This is a story about how well the supersaturations we measure in 24 hour urines reflect the average supersaturation in the kidneys of patients, whether supersaturations match kidney stones – match the crystals stone contain.
If they do match well, we can trust supersaturations as our guide to treatment.
If stones and supersaturations do not match well, what value can supersaturation measurements have?
They could mean nothing. Stones form over months or even years.
Can a few frames tell us about a movie?
It is also a story about my own past because Joan Parks and I did the work over 20 years ago. Though old, human observational data do not go out of date. The stones and 24 hour urines from those patients we studied long ago inform us today, still, as they did then. A good reason to study human diseases in humans.
In Principle They Must Match
As a physical fact the sole driving force for crystallization – supersaturation – must determine what crystals form. In turn, that physical fact makes the obvious prediction that urine supersaturations match kidney stones.
But, Do We Measure What Kidney Stones Encounter?
Although urine chemistries change with food and the weather, kidney stones accrue mineral like any other geological artifact and therefore reflect long term urine averages. In other words, physics demands that long term average supersaturations match kidney stones.
But do we measure long term average supersaturations? In practice, we measure supersaturation only here and there, a day at a time. Because we have only those few samples, we and the stones may ‘see’ different things.
So the seemingly obvious prediction that supersaturations match kidney stones really concerns sampling. Do clinical samples, a day or two here and there, really gauge those long term averages that produce the kidney stones?
This is why we should care if supersaturations match kidney stones.
If they so, what we measure means a lot.
If not, what we measure may not matter at all.
Supersaturation Stores Work as Energy
Supersaturated solutions store power in them like electric batteries. That power can discharge itself in forming a solid phase, kidney stone crystals. So our very 24 hour urine samples have instability built in. That instability can lead to premature discharge, loss of supersaturation in the very container, so we measure less than what was really present in the kidneys. Furthermore, losses may be random and blur the real signals so we lose resolution.
Solubility
You can saturate a solution easily. Simply stir it with an excess of any substance at a constant temperature, and eventually the concentration of the substance will come to some final and unchanging concentration. By excess, I mean some of the solid phase remains at the end of the process. We call the solution concentration at the end of the process the solubility for that substance at that temperature. No matter how much excess solute remains in contact with the solution, you cannot drive more of it into the solution.
If you perform the experiment with the same solid phase at different temperatures you will find that solubility rises with temperature. You can create the temperature solubility curve for that substance. Such curves have been created and published for many solute – solid pairs. For example, for the stone forming salts in water at body temperature.
A solution at solubility can remain stable forever. It has no forces in it to bring about change so long as the water does not evaporate.
But what about supersaturated solutions?
Doing work on a solution
Change of temperature
Because solubility rises with temperature, you can heat a solution at the solubility point and dissolve more solid phase in it. Essentially you move the system up on the temperature solubility curve. That does work on the system – heating it. If you take away the heat source, the temperature of the solution will fall. As it falls the solution gives up some of the energy stored in it by heating.
But, if you do it slowly and carefully, if you lower the temperature back down slowly, the solution may remain clear even though the concentration of the salt is above solubility. Some of the work you did in heating the solution remains as energy stored as supersaturation. This free energy of supersaturation will eventually release itself by forcing the dissolved salt into a state of lowered entropy – as a solid phase as opposed to a dissolved phase.
Evaporation
Suppose you return to the solution at solubility and pour off the clear part leaving behind the extra solid phase. Keep the temperature constant alone but evaporate water off the surface. For example, fan it, or blow warm air over the water taking care not to heat the solution. Do it slowly and the solution will shrink in volume but remain clear.
Once again, you did work on the solution, and part of the work transformed into the free energy of the supersaturation.
Kidneys do not evaporate water. They use free energy from metabolism of substrates to power transporters that move ions and therefore drive movement of water from tubule fluid back into the blood. Though the pathways are specific to kidneys, they do what you did when you evaporated water: store free energy in fluid as supersaturation.
Clinical Urine Samples
Because of the work kidneys have done, these contain free energy of supersaturation. They can change as that energy dissipates.
We let them cool yet they stay stable
When we collect 24 hour urines, temperature usually falls from that of the body to that of the wide world. Supersaturation must rise as in your experiment. But surprisingly, for up to several days, a urine will generally maintain very constant concentrations of calcium, oxalate, uric acid, phosphate, pH – all of the key variables that we use to calculate supersaturation. Such constancy makes 24 hour urines practical for patient care. Because of the constancy we calculate supersaturations assuming body temperature.
But why do they not collapse into crystallizations?
