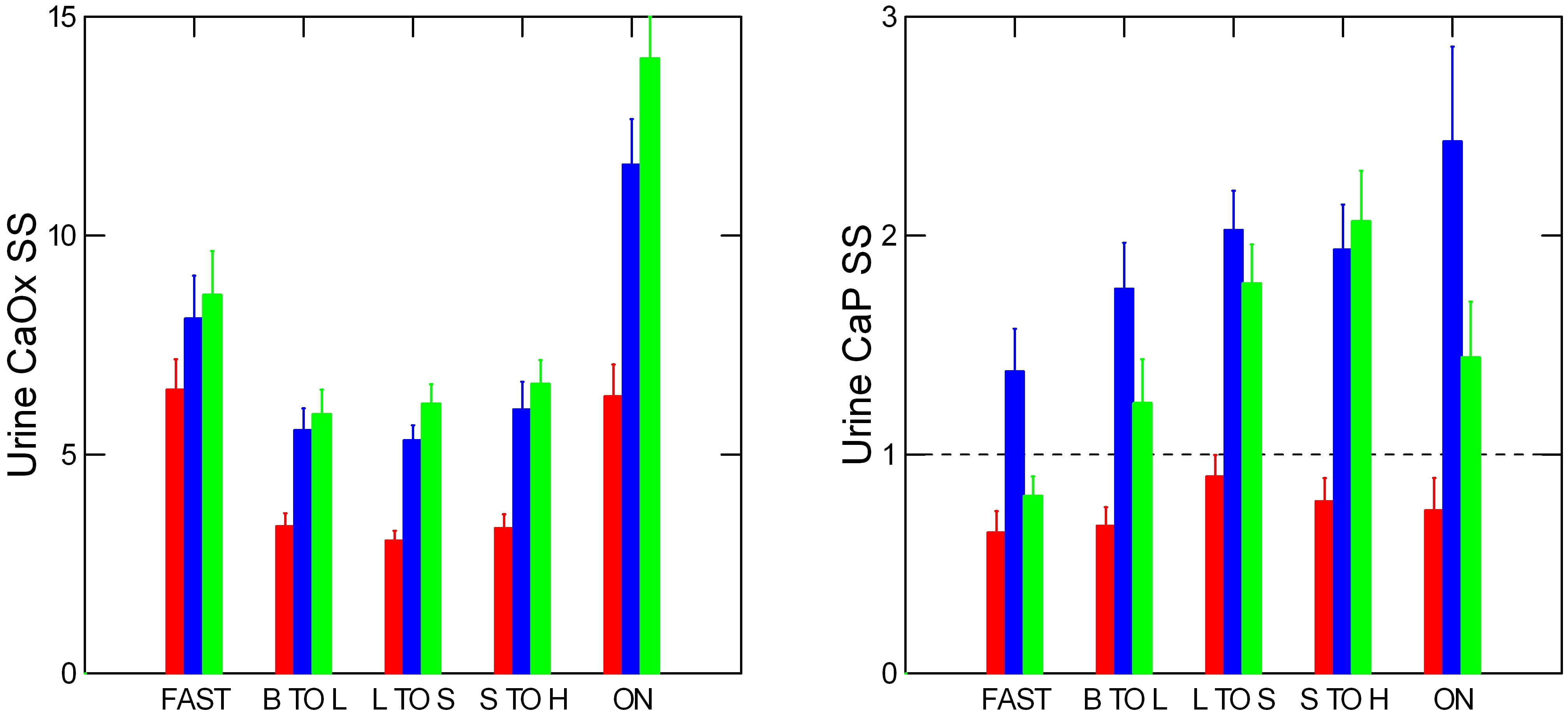 audience to listening before the grand virtuoso seats herself before the keyboard, all of the main components of supersaturations have had their hour upon the stage.
audience to listening before the grand virtuoso seats herself before the keyboard, all of the main components of supersaturations have had their hour upon the stage.
Calcium Supersaturations
As you might expect, calcium oxalate supersaturation is rather constant all day, but high at night. By contrast, that for calcium phosphate rises all day long and peaks at night.
But the dashed line at 1 tells a different story. Normal people do not produce an average calcium phosphate supersaturation above 1 – the point where crystals can form – whereas both kinds of patients regularly do so. Calcium phosphate crystals form the bridge between plaque and nascent new stones. Likewise, calcium phosphate crystals plug terminal ducts that can anchor new stones. Need we wonder that calcium phosphate supersaturations best separate kidney stone formers from normal people?
Uric Acid Supersaturations
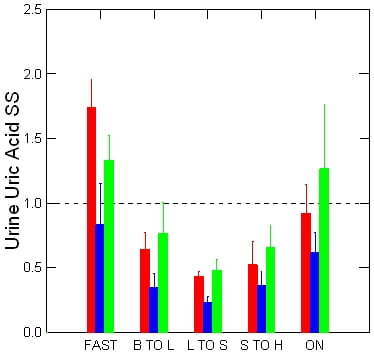 Being calcium kidney stone formers whose kidney stones contain no uric acid crystals, one should expect from our patients no significant supersaturation for uric acid above that found in normal people who of course produce no kidney stones at all.
