Our Tasty Villain
Salt, sodium chloride, part of our commons, our everyday, and central altogether in prevention of kidney stones.
My recent article on the use of 24 hour urine collections was about stone risk: Supersaturation, and the key 24 hour urine components that affect supersaturation – volume, calcium, oxalate, citrate, and pH.
Of these five components we think first about water when we treat patients to lower supersaturation: Fluid Prescription; Thirst for Variety; How to Drink Enough Water.
But, if we are wise, we should think next about salt, and how to lower it, because in so many people it is a key to treatment success.
It is key because sodium chloride strongly controls urine calcium and therefore the risk of kidney stones.
Likewise, it is key because high intakes can cause loss of bone mineral and raise blood pressure in some people.
I say sodium chloride because other sodium salts, like sodium bicarbonate, do not behave exactly as sodium chloride does. But this lack of generality is irrelevant, as it is indeed mostly sodium chloride we eat, and as we eat it entrain the problems this simple molecule can produce.
How To Read This Article
This topic is very complex and many will want only the general idea, some will want the details.
I have put brief summaries in each section. Some sections are all summaries.
They are sufficient to get the meaning of the whole article.
For those with an adventurous heart and a taste for the subject, the regular text is reasonably exacting and referenced.
If you take nothing more away from this article, think about this: Salt and its effects and fates are big stuff in human biology. That pizza is delicious, but that salt is something to think about.
How Does Sodium Get Into the Body?
It gets in with food.
That is the only way.
And, absorption of dietary sodium chloride is very complete.
A recent phase 1 trial of a drug 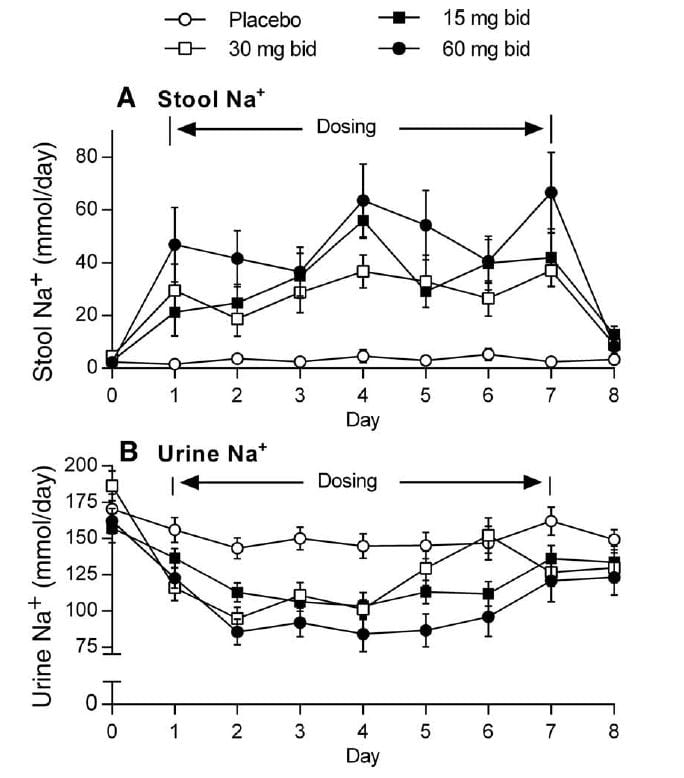 designed to reduce sodium absorption and thus protect people from the effects of their high sodium diets gives excellent quantitative information.
designed to reduce sodium absorption and thus protect people from the effects of their high sodium diets gives excellent quantitative information.
This illustration is from figure 3 of the paper.
On days 3 – 5, which show fairly stable values, urine sodium of the placebo group (the open circles) was about 150 mEq daily, stool sodium about 5 mEq daily. This means 150/155 or 96.8% of the diet sodium was absorbed into the blood.
The drug inhibited GI absorption, and as it did so more sodium was in the stool and less in the urine.
Given the total sodium in urine + stool of about 155 mEq/day, the placebo data probably apply well to most of us eating that much sodium, so one can say the 24 hour urine sodium is the minimum estimate of diet sodium intake for the day and stool losses are very small indeed.
How Does Sodium Get Out of the Body?
Some sodium gets out of the body in sweat. The amounts are modest in most of us, but can be huge in athletes and during heavy work
Intestinal losses are trivial except for bowel disease. We just showed that over 95% of sodium is absorbed into the blood.
Most sodium is removed by the kidneys, and the process is regulated and very complex. The sodium removed by the kidneys is what controls how much calcium we lose in the urine, and therefore the risk of calcium stones.
Non Renal Losses
Hard Data
Sweat losses are so variable what can I but try to establish some extremes.
Professional football players while plying their trade can lose up to 100 mEq/hour of sodium, meaning that in 1.5 hours they would have exhausted all the sodium in the diets of the people in the figure. Tennis players can lose about 38 mEq/hour on an average. Elite female cyclists lose about 60 – 80 mEq total sweat sodium in 3 hours of cycling – 20 to 26, Eq/hr.
Manual workers can lose very large amounts of sodium from sweating. Estimates from well acclimated healthy manual laborers doing a high level of physical activity at summer and winter temperatures lose during a 12 hour work shift between 200 and 260 mEq (16 – 22 mEq/hr). .
The rest of us, sedentary and unremarkable have less data to go on. The prior reference is to a review from WHO pointing out that only one study from 1936 could be found which documents total water balance in a person not exercising and living in a temperate chamber.