What lets urines store energy for days on end, when in a simple experiment with supersaturated sugar solutions sugar crystals form if your shake the container? We transport 24 hour urines on trucks, in airplanes; no doubt people shake them up all the time.
Urine contains abundant and varied crystal retardants
The stability of 24 hour urines far exceeds that of simple salt solutions because of retardants. They delay formation and growth of crystals. Some retardant molecules up kidney stone matrix. The urine proteome contains most of the kinetic retardants, but we know this vast mix more poorly than the flora of the ocean deeps. We know some of the retardant molecules. We do not know most of them. How they act together to retard crystallization remains for brave and tireless scientists to discover. Clinicians have no assays.
But we do know one small molecule, citrate, retards crystals. We measure it, we can influence its urine levels. Citrate links urine crystal retardants to clinical practice.
Do Supersaturations Match Kidney Stones?
Years ago we took on this problem and found an excellent match indeed.
But how can I pass by the immense effort hidden in those words? ‘Big Data’ offers a shiny image of perfect numbers all arrayed in tables waiting for smart young savvy folk to put up an elaborate query and watch new and remarkable patterns form themselves on multiple screens.
We have kept our data in computers since 1971 and our perfect tables of lab data wait on us. So we should have merely pushed a button.
The Work of Storing Data
Clinical data have messy elements. Sure, we had entered and stored the dates of each stone analysis and the percentages of each kind of mineral. Naturally, we could link lab data to the dates of analysis. And we could calculate percentages of minerals for all the stones in every person.
But as a solution stores work as free energy of supersaturation, computers store work of entry as free energy of information. Work demands people time that demands money, and money limits the work. That is how clinical data comes to depart from the ideal.
So, Joan Parks, who I introduced a short way back, scanned through hundreds of paper charts to find stone analyses not yet entered because late, or lost a while, or perhaps entered with a wrong date. We read about data integrity and all, but no one can publish clinical data safely without checking a lot. Joan did all that to make us our table, and the table made this paper.
Types of Kidney Stone Formers
Our Subjects
With considerable effort, Joan Parks gathered together our 1085 patients with reliable kidney stone analyses and grouped them by stone type. But first we excluded those with systemic diseases as a cause of stones leaving 585 to ask our question from. We had 24 hour urine studies for 67 normal – not patients – people to use as a contrast.
How We Named The Types of Kidney Stone Formers
Calcium oxalate (CaOx) stone former meant CaOx comprised more than 80% of the total crystal composition of stones analysed from a patient and no stone contained any uric acid (UA).