Being calcium kidney stone formers whose kidney stones contain no uric acid crystals, one should expect from our patients no significant supersaturation for uric acid above that found in normal people who of course produce no kidney stones at all.
In fact, our patients and normals hardly differ.
Uric acid kidney stone formers produce much lower urine pH values than these, and therefore higher supersaturations. But we have none to show from a controlled CRC environment.
Whether their daytime values dip I do not know.
Supersaturation Use
The principal maxim holds: Urine supersaturations of active kidney stone formers are too high with respect to the crystals in stones forming. Lower them. But the graphs tell us 24 hour urines show us only averages of a volatile measurement whose peaks, by night especially, far exceed the overall day’s average.
So supersaturation management requires we respond to what we cannot directly observe.
Because we see less than what happens, underestimate the worst of things. To lower the average by half seems impressive. But overnight supersaturation may not fall at all if fluids stop after dinner.
Physicians, patients: consider this. Judge carefully. Be bold with fluids, exert all energies to lower urine calcium, and oxalate, and to raise urine citrate. Remember, always, the perils of the night.
How Do We Lower Calcium Supersaturation?
We alter urine volume, and excretions of calcium, oxalate, and citrate. These four greatly affect both calcium oxalate and calcium phosphate supersaturations and can be changed through diet and drugs. Likewise, in epidemiological studies, they link directly to risk of new stone formation.
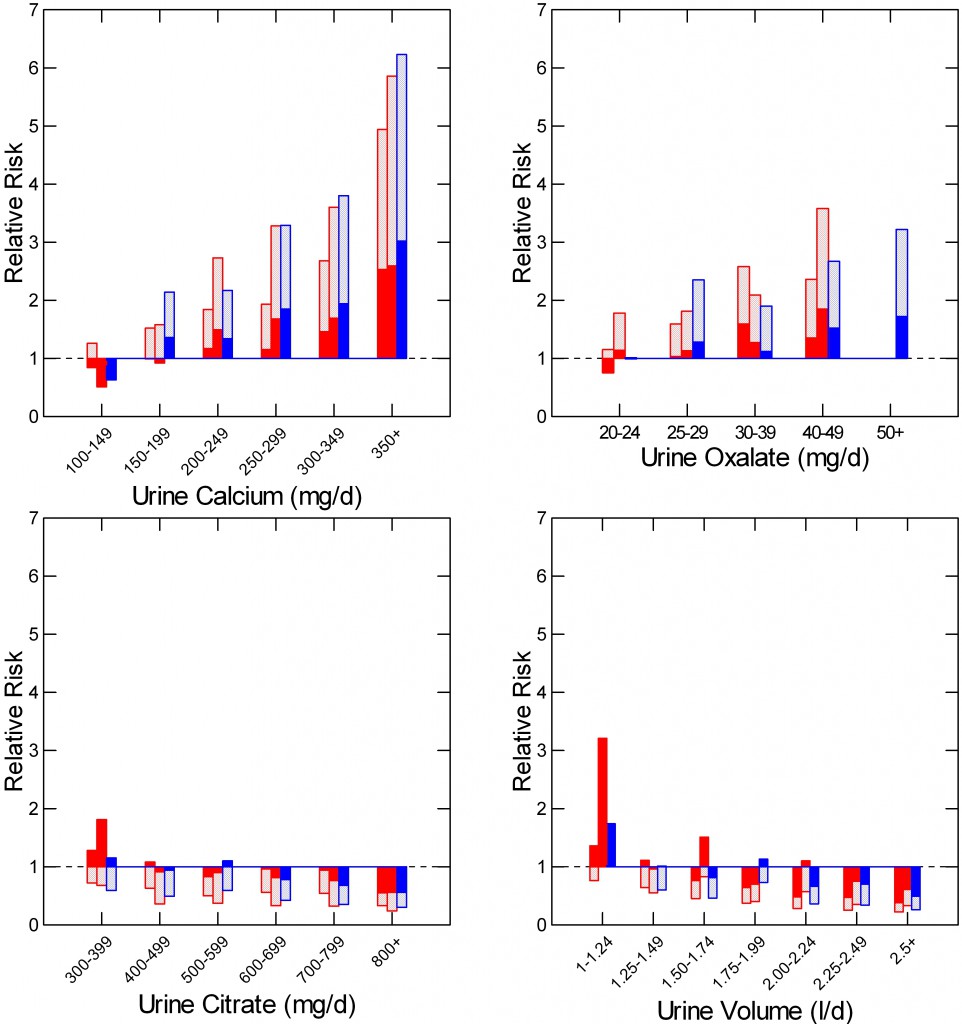
How Risk Was Ascertained
In two female (red) and one male (blue) cohort followed over decades, some people began forming kidney stones whereas more did not. Using 24 hour urine samples from both, from each cohort, Gary Curhan could estimate the average relative risk of kidney stones shown here for all four urine excretions at the top of each bar. The bottom of each light bar marks the lower 95th percentile of that risk for calcium and oxalate, whereas the top of each light bar marks the upper 95th percentile for citrate and urine volume. Red bars help visualize the tops and bottoms.