Insensible – non renal – losses of water were about 1000 to 1200 ml/24 hours or 41 – 50 ml/hr of sweat + respiratory water. This is much like the insensible loss figure Charles Pak found which I reported in another article which approximated 0.7-0.9 liters daily.
Complex estimates of respiratory water balance give estimates of about 270 ml/day of water loss at 15 breaths/minute. Sodium loss is zero.
This leaves about 750 ml/day as skin losses. Given normal ranges of sweat sodium concentrations of about 20 – 60 mEq/liter, this is about 15 – 45 mEq /day, a not trivial amount.
A Story One Of My Patients Told Me
A middle age African woman who lives part time in the US told me how much she dislikes the climate here as compared with central Africa where she keeps her primary home.
There, she said, she is always warm and knows she loses a lot of water and salt sweating. Her blood pressure is invariably low, she told me, at her physician’s office and at home. She by no means avoids salt. No one she knows there does so.
Here, she said, sweating is not so copious. She is freezing cold most of the time, and her blood pressure is high enough physicians have provided her with drugs to lower it.
People she knows who come from Africa and live here hate the climate much as she does. In her opinion, it is too cold and the lack of sweating is bad for them, and for her.
Renal – Urine – Sodium Losses
Whatever the complexities and uncertainties that lie between the sodium eaten and the sodium that is left over after sweating and intestinal losses, the amount in the urine is the true amount absorbed into the blood and not lost otherwise from the body – at least when diet intake and sweating losses are more or less constant.
Basically water is retained with salt, blood pressure rises, kidneys respond by removing the salt and water.
In some people blood pressure rises almost imperceptibly with high salt intake. In others it rises a lot.
Urine calcium rises in almost everyone with high salt intake, that is why we are interested in lowering salt intake to prevent stones.
How Sodium Gets Out in the Urine
I have already told about the nephron, filtration, and tubule reabsorption when we discussed how potassium citrate works. Here we need to add more detail.
Sodium is filtered in huge daily amounts: about 14,000 mEq (322 grams; 11.358 ounces or 0.71 pounds of sodium). Yes, about 3/4 of a pound of sodium. For sodium chloride this would be an astounding 812 gm or 1.79 pounds.
Of this, over 99% is taken back into the blood. The minute percentage left to go out in the urine is tightly regulated, and the regulation is designed to match urine losses to the net of diet sodium absorbed into the blood minus the amount lost in sweating.
In other words, on average, renal sodium losses precisely balance net uptake into the blood.
How Regulation works – What the brain knows and does
Sodium is mainly stored not in the cells of the body but in the fluids outside the cells – the so called extra-cellular fluid (ECF) which includes the blood and the fluid in the tissues that bathes the outsides of cells. A useful standard reference gives the outlines of sodium and its regulation. Bone is the other large repository, but that sodium pool is relatively static.
The brain monitors and zealously controls the concentration of sodium in the ECF.
If ECF sodium concentration rises, even a little bit, less than 1% above the level our biology dictates (140 mEq/liter) a hormone is released – vasopressin – which signals the kidneys to conserve water.
If serum sodium falls by even a trifling amount, vasopressin production is shut off and the kidneys lose water into the urine.
In the middle zone, when the sodium concentration is just right, vasopressin is secreted in its mid – zone, just as in the story of the Three Bears: Not too much, Not too little, but Just Right.
If you keep this in your mind all will be well.
As sodium eaten and retained minutely raises ECF sodium concentration, the brain lowers that concentration back down by using vasopressin to conserve water.
Therefore, sodium retained increases the volume of the ECF which includes the blood. When sodium eaten and retained falls – less eaten, more sweating – everything goes in reverse: Water is lost and the volume of the ECF, blood included, goes down.
How Regulation works – What the kidneys do
The kidneys act in a manner consistent with their monitoring the volume of the blood. How they do that is a matter for another time. But that they do is crucial.
As blood volume rises the kidneys lose filtered sodium into the urine.
As blood volume falls the kidneys conserve filtered sodium back into the blood.
Since the amounts of sodium filtered are vast compared to the amounts lost, the brain and kidneys have an overwhelming power to control sodium balance and therefore blood volume according to diet sodium intake and sweat losses.
They have such power because the total amount of sodium absorbed and excreted is in the range of 50 to 400 mEq/day and filtration is in the range of 14,000 mEq/day, so tiny fractional changes in filtration or in the reabsorption of filtered sodium can bring urine sodium loss up or down to match absorption.
Filtration can be altered by the volume of the blood.
Changes in reabsorption are mediated by hormones and the nervous system which signal the cells that line the nephrons.
What Tells the Kidneys What to Do?
Arthur C Guyton, perhaps more than other investigators has elucidated the effects of altered blood volume from changes in salt intake on the circulation and the kidney.
In general, as salt and water are retained, the volume of blood pumped by the heart remains constant but the blood pressure increases. Blood pressure has a powerful effect on the normal kidney to increase sodium excretion. It does so by increasing filtration and reducing reabsorption.
Filtration
Raising sodium intake (mEq/day) from a low to a medium and then a high level (20, to 200 to 1128 mEq; 460 to 4600 to 25,944 mg) raises filtration progressively. Although the effect is relatively modest, filtration is so massive that slight changes might alter overall urine losses.
Part of the effect is on the simple physics of filtering: Higher pressure increases it.
Part is the effects of angiotensin 2 which regulates the tone of the vessels that do the filtering. We recently detailed the whole process and recommend those interested read the post, especially that part concerned with blood flow and filtration.