We named as calcium phosphate (CaP) stone formers those in whom calcium phosphate comprised more than 50% of kidney stone crystals. By contrast, mixed CaP/CaOx stone former meant that CaP comprised between 20 and 50% of the crystals, the rest CaOx. Uric acid meant stones were entirely composed of that crystals, while mixed uric acid meant any uric acid in a calcium oxalate stone.
What We Compared
We have always obtained three 24 hour urine samples from each patient before starting treatment. We used these samples to calculate the three supersaturations – CaOx, CaP, and UA, and compared the values of each between the various types of stone formers and in contrast with our normal people.
Supersaturation vs. Type of Kidney Stone Former – Males
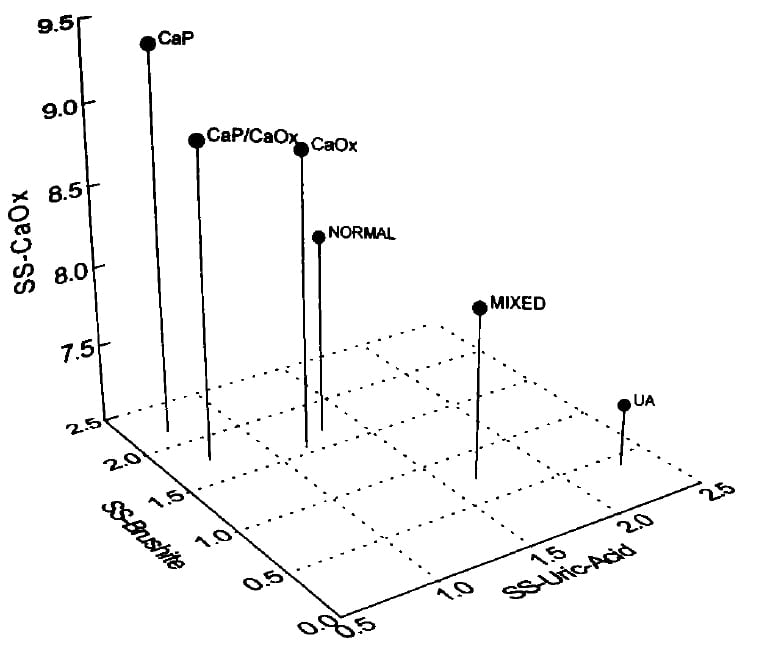
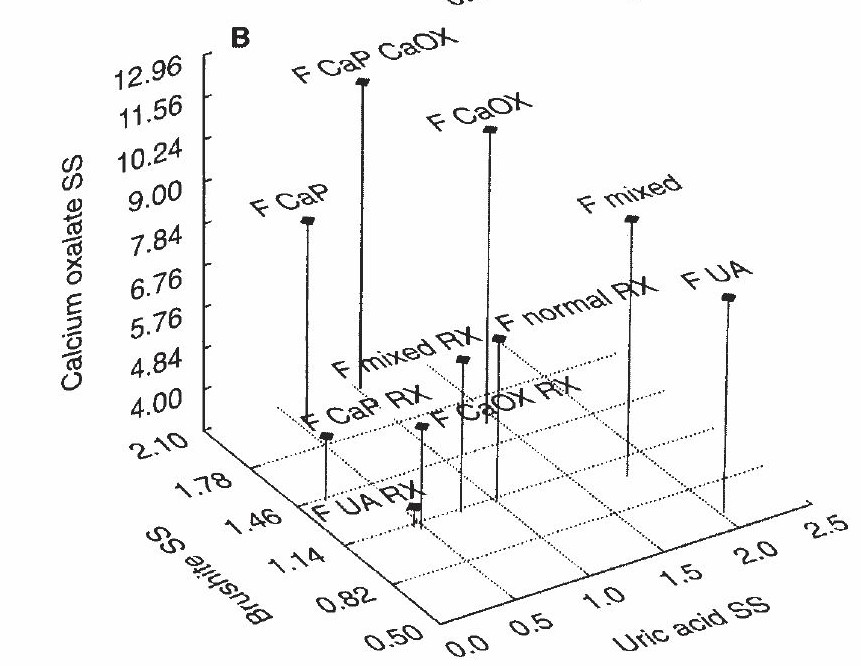
Read this 3D Graph With Me.
The pin ‘M normal’ represents normals on three scales. The point of the pin is at about 1.3 SS for uric acid (runs along the front of the graph) and 1.7 Brushite (calcium phosphate) SS. The height gives the calcium oxalate SS – about 9.
Use this as a reference point. M means males, and all pins are males, so I will skip the ‘M’.
Supersaturations Match Kidney Stones
Look at CaP – calcium phosphate stone formers – at the upper left. The pin point lies at nearly 2 on the brushite SS scale and nearly 0 on the uric acid scale but the height – calcium oxalate SS nearly matches the normals. This means a higher CaP SS goes with CaP stones.
Next to it, to the right, ‘CaP/CaOx’ means patients with a lot of CaP admixed in their CaOx stones. See where the pinpoint lies at CaP SS over 2, which matches for abundant CaP in CaOx stones. But the uric acid SS is higher because the urine pH is not as high as the CaP stone formers.
Now, look down at the ‘CaP RX’ pin. See how short it is? That is from treatment. But even though I – these are my own patients – lowered CaOx SS the CaP SS (pin point) lies at nearly 2! I lowered CaOx SS better than CaP SS.
Uric acid stone formers are at the lower right – very short and stubby, treated or not. UA, untreated uric acid stone formers have a uric acid SS of nearly 2 and, of course, a brushite (CaP) SS of only 0.5. Treated (UA RX) their UA SS is near 1. Those with mixed CaOx and UA stones lie far to the right at a UA SS of nearly 2.5. See how tall a pin represents them. They have considerable CaOx SS. Treated, their pin shrinks down and moves to a UA SS just about 1.
Finally, CaOx SS of CaOx stone formers is a bit higher than normals, and when treated their pin shrinks down a lot. But kidney stone formers’ CaOx SS does not exceed normal.
Urine pH Displays Considerable Stability over Time
Stones accrue mineral over long periods as compared to the single day of a 24 hour urine collection. Even so, with some modest variability, the pH dependent 24 hour urine supersaturations tracked reasonably well with patient stone types. This can only mean that urine pH, the main factor that controls CaP and UA supersaturations, remains more or less stable within individual patients over the geological time scale of kidney stone production.
Do Women Differ From Men?