 Urine Calcium
Urine Calcium
In all three cohorts – the two female cohorts in red and the male cohort in blue risk rises with increasing urine calcium. The bottom of the bar rides above 1 for all three groups above 200 mg/day of urine calcium.
Given this, if urine calcium excretion exceeds 200 mg/day one has a good reason to lower it to lower urine supersaturations. How involves diet sodium and use of drugs.
Urine Oxalate
As urine oxalate rises kidney stone risk increases pari passu. If urine oxalate exceeds 25 mg/day one can lower it using high diet calcium and food oxalate precautions.
Urine Citrate
Citrate runs backward from calcium and oxalate – it is against kidney stones because it can bind calcium and inhibit calcium crystal formation. Therefore I have plotted the mean value downwards with the upper 95th percentile at the top. When that upper percentile it falls below 1 levels are high enough to reduce excess risk to 0.
Uniform high risk – red bars above 1 – begins only when urine citrate falls below 400 mg, in either sex. As for calcium and oxalate, one can raise urine citrate with reasonable rationale when below 400 mg/day using citrate supplements and increased potassium rich foods and beverages.
Urine Volume
Just like citrate, higher volumes are protective so I have plotted the upper 95th percentile. Risk falls progressively as urine volume rises. Above about 2.25 liters daily the risk ratio is safely below 1. I have used this threshold in my analysis of how to prescribe fluids.
A Strategy for Clinical Supersaturation Management
Given the maxim, I act to lower supersaturations of active stone formers and aim for half or less of what I first find. I present the clinical complexities of such actions elsewhere. Of the main factors, I always try to alter all that raise risk using fluids and diet before drugs. Altering all creates synergies.
How Do We Lower Uric Acid Supersaturation?
I mean to say little for we need little: Raise urine pH above 6 and uric acid crystals will not form but may dissolve. No patient need form a recurrent uric acid stone. I favor potassium citrate over diet and beverages to assure stone prevention or growth.
How Many 24 hour Urine Collections?
How tempting to use one – cheap, convenient, and bespeaking of parsimony in medical practice. But I say shortsighted and wrong. One urine can mislead us, enough we miss the mark. One stone can cost ten or twenty urine studies, and as many days of pain and lost work.
We Compared Several Samples for Each of Many Patients
I measure 3 baseline 24 hour urines before beginning treatment. A single large practice collaborator
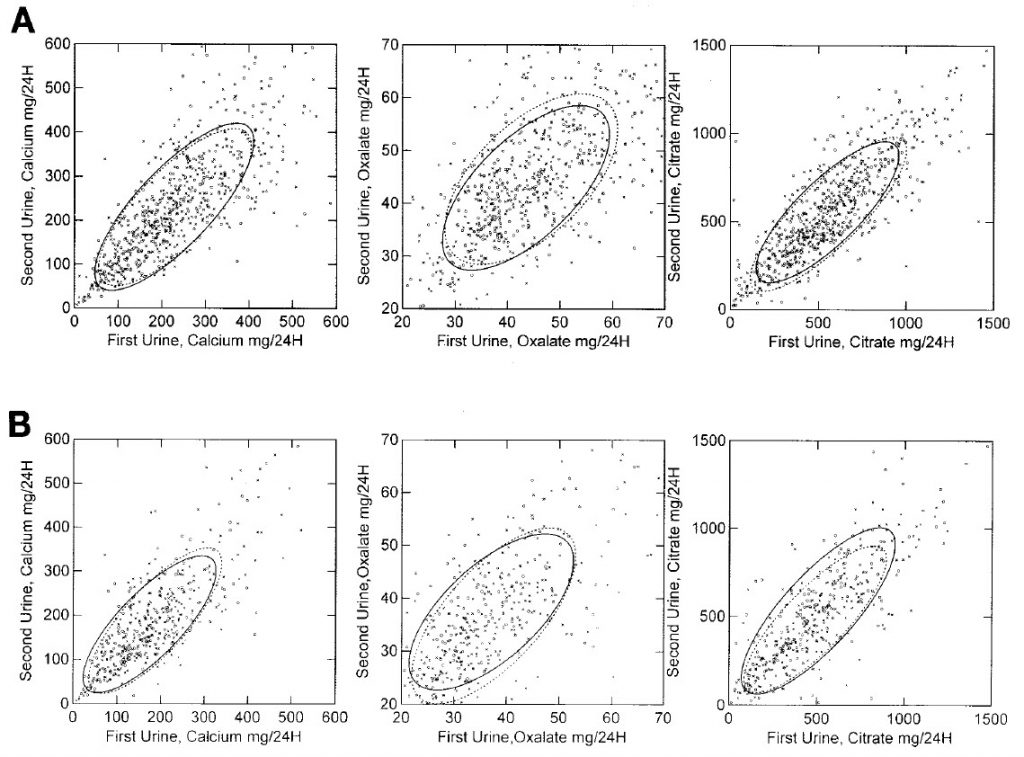 collected two such urines before treatment. We pooled the first vs. the second from the practice and the first vs. the third from my own practice asking how much variation is present between the first and subsequent collection.
collected two such urines before treatment. We pooled the first vs. the second from the practice and the first vs. the third from my own practice asking how much variation is present between the first and subsequent collection.