Reabsorption
A rising blood volume can increase blood pressure, and pressure itself can reduce reabsorption of sodium and promote urine sodium loss. While this publication concerns control of sodium balance with acute changes in pressure, chronic increase in pressure may well be similar in action.
Angiotensin 2 and epinephrine / norepinephrine which mediate sympathetic nervous system responses to changes in blood volume stimulate sodium reabsorption.
Both hormone types fall when blood volume rises and the converse, and it is these more than changes in filtration what appear to regulate losses of sodium in the urine.
When blood volume rises, the sympathetic nerves to the kidneys appear to promote sodium loss. The relative magnitude of this effect in humans is not clear.
Aldosterone, a steroid hormone, stimulates sodium reabsorption and increases in response to angiotensin 2: More angiotensin 2, more aldosterone. The link is to a recent publication which shows how widely in the kidney this hormone may act, but it also gives access to the older, more established and limited roles which are universally recognized.
‘Natriuretic hormones‘ are produced by the brain and heart. They can increase urine sodium losses in normal people but their role in sodium physiology is uncertain. Their initial promise as treatments for sodium retaining states has not been fulfilled.
Heavy reading for the interested.
Here are samples of very recent reviews concerning the main parts of the kidney responsible for sodium balance in response to diet. You will notice a distinct emphasis on angiotensin and renin which highlights the importance of that system.
These articles illustrate part of the transmission process of science within the profession.
At the foundations, are scientific reports, read by almost no one but a few peers in a field. Next are reviews like these – detailed analytical summaries by active scientists intended for a broader but expert audience.
Next would be chapters and reviews in more general books and journals, meant to disseminate results in a more summary way to scientists and physicians at large.
Next might be sites like this one, which aim to provide for a general audience but have their roots in the primary science.
Last is science reporting in the news by experts in communication who have enough science to be accurate.
Glomerular filtration: A great review of angiotensin and regulation of filtration by a famous expert.
Proximal tubule and angiotensin: Technical review of the cell effects of angiotensin in the earliest part of the kidney. We have discussed this segment in relation to the action of potassium citrate.
Distal Convoluted Tubule: Where aldosterone works, and where final regulation of sodium excretion happens.
Collecting ducts: Even further downstream, these late segments of the nephron tidy up the final amounts of sodium that leave the body.
Salt Intake Controls Blood Volume
Sodium intake affects the volume of the blood and the volume of the blood affects blood pressure and urine sodium loss, so that to get rid of more ingested sodium you must pay the price of higher blood volume and, if kidneys are not highly efficient in sodium removal, in blood pressure.
Prove it to Me
Anyone can prove the volume effect at home. Eat a lot of salt for some days and weigh yourself. Your weight will go up. Stop eating much salt for some days and weigh yourself. Your weight will go down. That changing weight is changing blood volume: 2.2 pounds per liter.
If you are inclined to experiment more, measure your home blood pressure for a week or two on a low sodium intake, then markedly increase your intake for a few weeks and measure again. Many of you will observe little or no change, others of you will observe an increase.
So What?
A certain effect of higher blood volume is an increase in urine calcium excretion, one of the key factors that controls calcium oxalate and calcium phosphate supersaturations, and therefore an increase in kidney stone risk.
A likely effect in stone formers is long term increase in bone calcium loss.
A third hotly debated but likely effect of higher blood volume is increased blood pressure, at least in some people.
Where are My References?
An astute reader will have noticed that my referencing has dwindled. I have made three assertions with minimal external references and not much comment.
The reason is that I plan to discuss these major issues in subsequent articles. Each issue is large and very complex. All I mean to accomplish here is detail how sodium balance works and how diet sodium can be derived from 24 hour urine sodium measurements.
The System Has Time Lags in It.
If you change your salt intake abruptly, it can take 3-4 days for the kidneys to catch up. So the 24 hour urine you collected may not represent your true state, being either on the rising or the falling side of the hill.
Likewise for a period of sudden increased sweating, or discontinuation of a usual workout routine.
Try to collect 24 hour urines during a time when salt intake is steady and represents your overall average.
Never collect during a brief intestinal illness when salt losses can be from diarrhea or vomiting.
Because of lagging urine sodium, blood volume and therefore urine calcium and even blood pressure respond roughly to a 4 day running average of salt intake.
So you can make up for a binge by a day or two of abstinence.
Sodium Balance
Because urine sodium losses follow blood volume and pressure, as diet or sweating change suddenly urine sodium losses must lag.
This is why when you eat a lot more sodium than you normally do your 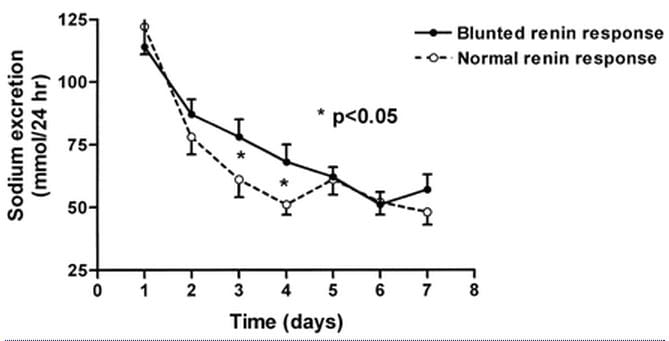 weight goes up: The weight is the increase in ECF volume that signals the kidneys to lose more sodium.
weight goes up: The weight is the increase in ECF volume that signals the kidneys to lose more sodium.
In this lovely experiment 67 patients with mild high blood pressure were brought from their ambient sodium intake (reflected in the 24 hour urine sodium on day 1) to 55 mEq of diet sodium in a clinical research unit.
