WHAT IS POTASSIUM CITRATE
We have reached the point in the evolution of this site where the main stone risk factors are introduced and detailed, and the importance of citrate established. I have written about the price of potassium citrate because many patients and physicians have told me that it has risen steeply in recent months, and I would like to be of help.
A LOUD DISCLAIMER
In this post I will mention beverages and medications by name. Let me be clear: I have no financial relationships with the companies that produce or sell the products I write about here. Likewise neither I nor my colleagues at University of Chicago receive any support, financial or otherwise from these companies.
WHAT DOES CITRATE DO FOR PATIENTS?
It Can Reduce Formation of Uric Acid Stones
Some patients produce too acidic a urine which raises risk of uric acid stones, and they need supplemental alkali to make their urine less acidic. The use of potassium citrate to make urine less acidic will prevent uric acid stones in most patients who form them.
It Can Reduce Calcium Stone Formation in Patients with Low Urine Citrate
Some patients form calcium stones because they produce urine that is low in citrate, a valuable inhibitor of crystal formation. Most of the naturally occurring inhibitors in urine are complex molecules about which we can presently do nothing. But citrate is a small and easily measured molecule which we can prescribe and which will increase the urine citrate in at least some patients. Potassium citrate lowers urine calcium excretion. In so doing it reverses a key kidney stone risk factor. In trials potassium citrate reduced stone formation.
Potassium Citrate is Preferable to Sodium Citrate
I have a long list of sodium’s undesirable effects. It can raise blood pressure in large numbers of people, especially with age. It raises the amount of calcium lost in the urine, and that increase of calcium can raise supersaturation and promote calcium kidney stones. High sodium intake can reduce bone mineral retention. But, it may be that the sodium in sodium bicarbonate causes less of these problems than the sodium in sodium chloride – table salt. So I offer sodium bicarbonate as an alternative – with reservations.
Because sodium produces problems of its own, we tend to use potassium citrate as the preferred medication, and generations of stone patients have taken it. In several trials it has reduced new stone formation when given to patients whose urine is citrate deficient.
IS THERE A COST ISSUE?
I am not at all sure why the pricing of potassium citrate has become a topic I often hear about from patients, doctors, and just about everybody in the kidney stone world. Certainly the price must have increased, but I cannot find data on the web to prove the point. I also believe Medicare and perhaps other insurers have altered the status of this drug in their payment schedules. Perhaps some of you know more about the problem than I do and are willing to share what you know by way of a comment.
I did find on inspection of the Medicare lists of drug prices by insurance plan that some plans appear to include potassium citrate pills in their formularies at a preferred level and charge as little as $10 for what appears to be 90 pills. Others do not do this and publish higher prices, often as percentages of the retail cash price. Once again, I hope those of you with experiences in purchasing the drug will share what you know.
CAREFUL SHOPPING LOWERS PRICES
Listening to agitated, and worrisome stories about inflated prices for potassium citrate, I decided to try to be helpful. A Google search for prices of potassium citrate yielded a few promising shopping sites, and on study of the prices I found some much better than others. Note that in the following sections I present a lot of prices and arithmetic. Sometimes, when the message is very clear the results are rounded for simplicity. I give the basis for every calculation if you want absolute exact answers to the nearest penny. Likewise, because we are comparing prices, I have chosen 4 pills daily as my cost basis. The actual range can be from 2 to 6 pills or even more daily, so you will have to adjust costs to your own prescription.
SAM’S CLUB
GoodRx gives what I believe is the clearest list of prices. On their site, Sam’s Club was least expensive at $145 for 180 pills or $0.805 per pill. A typical 4 pills per day treatment option would therefore come to $290/quarter, which is still very pricey. The site gives a long list of other stores whose prices are even higher. Everyday health offers an approximate price for Cytra-K and Polycitra K of $50 – $99, but I could not be sure if this was for a month and likewise how much medication was in a dose.