Data from my practice (outlined by a solid ellipse) and from the collaborator (dashed ellipse) calculated to contain 68% of the data points overlap almost completely. Men are on top, women on the bottom.
One Collection Will Not Do
Although values from the two urines correlate with one another, values for any one patient can diverge by a lot.
To see this, simply draw or imagine a vertical line at 200 mg of calcium in the lower left panel of the figure. It intersects with the bottom of the ellipse at about 120 mg and with the top of the ellipse at about 300 mg/24 hour. The first urine could place a patient at no risk for kidney stones from urine calcium excretion, the second place the same person as high risk. Since the ellipse contains only 68% of the data, the actual spread can be much larger.
Others Have Found The Same But Concluded Differently
Because the ellipses are narrow and point upward and to the right at about 45 degrees, other scientists have made wrong recommendations. Charles Pak said that given the correlation a single urine is sufficient. Doctors Eisner and Stoller came to the same conclusion.
But they are wrong. Their own data show the very same individual variability as ours. One urine can misleads physicians and patients. I hope Drs Pak, Eisner, and Stoller do not resent my concluding other than they did, and invite their rebuttal and argument. But to me their data look much the same as mine, and we differ mainly in how to interpret it.
Supersaturation and Clinical Judgment
The maxim depends on a few details.
You must know stone crystal types.
You must determine if new stones are forming – if patients are actively producing stones.
Patients must provide 24 hour urine samples that reflect life as lived. They may know what they did but you must ascertain if what they did provides an honest representation of how they live their lives. Especially, do their samples represent how they lived their lives when they formed new stones.
How low to push supersaturation depends on how many peaks one thinks may hide within a mean, and how high? This depends on how much you know about their lives as lived, their work, their habits.
Clinical supersaturation means to use a quantitative and powerful measurement guided by what I can only call clinical judgment. We do not teach that in schools, it is patients teach us, and, perhaps, time.
Cautions and Reservations
Cautions
In our clinical research unit, people drank as they chose but no doubt drank more fluently and perhaps more than usual during common life.
Although we found overnight and fasting volumes less than daytime, the world may produce other patterns. Someone doing orthopedic surgery, driving an 18 wheel Semi, teaching nursery school classes, or putting up house framing in the summer might have as low a urine volume by day as by night.
A clinical corollary: Ask your patient about work, driving, airplanes; tell your doctor about splitting 5 cords of wood for your winter fuel.
Reservations
All this refers mainly to urine volume but even more happens out on the street. Not everyone eats three meals a day. Some people snack or starve until supper. Many vary so much from day to day no urine sample will be ‘representative’.
Take what I show as an idealized view of things. Work from there.
Sources
For the purposes of exposition I re-envisioned and re-made published figures and tables from data referenced in Bergsland et al; Worcester et al.


At my age I find that I almost every night must get up to urinate at least once. Aren’t these the best times to drink some water (or some lemonade) and immediately upon waking up in the morning?
Dear Ludwig,you are right so far as I can tell. No better times could be found. Regards, Fred Coe
My 24 hour urine was well over 3 liters. I’m told this is abnormal, but I get well over 3 liters of fluids a day, sometimes up to 4.
Is this too much? I want to prevent stones (I am a calcium phosphate former) but I don’t want to create another problem.
Hi Joe, No it is not a problem. I would say it is a great way to reduce risk of more calcium phosphate stones. We have mentioned these kinds of volumes on this site. Phosphate stones can be frequent and troublesome. Be sure that with high fluids you have done enough. Sometimes urine calcium is very high and there are other measures needed. Your physician will have thought about this; be sure and ask. Regards, Fred Coe
Thank you so much for the reply!
My physician and I actually decided to use Litholink to judge my risk. I rate as “mild” currently. Only thing is, I have a normal calcium/creatinine ratio and according to him my urine phosphate level isn’t problematic, but slightly high. Calcium daily (roughly 22-23 hours) is still 329mg. I’ve been told by different sources that the ratio matter more than total excretion.
Thoughts?
Thank you,
Joe
Hi Joe, I popularized that ratio decades ago and wish I had not. Urine calcium levels of over 300 mg daily are high for anyone and certainly pose a real risk for stones. A common cause is high salt intake which is gauged by the 24 hour urine sodium excretion. High salt intake raises urine calcium and lowering salt intake lowers urine calcium. The high urine phosphate is from diet, and is not a stone risk in itself. Regards, Fred Coe
Thanks for the response,
Specifically, I saw mayoclinic list true hypercalciuria as being above 350mg daily, as well as a few other places I can’t recall.
Have you done any write ups on bone density? I know my urine calcium isn’t absurdly high, but I do worry about my bone density because of it. I’ve read in some studies with normal calcium intake that bone density is maintained.
Thanks for all of the info Fred!