It took between 3 – 4 days for urine sodium to fall into the range of intake – 55 mEq/day. Resting in the unit, sweat losses were evidently minimal. The two kinds of patients will be of interest to physicians who read this, but I shall pass over this detail for the moment. Notice, however, the patients with normal renin response came into balance faster.
You might say, sodium was lost from the body if urine sodium losses exceeded intake for all 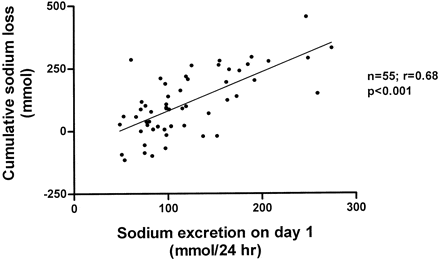 those days. The physiology of urine sodium regulation demands this happened.
those days. The physiology of urine sodium regulation demands this happened.
It did happen.
Here is the cumulative sodium loss balance over the experiment plotted against the excretion rate on day one – which reflected the diet minus sweat sodium value before the diet sodium was lowered to 55 meq daily.
Most lost sodium because they had been eating more than 55 mEq/day before the experiment. A few had been evidently eating less than 55 meq of sodium daily and gained – negative loss on the graph – on the experimental diet.
Of course, gain of sodium means gain of water because the concentration of sodium in the blood is tightly regulated by the brain as I have already mentioned.
The blood has 140 mEq/liter of sodium, so a loss of 280 mEq (look along the vertical axis) would mean 2 liters of water were lost. That is two kilograms or 4.4 pounds.
Blood Pressure
Blood pressure (vertical axis) fell along with falling sodium excretion (horizontal axis of left panel of figure) in some of the subjects. In fact in those with the normal renin response from the prior 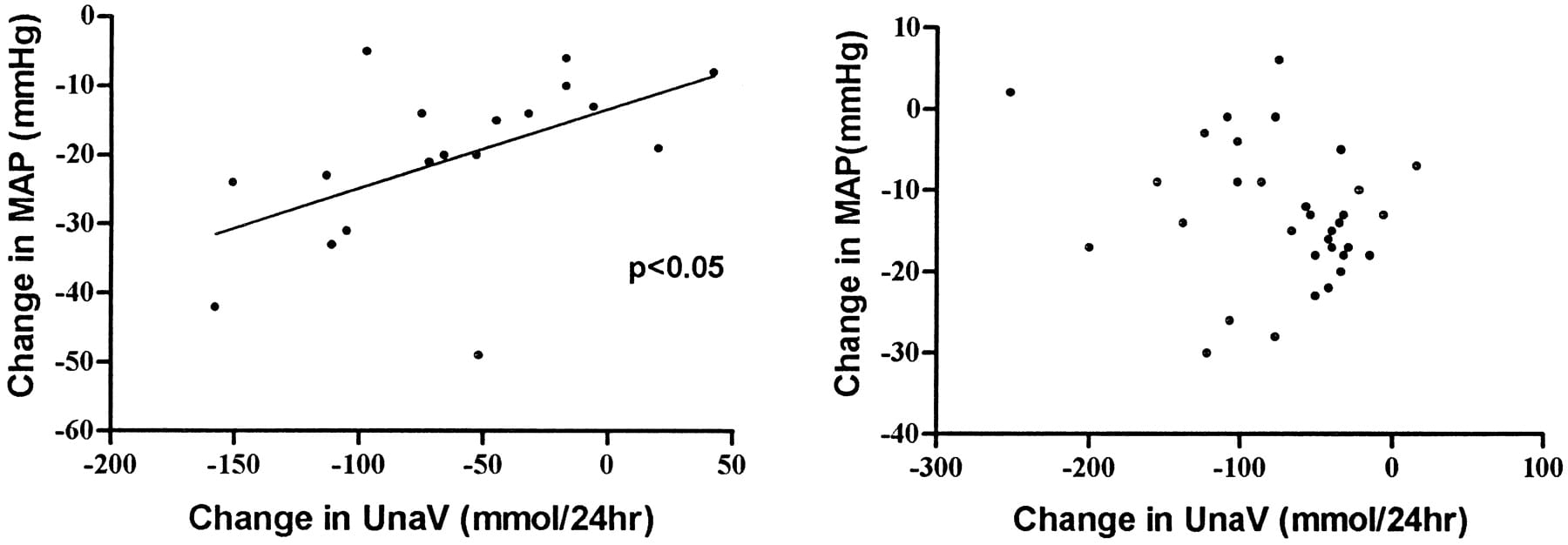 figure.
figure.
Among those without a normal renin response (right panel) pressure also fell, but the proportionality with urine sodium was absent and on average their pressure fell less markedly.
The main message: Lower sodium intake lowers blood pressure; variability among people shows how complex the system is.
Renin is an enzyme that controls angiotensin 2, the key hormone I spoke about just above.
So people with abnormalities in a key regulator of salt balance fail to show a ‘normal’ relationship between salt balance and blood pressure. In fact, those with a normal response simply came into salt balance at a lower blood pressure than those without a normal response.
The message is like that of Guyton: Normal kidneys and their controllers – like renin and angiotensin 2 – permit sodium balance with little change in blood pressure compared to the case where kidneys and their controllers are less supple and responsive.
The Lags Complicate Interpretation of the 24 Hour Urine Sodium
When diet is steady, the urine sodium is the net of intake minus sweat and GI losses, but when either diet or sweat losses are changing over a wide range the urine will tend to average things out.