CANADA
So far as I can tell, importing from Canada will not save you much money. I found Urocit K at $1.10 per tablet, which is higher than Sam’s Club. Another generic, K-Citra 10 was $0.79, which is about the same as Sam’s Club. Another less desirable canadian price was $0.52 per pill if you buy 90 pills, but it was for the 5 mEq size, 1/2 of the usual and therefore the corresponding price for 10 mEq would be $1.04/pill. Given that some costs must accrue for mailing, and there are issues with importing, I cannot see an advantage right now.
WHAT TO DO
Shop Well
Certainly web shopping is a good thing because in my modest and amateurish shopping efforts I found a tremendous range of prices. I am sure that many of you who read this post are far more skilled than I am at shopping for best prices. It is time for you to step forward and share your knowledge with all of us by posting a comment. Everyone will benefit and appreciate your contributions.
But even if you shop better than I did, retail pricing for this medication seems too high for most to afford. At even 4 pills a day, and at the best price I found ($290.00/quarter) we are over $1000.00 yearly for this one product. It seems to me that if your plan does not subsidize this medication, cost could be a serious issue.
Use Beverages
A useful publication reports the alkali content of commercial beverages. The ‘lemonade formula’ referred to on the graph is given as 1/2 cup ReaLemon© mixed with 7-1/2 cups of 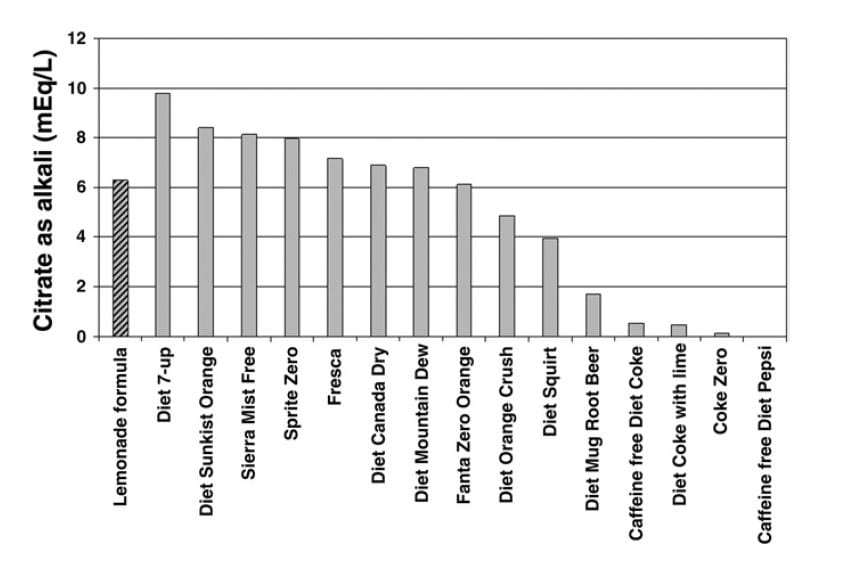 water and sweetened to taste with sugar or artificial sweetener. Diet 7-up was the winner with 10 mEq of citrate in a liter. A single Urocit K tablet contains 10 mEq of potassium citrate, as a comparison, so you would need 4 liters of the beverage daily to match 4 pills.
water and sweetened to taste with sugar or artificial sweetener. Diet 7-up was the winner with 10 mEq of citrate in a liter. A single Urocit K tablet contains 10 mEq of potassium citrate, as a comparison, so you would need 4 liters of the beverage daily to match 4 pills.
You Can Do Better
My colleague Dr. John Asplin has measured an additional group of products: Minute Maid Lemonade contains 10.3 mEq/liter of alkali, like Diet 7-up. Gatorade contains only 8.3 mEq/liter. But Crystal Light Lemonade contains 21.7 mEq of alkali, so it is the winner. Each liter substitutes for 2 potassium citrate pills, $1.60 a day, or $144 every 3 months.