No
Just like men, CaP stone formers cluster at the upper left and UA stone formers at the lower right, meaning that supersaturations match kidney stones. Treatment that reduced kidney stone production lowered supersaturations as one would expect.
Yes
But unlike the case in men, calcium oxalate supersaturation of calcium oxalate kidney stone formers exceeds normal. In other words these women made calcium oxalate crystals for their kidney stones and had a higher than normal urine CaOx SS to drive formation of those crystals. Men did not.
This sex difference about calcium oxalate stone formers arises from something the graphs do not show. Male calcium oxalate kidney stone formers produce higher urine volumes than normal men. Women display no such urine volume difference.
Adjust for Urine Volume
After a stone people usually drink more and come to clinics like ours, so we suspect urine volumes we measure exceed what preceded the stones. What do the calcium oxalate and calcium phosphate supersaturations look like adjusted for urine volumes?
CaP and CaOx Kidney Stone Formers and Normals
In the left and right hand panels we show volume adjusted urine CaOx and CaP supersaturations for normals (round symbols) and 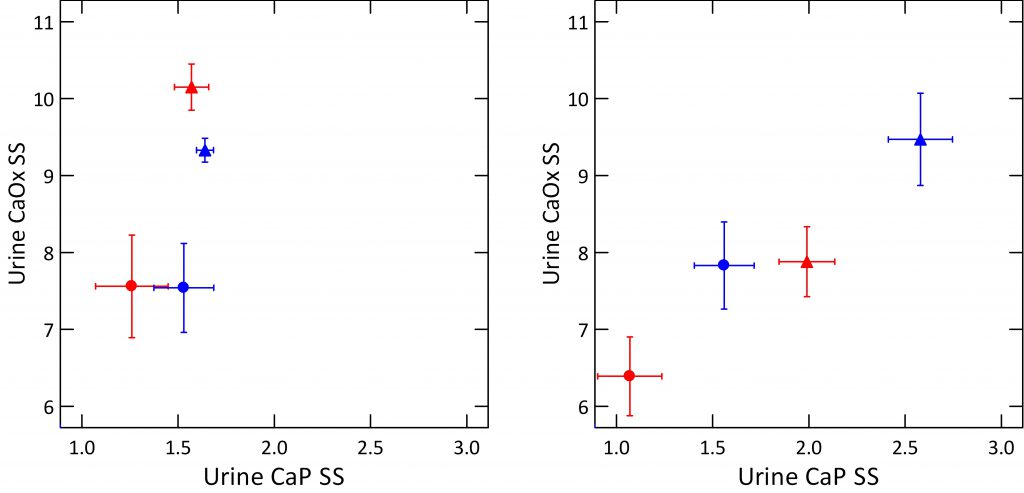 patients (triangles), women (red) and men (blue), with standard error bars to give a sense of variability.
patients (triangles), women (red) and men (blue), with standard error bars to give a sense of variability.
The left panel shows the calcium oxalate kidney stone forming patients. The normals have much lower adjusted CaOx SS but not lower CaP SS than the patients. You can see this from the marked separation in the vertical direction and how the points all merge together in the horizontal direction.
The right panel shows exactly the same things but for calcium phosphate kidney stone forming patients. Normal women have much lower CaP SS than stone forming women (compare red circles to triangles) and the same for men (blue).
The two panels are plotted to the same scales. This makes obvious the higher CaP SS and lower CaOx SS among the CaP stone formers, right panel) vs. the CaOx stone formers on the left.