This can be confusing.
You might come up with 200 mEq of sodium in a collection and remember that you ate very little sodium the day of collection and even the day before because in prior days before that you were eating a lot of sodium and were on the way down. So the urine can say what seems to be wrong but is in fact right.
The Lags Permit Forgiveness of Sin
Suppose you need to maintain a reasonably low diet sodium intake, 100 mEq/day as an example, and also want to enjoy eating out from time to time. We all know eating out means eating a lot of salt.
The averaging works in your favor.
That pizza or that Chinese dinner was great and salty. If the day before and the day after were specially low sodium intake days, the overall demands on the kidneys would be for excretion of the average over the whole 3 – or even 4 days. You can make up for and prepare for high sodium intakes.
Things I Say to Patients
Some patients express incredulity when confronted with the results of their 24 hour urine sodium excretion results, and point out that they eat very little sodium.
For them I always offer a few observations.
Because of lags, it is always possible that the 24 hour urine reflects diet a day or two before the collection, so memory of the collection day might conflict with results from the collection itself.
Otherwise, the urine sodium excretion is, more or less, the net of intake minus sweat losses.
Sodium is an atom, and therefore our bodies can neither make or destroy it.
There is no way to lose sodium in the urine in excess of intake except for the few day lag I have already detailed.
Bottom line: If you lose it in the urine you ate it.
A Great Site For Finding Low Sodium Foods
Theory is wonderful but how do you put together real low salt meals?
The common response is ‘Read Labels’ but that is not so ideal. Stores are busy, there are a lot of labels, and your feet get tired.
Here is a website I found that really offers a lot of alternatives. For example, there is a daunting array of dairy products with modest sodium contents. Take a look.
Here is another. It has a broader reach, being about ‘heart healthy’ foods. But I found a good range of low and sodium free products. I would not mind suggestions for more commercial outlets. Let me know.
A Few Closing Remarks
Sodium physiology is arguably among the most important topics in all of medical stone prevention and moderation of high salt intake one of the more important components of an intelligent stone prevention program.
What Is A Good Goal?
The US Government CDC has recommended an upper limit of 100 mEq (2300 mg) of sodium daily for all of us. For older people or those with high blood pressure the ideal is 1500 mg (65 mEq) daily. The American Heart Association has adopted the same guideline.
Why Have I Not Finished the Presentation?
If reducing diet sodium lowers urine calcium why have I not presented proof of that and also shown the mechanisms by which this happens?
Why not more about blood pressure?
Bone?
Because these are long stories and if I put them here this article would become a book.
The calcium story is at the very center of stone disease and its prevention, and this article and the prior one on calcium as a risk factor are my toe in the water – my few seconds on the high diving board before jumping.
In the meantime, think about salt intake and that for most stone forming people less is better than more.



Highly informative! Exceptionally clear, too. I’m 74 & generally very fit. This spring, over a period of several weeks, I underwent 4 hospital procedures to bust up a big, dense stone (2 endoscopies with laser & stent bookending 2 extracorporeal shock-wave lithotripsies), and now I’m studying how to prevent a recurrence. You’re a big help here. My 24-hour urine collection, by the way, showed that my sodium and protein values were somewhat high, with everything else being normal, so I’m newly alert to the hazards of sodium, but your article does a great job of particularizing those hazards. Many thanks.
Thanks, John. That protein in the urine may just be from blood because of all those procedures. Be sure you know what the stones are made of, and check out the stone risk vs. 24 hour values from Gary Curhan’s work. What used to be called normal may not be. Regards, Fred Coe
Fred, thanks for doing this. Since retiring from Nephrology, and still doing GP telemedicine, I haven’t thought about this so much recently. However, your estimate for sweat loss may be really quite high. Think for a moment about doing sweat chloride to diagnose Cystic Fibrosis: Over 35 meq/l would essentially diagnose CF. I recall old experiments, in which initial NaCl excretion on severe exertion in hot climates (chain gang in AL), is rather high, but adapts within a couple of weeks to vanishingly small. The consequence of that overestimation, which you and hundreds before your have made, has resulted in a religious use of “1/2 Normal saline” as a “maintenance solution”, which makes no sense at all. And as a Pediatrician teaching F&E, and as a Nephrologist treating hypertension, was the bane of my existence for years. Best regards, Jon
Thank you, Jon. Being, as you probably know the least athletic of people, it is hardly my place to argue with the papers I cited. But you do raise an important point: Over time, adaptation may reduce these losses. I did not find such data in the papers. But – perhaps you have some references, and if so I am more than pleased to either update this article or put up another linked to it.
I trust your retirement is going as well as your distinguished and admirable career. All the best, Fred
I like the synopsis and technical details in your writing. The issue of sodium intake as part of a stone treatment program is one that I have been trying to understand for my situation. I am getting conflicting advice on whether reducing my sodium intake would have any beneficial effect for me. I have had intermittent recurring CaOx stones for a little more than 25 years. A recurrence interval of once every year to a year and a half; most of them pass with just a little discomfort, and only a few have been painful enough to go to the ER. My stone issues began a couple of years after ileostomy surgery to treat ulcerative colitis. The presence of stones has been the only diagnosed side effect from the removal of my colon. Because the primary function of the colon is to reabsorb water, salt, and other electrolytes, it seems to confound doctors trying to understand whether reducing sodium would help in my situation. Some say it will definitely make a difference, others have said it will only make a difference if my urinary calcium is high (which is not expected in ileostomies). My recent 24-hr urinalysis had a volume of 2700 mL, 84mg/24 hr of urinary calcium, 78mmol/24 hr of urinary sodium, and a urinary pH of 5.3.