Joe
Hi Joe, the answer always depends on the exact question. If you are doing research on hypercalciuria and want to identify people whose urine calcium excretions are above the 95th percentile of normal, this upper limit is perfectly fine. If you want to know at what level of urine calcium risk of new stones begins, you need some kind of epidemiological study linking the two. The best so far is by Gary Curhan. IN the linked post I have inserted my graphical redaction of his work along with an explanation of how to interpret his data. Essentially stone risk increases significantly at urine calcium excretions above 200 mg daily and risk increases progressively above that point. Regards, Fred Coe
If I have read this correctly, due to some normals being in the >200mg calcium excretion group, you could theoretically excrete <350mg calcium and not be/never be a stone former, correct?
I know there is a higher risk/chance that you are, in fact, a person that forms stones, but that doesn't necessarily mean you are one, does it?
Hi John, That is a great question. Urine calcium is a graded risk factor, and risk can be detected above 200 mg/day. By 350 mg/day risk is higher than at 200 mg/day. Stones are events, so in any one case you cannot predict very well what will happen. Say you park your car in a high crime area – lots of car thefts there – and do it every day. Your risk of theft is higher than average, but your car may never be stolen. If you had a fleet of cars, and parked them all in such an area, it is probable that your rate of car theft would be higher than the fleet owner who was more prudent. Urine calcium excretion expresses itself in actual stone risk, so far as we know, through increasing urine supersaturation. This adds yet another layer. You might be someone who drinks so much that 350 mg of calcium/day does not raise supersaturation enough to cause stones. That would be like equipping your car fleet with very sophisticated anti – theft equipment, or posting a guard. Risk is only risk – probability of an event. Prevention of stones is by reducing risk. Regards, Fred Coe
Hi Fred,
I am sorry to bombard you with questions, but I recently had a 24 hour urine, and wanted to get a better idea of what is going on and what the results mean.
I read through this again. I am 24 years old, never had a stone, no family history, CaP crystals ++ found only a couple times. However, the CaP SS rule that normals never make anything above 1 is curious to me. Do you mean normal as in they can’t have a kidney stone of the CaP type without that SS, as in, if you have CaP SS above 1 you are definitely a kidney stone former? Can my SS tell me I am forming a new stone if it is in fact above 1? And could it be that non stone formers could have CaP SS >1?
By the way, my urine citrate is 564g/day. Would you say this a good amount of protection, even if I have mild hypercalciuria (>300mg and <350mg)?
I hope I am not a bother but I have one last question that is the most critical one I think. If the CaP SS is in fact above 1 on a daily basis, does that mean a stone is eventual? Is it possible to have SS above 1 and never form a stone?
Thank you for all the help Dr Coe!
Hi John, It is this way. If you form cap crystals in your urine the urine SS with respect to those crystals has not only exceeded 1, it has exceeded the upper limit of metastability. We have a lot on this problem on the site. As an average, in our experimental studies of people all eating the same diets, normals did not supersaturate their urine with CaP because they are not hypercalciuric. You are – urine calcium over 300 mg/day. So you are a hypercalciuric non stone former who sometimes probably lets urine volume fall, or has a brief spell of high pH – could be anything in the diet with an alkali load – and forms crystals. Being hypercalciuric, you have increased risk of becoming a stone former – see Curhan’s data in the hypercalciuria article. But, you may easily escape as risk is only risk. What would I do? Keep your sodium intake down as much as you can, and keep your urine flow up – fluids of all sorts – as a safe pair of precautions. If you develop urinary bleeding, or pass stones someday, it will not be overly surprising and treatment will just be what I have already said and, depending on how many, added medications. That ‘someday’ may well never come. Regards, Fred Coe
Thank you Dr Coe. I read the metastability article and I saw that CaP SS can get up to 4. That is mind-boggling.
I am glad to know though, that according to the data (correct me if I’m wrong) typically the ones that will get CaP stones are above 2. I am glad I am not in that range.
I have been keeping sodium low but I don’t want to harm myself nutritionally. Is there are safe lower limit? I don’t want to drop to bad levels.
Thank you,
John
Hi John, It is not quite that way but almost. Some people are prone to crystallize so SS of just above 1 is enough. This is what the upper limit of metastability is about. As for sodium, the recent CDC and AHA recommendations are 1,500 mg daily for an ideal, 2000 as an upper limit. For someone without heart disease, kidney disease, or similar there is no reasonable lower limit – for example 1,500 mg is now recommended and 1,000 is not a problem with the above provisos. The issue is that you are not a stone former, so lots of water, and perhaps the 1,500 mg sodium diet should be plenty. Regards, Fred Coe
I’ve seen one small study that treated uric acid stones with sodium citrate, 6/8 patients removed their stones over an extended period of time, if I remember right their average urinary ph only was just below 7, so perhaps not high enough. I was aggressively treating stones I had with sodium bicarbonate, and I think it took a 5 days to eliminate them(I would see tiny crystals in my urine). Its seems to me that urinary ph can affect calcium in another way. One little talked about buffer system is the bone buffer, where calcium phosphate is released to conteract acidity(the other buffers being bicarbonate in the blood and phosphate intracellular). Excercising causes calcium phosphate to be released(via parathyroid hormone), then after excercise it is sequestered again. I believe I’ve seen that thryoid hormones can also affect the parathyroid(which is usually why glands are connected, like the adrenals(cortisol maximizes conversion of noradrenaline to adrenaline). Anyone check if hyper or hypothyroidism is related to stone formation(best to check body temp not actual hormone levels)?