A Look At Only Calcium Kidney Stone Formers
Perhaps this last point becomes more clear if I plot 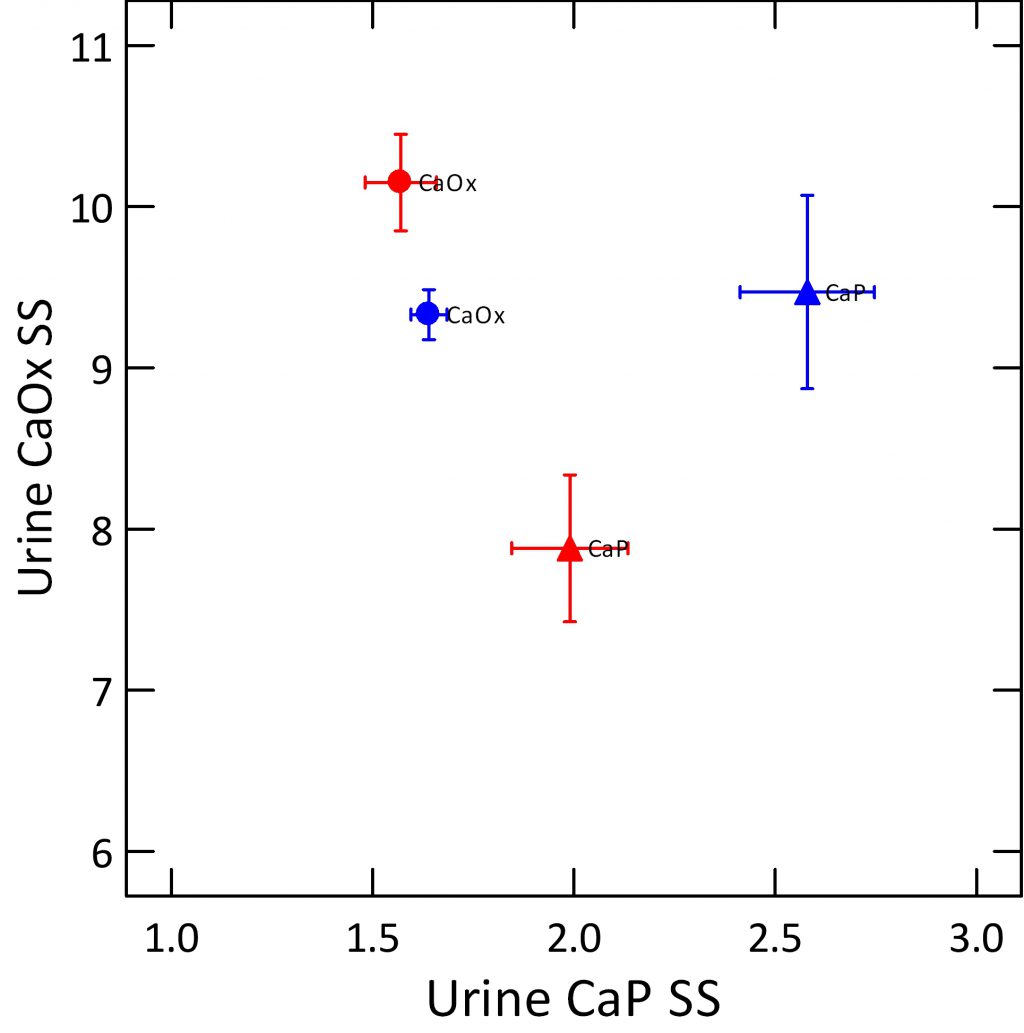 only patients, only calcium stone formers, and label the points by the kinds of kidney stones they form. For women (red) CaP kidney stone formers (triangles) have much lower CaOx SS than do women with CaOx kidney stones (red circles), and modestly higher CaP SS. For men (blue), the CaP SS is much lower among CaOx patients, but both types of stone formers produce considerable CaOx SS.
only patients, only calcium stone formers, and label the points by the kinds of kidney stones they form. For women (red) CaP kidney stone formers (triangles) have much lower CaOx SS than do women with CaOx kidney stones (red circles), and modestly higher CaP SS. For men (blue), the CaP SS is much lower among CaOx patients, but both types of stone formers produce considerable CaOx SS.
What About Uric Acid Stone Formers?
No need to make a graph for them. They stand so much apart from the others confusion is minimal. They do this because urine pH has such power over supersaturation, supersaturation seems to cause crystallization without much effects from inhibitors.
Could We Reproduce This?
Who We Studied
A group of practices that used Litholink during its early history as a private small business provided equivalent data so we could ask the same question using different sources.
The six practices ranged from NY to Nebraska, meaning 24 hour urine samples traveled via trucks and planes to one laboratory whereas all of the samples I already showed you were delivered by patients to our laboratory the day the collections ended. Also, the practices differed from our research center: more heterogeneous patient groups and physicians, different geographical locales.
What We Found
Even so, results match ours, and supersaturations match kidney stones.