Based on your discussion of how urinary sodium can lag behind salt intake by several days, the recommendation from one of your previous posts regarding taking multiple 24-hr tests, not relying on a single one for a complete diagnosis, and looking back at my fluid intake data before and after this test, I am not sure how representative these test results are. My diet remained relatively steady prior to the sampling, but for several months prior to this test sampling I averaged 48 oz. of fluid intake daily. Then two days prior to the test I increased my fluids to 58 oz., one day prior to 70 oz., then the day of the test to 102 oz. The doctor stated that I needed to produce a minimum volume, and not knowing how much intake was needed for the minimum volume, I maximized intake to make sure I would exceed it. So I am not sure if the substantial change in volume over a few days might influence the interpretation of the results. Since this testing was completed I have now been averaging 70 oz. per day, which is just below the rule of thumb of 1/2 oz. of liquids per body weight pounds per day for me (weighing 160 lbs).
I would be curious of your opinion in my non-typical stone case.
Hi Jeff, thanks for posting this important set of issues on the site. Removal of the colon causes major GI losses of sodium bicarbonate and water. Because the beginning of the colon – the cecum – is a major area for calcium absorption, and because of sodium depletion (your 24 hour urine sodium is 78 mmol/day) urine calcium is generally low as it is in your case. The low pH of 5.3 reflects the loss of bicarbonate. Urine oxalate is usually normal and urine citrate low. So calcium oxalate stones occur because of low urine volume which concentrates calcium and oxalate and raises supersaturations. The low pH can also lead to uric acid stones. Treatment is as you are doing – very high urine volumes and, if uric acid appears in stones, potassium or sodium citrate or bicarbonate to raise the pH. Your case is not untypical, it is just that this site is in evolution and we have not gotten around to stone disease from bowel diseases. Warm Regards, Fred Coe
Hi Fred,
Thanks for the article. And my condolences to Jeff, having a stone at least every year and a half must be difficult.
I am curious though, has anyone figured out or been able to explain the difference between people such as myself, with high calcium excretion and no stones, and people with incredibly low calcium excretions (excluding GI complications) that make stones? I wonder why it is there are so many like me that remarkably have no stones (recent CT scan proved this to me) and why there are so many men and women with more than safe calcium excretion but make stones often.
I know they’re making crystals, but why am I not, and why do they?
I appreciate you satisfying my curiosity Dr Coe!
Thanks,
John
Hi John, I suspect it is the myriads of inhibitors in urine. That is yet to be proved. Regards, Fred Coe
I was a chronic stone producer. I used to have great salt cravings and what I called ‘salt sensitivity’ because things tasted really salty to me. I stopped eating gluten and I no longer crave salt, or have that sensitivity. Have you studied the relationship between salt and sugars. particularly in the form of gluten?
Dear Nancy, one thing salt and sugar have in common is that both are probably bad for us in excess. Sugar and salt both raise urine calcium losses, and sugar excess is a prime cause of obesity. Regards, Fred Coe
I’m really enjoying several of your articles, Dr. Coe.
I’m curious about natriuresis of fasting — also in the context of ketogenic diets. Why do the kidneys dump sodium during a fast and carbohydrate restriction? Apparently insulin regulates sodium retention, and the kidneys cover ketone anions with sodium and perhaps other cations.
It seems very dangerous in terms of health effects. Almost like a maladaption in evolutionary terms.
I currently carrying a small kidney stone after several months of a mildly ketogenic diet, and that helped me find your wonderful site!
Hi Doug; Simply fasting without sodium intake will result in net sodium loss because of the time delay in kidney adaptation to sodium intake. Ketones are anions and those not reabsorbed will be lost with sodium. As volume depletion increases, and aldosterone levels rise, potassium is lost instead. Ketogenic diets will do this, too, and presumably could cause potassium depletion, but they are typically high in potassium being made up of meats. The stones you formed during your diet could be uric acid – acid urine – or be from inadvertent fluid depletion. Regards, Fred Coe
Thanks, Dr. Coe!
My stone hasn’t been recovered yet, but my urine was alkaline (8.0) when admitted to the ER, and I ate a lot of nuts on my diet, so I suspect CaOx. Both types appear to be not-uncommon in studies of patients on ketogenic diets, but you’re right that the risk of uric acid stones appears to be higher.
I also think you’re right about dehydration, but my urine was never dark, which is why I suspected it might have to do natriuresis. Lower blood volume, normal sodium concentration, but higher concentration of other solutes?
Hi Doug, A pH of 8, if correct, is very alkaline and is compatible with infection or more than simply a ketogenic diet. Regards, Fred Coe
I also am on the ketogenic diet and was diagnosed with kidney stones a few months ago. The keto diet stresses the fact that pink Himalayan salt has the minerals, potassium and electrolytes needed when doing keto, however, now being a stone former I’m told to lower my salt intake- so my question is, what the difference between regular table salt and pink health beneficial salts? And what about the recommended sole water? I’m stuck and I’m not sure what to do. Please help me figure out what to do. Also I was just prescribed potassium citrate to take daily now. Also I don’t eat processed foods- so no added salts hidden . I only consume veggies, meats, eggs, good fats, and water or lemonade. Also for sweeteners what’s a good ketogenic approved sweeter low in oxalate. I was using stevie but won’t be now. So mucheck confusion.
Hi Chelsy, I believe I answered a version of this/ Regards, Fred
If you got to the point sooner the info would be a lot more tastier. for us non- readers ?