Hi Jim, Uric acid crystals are unstable at pH values much above the pKa of 5.3, so any way you raise the pH you prevent uric acid stones. I have no real data on dissolving uric acid stones clinically, so thanks for the reference – can you please edit your post to include the reference itself? As for urine calcium and alkali, the effect is certainly on the kidney and the effects of neutralizing diet acid on bone mineral loss is controversial and a subject we have not approached on this site. I will do this over time, but I assure you bone experts do not fully agree on whether alkali prevent bone mineral loss. As for exercising and PTH, I am not aware of this physiology – references? Hyperthyroidism does raise urine calcium and is mentioned as a cause of stones. Body temperature is not really a proper substitute for measurements of appropriate hormone levels, I am afraid. Regards, Fred Coe
radioactive iodine treatment of hyperthyroidism will effect the parathyroid.
Hi Gem, I agree. A recent article points out an increased risk of hyperparathyroidism and older articles noted this, too. Fred Coe
Dear Dr: Is the 24 hour urine collected with any acidificant?, or must be collected without preservative?. This cuestion is respect the variation in pH.
Regards
Hi Victor, At University of Chicago and at Litholink 24 hour urines are collected without addition of acid so net acid excretion and pH can be measured. Steps are taken in the laboratory to assure accurate assays. Preservatives are added to retard bacterial growth and these must be chosen so as not to interfere with the measurements. For all these reasons, variation of urine pH can be accurately assessed. Regards, Fred Coe
Thank you Dr Coe for the excellent article. Cant tell you how much helpful this is . IS there any chance that the EQUIL2: a BASIC computer program for the calculation of urinary saturation is available for use ?
thanks
Hi Amit, The program is open source but no one curates it so you might find it on the web but the version you find may not be correct. If you are a physician or scientist interested in supersaturation calculations, I am sure you can secure a proper version. If it is simply for your interest as a stone former, I would not recommend putting yourself to any extra effort as commercial vendors regularly calculate supersaturations accurately for you. Regards, Fred Coe
Hi Dr Coe,
I read this article with great interest due to my own current situation, the crucial message at the end giving me a wonderful feeling of being understood at last. It is also understandable to someone who is a lay person. Although not directly connected to kidney stone formation my question is relevant to this article I believe.
A little background information for context. I am currently being tested for primary hyperparathyroidism (I drink lots and lots of water in an effort to prevent stone formation and I eat hardly any calcium containing food to keep calcium levels low). One of the tests was a 24 hour urine collection (creatinine was also collected during the subsequent 24 hours), needless to say, because of how much I drink, the actual volume was high. After this test I was told that my results were suspicious of FHH (Familial Hypercalcaemia Hypocalcuria, as you no doubt know. I have only had this one 24 hour urine test and am now being sent for a second opinion.
My question is – how much do factors such as urine volume, medications (aspirin), not keeping the sample cold, food intake (low calcium diet), low sodium diet, vit d deficiency and stress influence the result gained from such a sample?
The crucial message at the end of the article answered my other question which would have been – do I need more than one 24 hour urine collection.
Thank you
Sorca
Hi Sorca, FHH is a genetic trait of high serum calcium and very low urine calcium, and is not in itself a cause of stones but rather a confusion with primary hyperparathyroidism – which has a high urine calcium. Serum PTH levels cannot differentiate FHH and PHPT. Low calcium diet has no known place in modern stone prevention, so doing that is not of help. Proper testing and organization of your information is crucial so things are not confused. Take a look at a reasonable approach. Here is another summary. This approach may not be ‘the best’ nor even perfect but it does work and will get you proper prevention if you follow it. Regards, Fred Coe
Hi Dr. Coe,
I’ve never had kidney stones, but I do have small crystals that come out of my skin-they form in large amounts when I have any sort of green drink (spinach or Swiss Chard), or when I have excess Vitamin C. They are sharp and itchy, and while sometimes they come out as sort of a dust, sometimes they are bigger and a red rash forms around them. I have had chronic UTI’s since childhood, and was told five years ago that I have Medullary Sponge Kindney. It showed up on a CT scan for a ruptured ovarian cyst. I was told MSK is usually not a problem, but now I’m wondering if the UTI’s and my strange skin issues could be related. Is this possible for oxalate crystals to come out of your skin? And if so, what course if investigation would you recommend? And should I be seeing a nephrologist for the MSK?
Thank you so much!