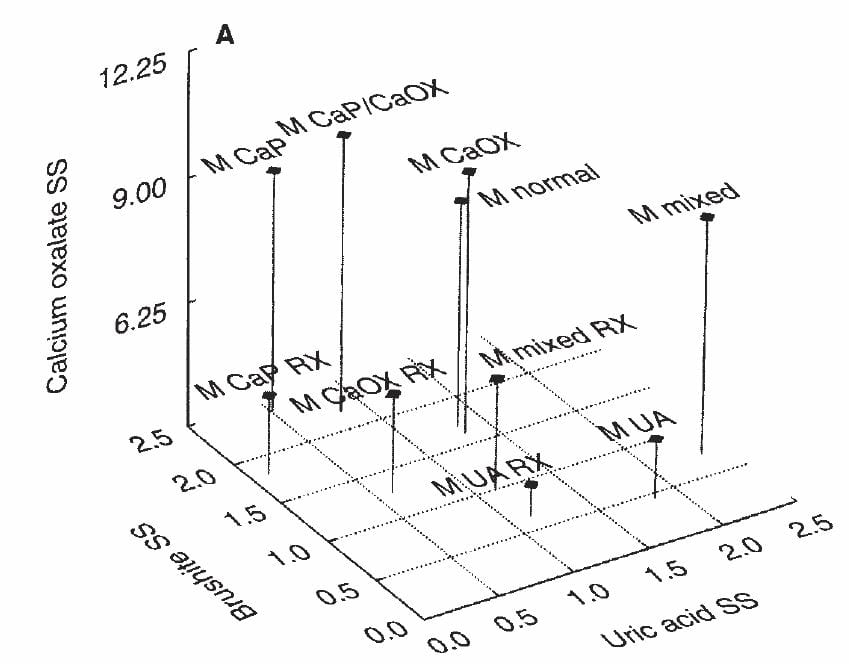
 Uric acid kidney stone formers (UA) lie at the lower right, meaning all UA supersaturation. CaP kidney stone formers lie to the far left, with high CaP supersaturations. CaOx kidney stone formers occupy their middle ground and like the CaP patients have considerable CaOx SS as well as CaP SS. Normal people are borrowed from our normals, so look more or less the same.
Uric acid kidney stone formers (UA) lie at the lower right, meaning all UA supersaturation. CaP kidney stone formers lie to the far left, with high CaP supersaturations. CaOx kidney stone formers occupy their middle ground and like the CaP patients have considerable CaOx SS as well as CaP SS. Normal people are borrowed from our normals, so look more or less the same.
As if the exigencies of sample transport and differences in patients and centers and physicians count for nothing, results confirm what I already showed you. So we can believe that supersaturations match kidney stones.
Exceptions Occur
Types of Exceptions
Among our clinic patients and those in the Litholink network we could identify two kinds of individual divergences between supersaturation and kidney stone type. Type 1 divergence meant absence of stone crystals to match a urine supersaturation, and Type 2 meant absence of urine supersaturation to match a stone crystal.
Type 1 Divergence Reflected Low Urine Volumes
When the discriminant analyses and computations had ended, we found some patients who had exceptionally low urine volumes supersaturated their urine with respect to all three kidney stone crystals yet their stones contained only 1 or 2 of the three. Even so, the main stone component was matched by a corresponding high urine supersaturation. Essentially low volume supersaturated urine generally. But that only some phases appeared make us suspect the low volumes as sporadic or inconstant.
Type 2 Divergence Reflected High Urine Volume
To find a phase sans supersaturation upsets our primary conclusion, so this type of divergence weighs on our minds to now. We found it in 13% of patients at our center and in our network study. Mechanistically the patients had high urine volumes in relation to their urine calcium excretions and this volume to calcium disproportion reduced CaOx and CaP SS. We guessed that increased fluids and diet calcium restriction in response to kidney stones caused this important divergence, but could not test the guess, to this day.
What Is The Answer?
After all the numbers have been put in their places, 24 hour urine supersaturations look pretty good.
Supersaturations match kidney stones – they match the crystals those stones contain.
So, give or take some issues around the edges 24 hour urine supersaturations broadly reflect those long term geological averages that control how kidney stones form. That they do so yet are themselves very brief samples of life as lived means that urine composition has considerable stability in any one person.
What Does The Answer Mean?
The 24 hour urine supersaturations we measure in our patients, the numbers patients can see for themselves in their own laboratory reports mean a very lot. They bring to my mind the ancient discipline of celestial navigation which, howsoever crude, guided those early bold sea captains round the Cape and the Horn, and over the whole world of the ocean sea.
As they did, we have our few fixed points of light, our scant measures to guide us. From what I have shown you, they are firm enough to trust. If the future grants us the perfected nautical chronometers of the nineteenth century or the miraculous GPS that tells us everything, right now we can make do as they did who caught the moon’s transit and found their way over the vast, indifferent, widow making sea to their distant and long desired landfall.
The supersaturations make us our way to prevention.
Use them.
Joan Retired
How could I end the story without a note about who did all the work?
After over 100 papers on stones, Joan retired to pursue writing novels, some quite good.