I am trying, Joe. You know how professors are. Best, Fred Coe
Thank you so much for the article, it helped me understand the process very well. Clear and concise, I thought.
Dear Dr. Coe,
Does my sodium intake have any impact on urine pH? i.e. if I lower sodium in my diet, does urine pH go up or down? Or does it have no impact at all?
Thanks
Nihal
Hi Nihal, urine pH is not very responsive to sodium intake. Perhaps a very low sodium intake might lower it slightly, but I have no data on the subject. Regards, Fred Coe
What is the difference between pink Himalayan salt and regular table salt? I also am on the ketogenic diet and have been told to increase pink salt intake due to potassium and electrolytes loss. Then was diagnosed with stones. So what do I do? I don’t eat any processed foods with hidden salt or sugar. I only consume, veggies, meats, healthy fats and small amounts of berries. Water and lemonade, and heavy cream. For sweeteners I’m lost because it cannot have sugar and i was using stevia now it’s high in oxalate. I’m so confused on what to do. Can someone help me understand this? Please I just was prescribed potassium citrate. And then there was the sole water talk about why those on a ketogenic diet need it… so what to do,
Hi Chelsy, Here is my suggestion to you. The best course is a plan, and here is a good introduction. Let me know, Regards, Fred Coe
Thank you Dr.
Dr. Coe,
Pleased be advised that the lowsaltfoods.com site that you provide as a reference is no longer accessible.
Hi Milton, thank you very much. I asked Jill Harris to check the link and we will replace it. Best, Fred
Dr. Coe,
Thank you for the very in depth and detailed articles.
Could you please comment on the article by O’Donnell et al in NEJM, 14 Aug 2014:
https://www.nejm.org/doi/full/10.1056/NEJMoa1311889
Your comments might make an appropriate addendum to this article of yours on salt.
Please also note the differences between conventional diets and low carb/keto/carnivore and the effects of those on Na and K requirements. The latter nutrition styles result in much lower circulating insulin levels which seem to drive a significant increase in urinary Na loss by lowered signaling of the distal tubules by insulin for reuptake of Na. Also, what other effects are exerted by lowered insulin on the other various nephron structures?
It seems that on a ketogenic macronutrient diet, the electrolyte balance can become disturbed without adequate Na replacement, and this can lead to excessive urinary water loss and elevated risk for hyponatremia, both chronic, and acute exercise induced.
There are at least two other inquiries in this thread relating to ketogenic diet concerns, and I am very interested as well since I have been eating keto style for quite some time. This might be a good topic for another article, and I hope that you would consider.
Hi Dennis, The article is a large observational study of urine sodium estimates vs. mortality and showed – as other studies have – a J shaped curve with death rate rising at the high and low end. They did what they could to remove effects of reverse association – sick people eat less salt but die faster. On the other hand, in trials, no evidence exists for increased mortality from lowering sodium intake, and evidence for better cardiovascular outcomes is likewise lacking – too little time, I suspect. Maybe the trials and epidemiology should become part of an article, and thank you. As for ketone inducing diets, the ketones themselves obligate sodium and potassium losses because non adsorbed anions in the renal collecting ducts. I have not written about this, as ketogenic diets are not part of kidney stone prevention. But perhaps this topic could be folded into the proposed article. I appreciate your thoughtful prompt and if I write the article I will try to remember to acknowledge your suggestions. Thank you very much, warm regards, Fred
Dear Dr. Coe, I love your writing, such a great combination of humor, humility and profoundly good information! My question for you: I assume sodium is the main culprit in these salt discussions. Is there any downside for us stone formers in using Potassium Chloride as a salt substitute in our cooking?
Hi Doug, and thanks. It is fine if the taste works. Be sure your have no special diseases that might compromise potassium loss – chronic kidney disease, diabetes, certain drugs. If you are merely seasoning with KCl, I see no problem. Yes, it is the sodium that is the culprit. Regards, Fred Coe
Daughter has dysautonomia with POTS. Increased salt has certainly helped her control low blood pressure symptoms by increasing blood volume (and thereby increasing BP). No stones yet, but to reduce that risk, is there another cation that acts as sodium does for increasing blood volume but doesn’t run the risk of kidney stones?
Hi David, Sodium per se is not a cause of stones. People with genetic hypercalciuria have an abnormal sensitivity to sodium so their urine calcium increases with salt loads, but in your daughter’s case there is no reason to suspect that. No cation can substitute for sodium in her situation. Regards, Fred Coe
my wife getting kidney stones so often i think twice in a year.
please tell us what causing her kidney stones. she is eating normal food. please tell us food diet to avoid kidney stones. please mail your answer. we are in need of solution. please help
Hi Prashant, Something causes her stones, and prevention requires one know what that is. Here is my best article on how to evaluate for cause. I hope this helps. Random food advice will usually fail. Regards, Fred Coe
Hi Dr. Coe,
I too have benefited greatly from your published articles & opinions. I’ve had issues for 18mos w the same kidney stones, pain going from bad to worse & more often. My diet hasn’t changed, salt levels normal & cannot take the potassium chloride bc it was stated my stones are the most rare, & would make worse vs benefit from a 24 hr urine able to collect small fragments. I’ve tried everything to get these bilateral stones to pass bc being 4’11/90lbs low blood pressure this shouldn’t be difficult but they won’t. They are both 3mm & the left moves & when in lower pole is excruciating. What other options do you have that may help pass? I drink approx 90oz of H2O daily, added small amt of cranberry concentrate & take cranberry vitamins now due to their potency (from a reliable company I worked for made on site). I eat on a 7 hr fast daily & only for 10 yrs of having a partial colectomy. I’m open to anything bc the urologist will NOT remove the stone (due to the size) & non obstruction (bc it won’t pass) & will go to another including medical schools if necessary for most recent literature opinions (my family are the wrong drs but their suggestion I live outside NYC) Have Litholink sample to give & nephrologist stated even if a rare kidney disease it’s untreatable. Bun & creatinine are fine but the ratio is 27ish. Not jumping to conclusions but could my kidney be failing? They have also become smaller in size from 10.5 to 9 over the 18mos. Again, not sure it matters. I’m begging for how to get just the 1 out for relief, & w a healthy diet in place, but 20lbs of weight loss. Not GI related.