Hi Paula, Oxalate is in vanishing concentrations in blood so I doubt these are calcium oxalate crystals. I know of no relationship between MSK and this kind of skin complaint. But with MSK is usually multiple calcifications in the kidneys, so perhaps high oxalate foods are not a good idea. UTIs are well known associates of MSK, however. If there are indeed multiple kidney calcium deposits the seeing a nephrologist interested in stone disease would be a very good idea. Regards, Fred Coe
Dear Frederic Coe, could you help me to find EQUIL2 Program
I wanted to incorporate it to an application – make so to say more popular 24 hour urine Analysis
Thanks
It is open source. I do not know a specific website with it. Be careful as there are many versions and some have out of date constants. If you are planning a product you need to work with someone with expertise in the calculations and constants to be sure the product is not giving faulty answers thus opening things up to a product liability lawsuit. There are also regulations concerning presentations of clinical laboratory data that might affect patient care. Regards, Fred Coe
Hi Dr. Coe,
Having been a CaOx stone former for a decade now, I was so fortunate to find the excellent research and articles that you and Jill Harris have written on the University of Chicago kidney stone website. My first two Litholink 24-hour urine collections pointed out my glaring dietary and water consumption issues which I have taken great strides to correct. My most recent 24-hour collection showed vast improvements in all factors (except one) which my urologist described as “amazing”, with the one exception… my Ca24 is still high, prompting my doctor to declare that I am still forming stones. Having ruled out problems with my parathyroid, he has prescribed chlorthalidone and potassium CL.
Do you have any ideas as to why my Ca24 would still be high?
What are the potential side effects of chlorthalidone and potassium CL?
What alternative approachs should I consider?
Your thoughts and opinions would be greatly appreciated.
For your reference, My stone risk factors are as follows:
Vol 24 = 3.44
SS CaOx = 3.05
Ca 24 = 492
Ox 24 = 21
Cit 24 = 1024
SS CaP = 0.97
pH = 5.876
SS UA = 0.59
UA 24 = 0.779
Dietary Factors are:
Na 24 = 144
K 24 = 83
Mg 24 = 169
P 24 = 1.438
Nh4 24 = 44
Cl 24 = 158
Sul 24 = 58
UUN 24 = 16.53
PCR = 1.3
Hi Dan, Obviously I cannot interpret these numbers in terms of your care as I do not know you and have no knowledge of all the factors that might matter here, so this is just parsing the numbers and saying what is in the articles on the site. The high calcium may be because the slope of calcium on sodium is higher for you than usual, and less sodium, still might lower urine calcium. Hour protein intake is very high at 1.3 gm/kg/d and lowering it to 0.9 might lower urine calcium. Your urine citrate is very high yet hour urine pH is not high suggesting insulin resistance – Are you overweight, are your fasting blood glucose values high normal etc?? High sugar intakes can raise urine calcium, do you eat a lot of sugar, do you make smoothies? You do not show a urine creatinine value – is the sample over collected? These are thoughts for your physician and you to think about. Let me know, Regards, Fred Coe
Thank you Dr. Coe, I really appreciate your quick reply and your invaluable insights. I will take this information forward to my physician. I am about 25 lbs overweight and do consume too much sugar as well as a lot of protein. I guess it’s time to get serious about reducing both the sugar intake and my weight, while still focusing on a further reduction of sodium intake. My urine creatinine value is 2180.
Warm Regards,
Dan Metteer
Dr. Coe,
Last November was my first kidney stone encounter, after 2 ER visits within 24 hours. I was able to pass the stone three days later, although somehow it was not caught in the strainer. I since have changed some habits, am drinking way more water, and last night I felt some of the same symptoms. panic! This morning I seem to be fine but have been reading everything I can and came to your excellent information. Since I have no idea what kind of a stone it was, is the next step 2 24 hour samples? Do I go to the urologist for that or my local family doctor? Thank you so very much!
Hi Debbie, Your physicians can order for you and indeed should. Be sure a blood is drawn to check for systemic causes of stones. Here is a plan for you. Regards, Fred Coe
Dr Coe,
I have read your wonderful articles.
Thank you!
Jill Harris told me to contact you directly. I have taken her Kidney Stone Prevention diet course in early 2019 and I continue to be part of her phone calls.
She reviewed by Litholink 24 hour urine results from July 2019 and my Mayo Clinic 24 Urine super saturation from January 2020. She told me she has never seen results like mine. My 24 urine calcium is over well 500 close to 600 and my urine phosphorus 1.83
I went to Mayo Clinic Arizona to a Mayo Nephrologist Dr Keddis in May 2019. I started Chlorathalidone 25 mg as Dr Keddis told me I have IH and I’m a kidney stone factory. She also told me I now have CKD stage 3.
I had started eating the kidney stone diet in March 2019. low sodium, low added sugar,
Less than 25 grams, calculated amount of protein, low oxalate less than 100 mg. I continued to drink lots of water. I was so hopeful my results would be improved in July and they were worse not better! I wept my Mayo nephrologist has never had a patient like me before and after 3 months took me off Chlorathalidone to let my body detox. My repeat 24 hour urine in January was the same! Her nephrology nurse practitioner called me a zebra patient. Dr Keddis is writing a case study on me to present to other Mayo doctors to see if someone can figure out how to treat me. I have also been to many other Mayo doctors to find answers: Endochrinologist, Allergy Asthma Immunologist, Dermatologist, Geneticist because I also had severe reactions including I also developed severe eczema from the Chlorathalidone and it also did not stop my calcium loss! No one has answers for me! I did find out that my serum baseline Tryptase level is elevated but they ruled out Mastocytosis.