The article on supersaturation, although academically interesting, does not give specific suggestions on what might be most helpful to patients with higher than average supersaturation of CaOx or CaP. Increasing urine volume will lower supersaturation, but is there any benefit to HCTZ if the patient’s 24 hour urine Ca is not high, or is there benefit to oral calcium or dietary restriction of oxalate if the patient has normal 24 hour urinary oxalate excretion?
Thanks for such perceptive and useful questions. When urine calcium is normal depends on the definition. I prefer using statistical risk estimates as a basis for deciding when urine calcium is high in a stone former, and that would place the upper limit – when risk begins to increase – at about 200 mg/day. Likewise for urine oxalate, but I am afraid that risk begins at around 25 – 30 mg/day so one is always hoping to lower its excretion rate. As a clinician, like you, I use fluids first, and hope to reduce supersaturations for CaOx and CaP by about half. Often, the amount of fluid required is quite high and not achieved in practice. When urine calcium is not above conventional limits – 250 mg/day for women, 300 mg/day for men – but above 200 mg/day in either sex, I try to lower sodium intake to below 100 mEq/day as a first step and then add a low dose of a long acting thiazide like drug such as chlorthalidone 12.5 mg daily or indapamide 2 mg daily. I always try to raise diet calcium to around 1000 mg from foods, in hopes of lowering urine oxalate and, incidentally, lowering risk of bone mineral loss. Likewise in all cases I look for sources of high diet oxalate. When urine calcium is below even the statistical risk limit of 200 mg/day, I still try to lower diet sodium intake to below 100 mEq/day, but decline to use thiazide like drugs. This is personal preference as we have no relevant trial data. I should mention that the site is in evolution, and sections on hypercalciuria, as a example, are yet to come. One day, when all of it is finished perhaps some of the excellent questions you have posed will be answered more organically. Regards, Fred Coe
As always, a clear and thought provoking discussion.
A few comments from an interested but mostly uninformed observer:
What I the critical size of oxalate crystals in urine, or of apatite crystals if these are the nucleation sites for oxalate crystals as you suggest they might be? Given the complexity of crystal growth in the presence of many and unknown inhibitors, I suspect that there is no simple answer, but I ask because in my experience the behavior of very small (say <500 nm) crystals is unpredictable, and thermodynamic theory a poor guide. For example Ostwald ripening, in some cases at least, is a thermodynamic fantasy that real crystals don’t obey. Hematite (Fe2O3) nanocrystals incubated in their parent liquor at saturation became finer, not coarser, as large crystals (ca 300nm) preferentially dissolved and are replaced by a new generation of smaller (ca 50nm) crystals. This seems to be because the larger crystals had more and larger defects, which negated the increased stability they otherwise would have had from their lower relative surface area.
Have there been any isotopic studies of kidney stones? I know that there haven’t been any for Ca isotopes, but perhaps someone has looked at O or C. I ask because isotopes could record information about the kinetics of crystal growth and the actual conditions of growth, which may not be the same as what you see in 24 hr urine. For example, the isotopic offset between total urinary and mineral Ca should be less in crystals that form rapidly than those that form slowly.
I do not know if there is a critical size for stone crystals because of the extreme pressures that urine proteins exert right from the beginning of nucleation. Much of what we know comes from in vitro modelling which is never really exact in relation to the situation in kidneys. I know about one isotope study in kidney stones by professor Kok using – I believe – calcium isotopes to date stone formation via swings in isotope abundances from atomic testing. With respect to differences between urine and stone I know of nothing. Of course urine conditions vary constantly between people and within a person over time, but perhaps isotope ratios do not vary so this kind of information might be available given stones. Stones are abundant. Regards, Fred Coe
There is a geological analog to this. The ocean is supersaturated with CaCO3, but is metastable because of poorly known proteins and other organic molecules excreted by organisms. There are a few places where CaCO3 spontaneously precipitates from seawater, but for the most part it is removed by organisms that exploit the supersaturation to build skeletons.
There is also a normal human analogue: Blood is metastably supersaturated with respect to calcium monohydrogen phosphate but tissue bound and circulating inhibitors prevent soft tissue crystallization most of the time where as bone exploits the saturation to add new mineral as part of bone turnover. Fred Coe
The stability of 24 hour urines is far greater than that of simple salt solutions because of its retardants which are, at least in part, the molecules that make up stone matrix.
Hi, I think you are right, and thank you for the remark. I have done something with this idea on the site. Regards, Fred Coe
Whether the stone is soluble when we maintain concentration below supersaturation in urine? Or it is irreversible? . I have 2 cm size kidney stone in right side.