Thank you again,
BB
Hi Blair, You do not mention the kind of ‘rare’ kidney stone. The partial colectomy catches my eye, sometimes they cause odd stones – like ammonium urate etc. -Could you write back what the stones are made of? What was found in your 24 hour urine samples?? Regards, Fred Coe
2nd comment please disregard 1st clicked submit too early:
Hi Dr. Coe,
Thank you again for responding . As high level as possible: partial colectomy is going on 11 yrs. w same diet; have no issues to date with the procedure, elaborating first stone occurred 4 yrs prior, a 4mm & 3 mm first 2 has lithotripsy, stented for 10 days until the 2nd was taken care of. Neither were the typical Calcium stones where my diet impacted or needed to change.I was told they were “crystals”. Latest 24 hr revealed stones are struvite, brushite,& alkaline state urine which I’ve remedied w small ants or citrus, but I drink approx 80-100mL H2O daily. Current urologist REFUSES to do any procedure due to 1. Size of stone (either 2/ 3mmm stones or 2.5 depending on CT or sonogram report). His size chart begins @ 4mm. Again I’m tiny. 4’11 93 lbs & over the months have gone over all results to also see any changes. Salt/Sodium levels are normal = 126 ammonium =24 normal. The highs were Brushite @ 4.56 and Struvite @ 118.41. Started cranberry capsule (I personally know the vitamin comp bc of working there made on site & legit as some aren’t). & the excruciating pain has subsided. Not gone totally but I can breathe most days. Psychosomatic or not I’ll take that as a win. Thyroid cancer & hoshimoto’s thyroiditis (2nd autoimmune disease) left D25 Hydroxy at 4.6 add D2 5000 1x to everything. The partial colectomy never gave issues with malabsorption any weight issues so I gave what I could to ask what would make these “a possible” correlation everyone knows about yet do nothing about? And 1 last oddity is cholesterol was over 300 with triglycerides at 222. Genetics yes, unsure if another possible piece of a puzzle. That’s it for 1 offs
Lastly, asked how I can prevent these stones on my end; norm treatment =Cal Phosphate tabs but this is not a luxury to take advantage of for me. If you’re familiar with Litholink & having the last 24hr picked apart 1 last time. You’re the last dr able to be straightforward without judgement & I’m desperate. I obtained all radiology films written diagnostics results, urine results to ensure all ducks were in a row in the hopes someone hears me or even looks at the whole puzzle or may have hope for the missing piece. If you have questions which may be answered faster & more detailed a phone call please don’t hesitate because I’m literally exhausted @. 41 & want to enjoy life. My # is 972-987-9980 and free anytime. I’m hoping you have more questions!! Thank you again for the time.
Blair Bodrato
Blair_bodrato@yahoo.com
Hi Blair, It sounds like you want me to do a more detailed analysis of what is causing your stones. The only way I can do this is as a medical consultation that can be telemedicine for convenience. My secretary’s phone is 773 702 1475. I will copy your note to her and include her as an introduction. Regards, Fred Coe
Dear Dr. Coe, thank you for this web site!
As a background, I am 53 yo 250 lb male, I passed my first small stone 3 years ago, it was Ca-Oxalate and the second stone last month, it was removed by URS and it wasn’t collected for testing. 24h urine showed high Ca and they forgot to test Oxalate!
Most of this article on Salt is beyond my comprehension and I do have some questions.
Is sauna good or bad for stone formers? I lose 2-3 lbs in sweat there and I like to go 1-2/week. I am making sure to drink extra water on those days. If I will go on low sodium diet, am I risking losing too much Na through sweating?
The same question applies to heavy sweating during exercise?
I recall reading in Waterlogged book that the rate of sodium loss through sweating decreases during long endurance sport events. Does it make sense to you?
Thank you very much again.
Hi LK, There is no substitute for 24 hour urine testing. The net of sodium intake – sweat loss shows up in the 24 hour urine and that net is what drives urine calcium loss thence stones. I gather urine calcium is high, pity about oxalate!, I guess urine sodium was not measured. I suggest your physicians use a national vendor that does 24 hour kidney stone testing. Litholink is the very best of there and will give the calcium and oxalate and sodium and all the rest. If you collect under conditions that you want to live with, you will know about the net of sodium. Best, Fred Coe
Thank you Dr. Coe for the reply.
Any lab recommendations for 24h urine testing in Canada (Toronto)?
My 24h Ca was 8.07 mmol/d and Sodium 139 mmol, I am working on bringing the latter down. I self diagnosed IH 🙂
I will have to see how many 24h tests my physician is willing to order… Thank you again.
Hi LK, That urine calcium is indeed very high. Perhaps lower sodium (1500 mg) will reduce it, perhaps you might consider thiazide as well if stones are active. Regards, Fred Coe