I have had 3 Kidney stone surgeries. One in December 2015 and two in 2019 the last at Mayo Clinic Hospital Phoenix.
Kidney Stone Analysis
Ureteroscopy surgery with basket stone removal no Holman laser used!
KIDNEY stone from left kidney
From remaining portion 1.5 cm stone
September 9, 2019
Mayo Clinic Hospital Phoenix
80% Calcium phosphate (apatite)
20% Calcium oxalate dihydrate
Also my stones had 4 + Enterococcus when cultured at Mayo
March 19, 2019
Banner University Medical Center Phoenix
Bilateral ureteroscopy Holman laser lithotripsy surgery
No stone Analysis done on stone from Left kidney.
From Right Kidney/ ureter
5 mm stone
40% Calcium Oxalate dihydrate
60% Carbonate Apatite
February 17, 2019 17 small stones passed right after stent placed in right kidney/ureter/bladder
I was an inpatient at Banner University Medical Center on Kidney floor I asked for them to be analyzed but they were analyzed!
I also had a second Pyelonephritis enterococcus after my stent was placed in February 2019 before Kidney stone surgery in March 2019
December 14, 2015
Left ureteroscopy Holman laser lithotripsy surgery
Banner University Medical Center Phoenix
From left Kidney
1.9 cm stone
10% calcium oxalate dihydrate
90% Carbonate Apatite
Dr Coe, do you have any ideas of how help me and what is going on with my body?
Dr Keddis thinks maybe my body is losing phosphorus and is signaling my body for more phosphorus and my body is also releasing calcium. My serum phosphorus levels on Chlorathalidone became very low below the reference range.
I resently in late January had a very severe delayed allergic reactions to Augmentin prescribed for another reoccurring UTI this time it was Klebsiella pneumonia.
I look forward to hearing from you.
I hope you can help me!
Thank You for reading my message,
Ruth Angle
Hi Ruth, It seems you have a more marked urine calcium loss than is usual in routine genetic hypercalciuria, and perhaps there is some notable low serum phosphate on and off. You do not mention serum calcium or PTH; I presume fasting morning serum calcium levels are all normal (below 10.1) and serum PTH is not low (significant diseases lower serum PTH and raise urine calcium) and that your level of serum 1,25D has been measured and the value is not exceedingly high. Urine phosphate reflects diet phosphate, so the amount in the urine is of no special significance. The fraction of filtered phosphate in urine is sometimes helpful in that rare cases of very high 1,25D can arise from reduced kidney phosphate conservation. On the other hand we have published that routine stone formers tend to have less than normal serum phosphate levels. I also presume your bone mineral density has been measured and is not low and is not falling over time – progressive bone disease can cause what you describe, and that you are not diabetic or markedly insulin resistance as both can do the same. I guess in saying this I am saying that evaluation of marked hypercalciuria is complex and a lot of causes need to be considered. Perhaps your physicians have already done all I have said, and also have looked at your magnesium handling (low serum magnesium can be a clue). From here, lacking details I cannot be of much help. Perhaps you might want to review your medical results against the few things I have noted and find a clue. Regards, Fred Coe
Hi Dr. Coe.
Thank you for your series of informative articles.
My lab uses Quest to process Urorisk results, and Quest does NOT provide supersaturation values. I know only the AMOUNT of calcium, oxalate, calcium oxalate, sodium, phosphorous, citrate, potassium and uric acid I produced (among other constituents), and the volume and PH of my urine. Is there a way I can calculate supersaturations myself, or is there a website to guide me? I do understand a computer is usually used for the complex calculations because many factors interact.
Do you recommend another lab for future 24 hour urine tests, one that provides supersaturation information?
Thank you.
Hi Pamela, If I may permit myself a brief moment of candor and perhaps impoliteness, I may say it is wrong for a commercial vendor to offer kidney stone testing in 2021 without supersaturation calculations and patient/physician oriented interpretation thereof. Use Litholink. I founded it, sold it to Labcorp in 2006, and own not one share of stock nor receive one penny from them. So I am free to say they are superior. Quest need not offer an inferior a product – they are a fine commercial lab. They should upgrade their kidney stone offering. Regards, Fred Coe
Dr. Coe
This is a wonderful article I read on clinical supersaturation. Could you please provide us with any contact point for accessing the program Equil2 or Equil93?
Many thanks
Hi Shini, It is not available, unfortunately as an open source. Regrets, Fred
hi Dr Coe.
i have a question
is there any standard reference range for calcium oxalate , calcium phosphate and uric acid stones Relative Supersaturation ?
i mean RSS normal range for these stones ?!
is there any reference range exist ? i can not find it anywhere in internet or articles.
Hi Sal, Here is the article you want. For Calcium oxalate stones, risk begins at CaOx SS above 3-4, or so; for calcium phosphate and uric acid risk begins with SS above 1. SS is a graded risk factor. Regards, Fred