Hi Raj, uric acid and cystine stones can and do dissolve. Calcium oxalate stones hardly ever, and calcium phosphate likewise. Regards, Fred Coe
Hi I am a patient and chronic calcium stone former. I found this site when trying to determine how I might be able to improve things. My urologost and I have struggled to manage things over the last few years as I am always producing and passing small to medium size (5mm) stones. While your language is geared towards medical professionals I found your analysis interesting and relevant.
Hi Jacob, I guess the most valuable way to progress matters is to think about a strategy for prevention. I have several linked articles on this topic, and this link ‘links’ to them all. Basically you need to know the crystals in your stones, know the supersaturations in your urine that are relevant to those crystals, and take steps to lower them. Have a look and let me know. Regards, Fred Coe
Dr Coe,
What if susperstation Caox and Cap are both LOW as well as urine calcium but you still are an active stone former? I am specifically referring to Cap stones.
Hi Kori, Since crystals follow physical laws and there is only one physics what you describe is complex. When you say supersaturations are low, compared to what? For example, in some people any CaP supersaturation is enough to create new CaP crystals. In anyone, any supersaturation is enough to support growth of existing crystals, and such new growth can break off and create ‘new’ stones. For this reason, if you are active with ‘new’ CaP stones perhaps SS for CaP needs to be below 1 in a 24 hour urine but so low in 24 hour urines that during the day it is not above 1 at any time. I have not said that on this site before, but it is crucial for practice and I really should add the point in the articles. Regards, Fred Coe
Dr Coe, How is supersaturation calculated from a 24 hour urine sample?
Thanks
Satyarth
Hi Satyarth, Take a look at my video that tells about the whole matter. Let me know if it works for you. Best, Fred
Dear Dr. Frederick Coe thank you once again for a thought provoking article.
I see the supersatuation levels for me (cAP) remains the highest of all formers
How much does high pH play into this? Also, we’re any tests, anytime, cases more complicated stone formers. Those of us with deformed drain tubules? Can pooling in the kidneys effects these success rates.
If not, it would certainly leave those of us that do consume two litres daily baffled.
Do you think a kidney pooling defect could muddy the results? Do msk patients have a pooling defect thereby rede ring these results valid for simple stone formers. I also take two.5 mg of indapomide, and 20mg daily of amino ride. To combat the indapomide potassium wasting.
But I believe supersaturation is not nearly enough to combat makers. Yes supersatuation but something else too!!!how else do you fight a birth draining defect ?
I fight with every tool there is. Water in my minds eye been the best but how do we fight dirty little draining pool’s
In so much gratitude, Laura Bousada
Hi Laura, The poor draining you speak of would be MSK, and I do believe the lower the supersaturation the less the crystals. But, if that is what you have and many crystals have accumulated they will not dissolve. So, water matters; the indapamide, too. Better said – whatever the reason for crystallization supersaturation is always the primary force that permits crystals to form, so everything we can do to prevent stones ultimately works through lowering supersaturation. Warm regards, Fred
Some hospitals require that the 24 hour urine collections be refrigerated. Does this practice influence the measurements?
One does not advise it. The main tests of stability have been done without deliberate cooling. Regards, Fred Coe
How much importance one should give to improvements in citrate levels if parrallel improvements in saturation index and ph is not seen. In some of patients this was the respose to treatment after nalysing 24 hr sample. Also these pts showed persistance of acidic urine with significantly low levels of Magnesium and pottassium , even with improvement in citrate levels
Hello Dr; an interesting question. Citrate probably matters a lot as an inhibitor of calcium salt crystallization. So a rise in citrate has theoretical value at a constant supersaturation. As for unduly acid urine despite potassium citrate I have seen this in metabolic syndrome- urine citrate rises higher and higher and pH remains low. The urine potassium can be low because of type 4 RTA. Regards, Fred Coe
HI Dr Coe. Thank you very much for all your articles. I am a nephrology fellow in Boston and got interested in stones. I was wondering what are your thoughts on JESS saturation index vs SS by equil 2. I believe litholink also uses equil 2, but from some basic reading, I got the impression that using JESS has no clear downside and is potentially more accurate?
Hi Nikhil, Jess offers some benefits concerning the calcium phosphate species but neither I nor Litholink uses it. The system is proprietary and the costs are not so low. The interface is not very friendly and one cannot easily automate data intry from production into the system nor get the final results out in usable form – easily. So, if I were specially interested in phosphate complexes and had a grant to cover Jess and some people who wanted to fool with the interface I would surely use it. Right now none of these things apply. I believe John Asplin at Litholink has used Jess for some personal work, and you might want to email him. Warm regards, Fred