We know About Classic Crystal Light
Crystal Light beverages include teas and other drinks. Our measurements refer to the classic or standard lemonade beverage. In what follows all of my remarks at bounded by that limitation. For example, I do not know if liters of the Crystal Light tea might contain excessive amounts of oxalate.
The Prices of Crystal Light
I did not research the price of Crystal Light Lemonade extensively, but Crystal Light Lemonade Pitcher Packs – 3-Pack – are $27.95 at Amazon. Each 3 pack provides 96 quarts of beverage. Each quart is about one liter (0.946 liters to be exact). The cost is therefore $27.95/96 or about $0.29 per 20 mEq (2 pills). This comes to $0.58 daily or $52 every three months. The Amazon site points out that prices might be lower at other stores. Please comment on the best prices you have found so everyone can benefit.
It is Not Just How Much Citrate is in the Beverage
You may have read, on a label or in a scientific paper, that some of the beverages I have listed contain quite a lot of citrate, yet we show them as inferior as an alkali. The reason has to do with the form of the citrate. If the drink is made up in a very acidic manner, much of the citrate is citric acid and will not produce alkali in the body when metabolized. It is only when the molecule is citrate itself, not the citric acid, that it can benefit you as an alkali. The graph and the additions by Dr. Asplin present the true alkali content.
Be Wary of Sugar
The beverages are mainly diet so they do not add to your caloric burden. If you sweeten them, or lace them with fruit juice, or add fruit juice or other flavorings to baking soda – see below, you will be adding calories to your diet and that may not be ideal.
But apart from weight gain, sugar has undesirable effects specific to kidney stone formers: It raises urine calcium losses. Even worse, as the article points out, urine flow rate falls as urine calcium increases, so supersaturation rises for two reasons.
What About Sodium Bicarbonate
It Has a Lot of Alkali for the Money
Baking Soda
According to Google, a teaspoon contains 4,500 mg of baking soda (sodium bicarbonate). Given the molecular weight of 84 mg/mEq (each molecule is one mEq of alkali) the teaspoon contains 53 mEq of sodium alkali. In principle, therefore, one can get alkali for nearly nothing by way of price. According to Dr. Asplin, who has – unbelievably – determined such matters, a teaspoon can contain up to 6,100 mg of baking soda depending on packing and whether the teaspoon is level or heaping.
To get 20 mEq of alkali from baking soda would require about 1/3 teaspoon. Given the variability of what a teaspoon holds, and the sheer problems of fractions of a teaspoon for every dose, I strongly recommend we abandon the remarkable cost savings from baking soda and use sodium bicarbonate tablets, which are very inexpensive and measure out the dose for you.
Sodium Bicarbonate Tablets
You can buy sodium bicarbonate tablets OTC and they are cheap. Concord, via Amazon, sells one hundred 650 mg tablets for $14.95 ($0.14 each). Rugby sells 1000 tablets of the same size for $25.77 ($0.026 each). Because each tablet contains only 7.7 mEq of alkali, it takes about 3 to match 2 K citrate pills (I realize 7.7 times 3 is 23.1 mEq but it approximates 20 mEq and the difference is not important). But that is only $0.075 for the three. So the price can come way down with this form of alkali.
It has a lot of Sodium, Too
But, alas, the 1/3 teaspoon, or the three 650 mg pills, deliver 20 mEq of sodium for each 20 mEq of alkali. The extra 20 mEq of sodium is 460 mg, about 20% of a full day’s sodium intake. For the 40 mEq (4 potassium citrate pills) we have used as a benchmark thus far, it is 40% of a full day’s sodium intake.
Whereas I am unconcerned to recommend beverages as replacements for potassium citrate pills, I have considerable reservation about sodium loads for reasons I have already mentioned and repeat here for emphasis. Excess sodium intake can raise blood pressure in those who are sensitive to salt. Although we have not as yet discussed urine calcium losses as a risk factor for stones, sodium loads will raise urine calcium, and are therefore not beneficial in that respect. If you are taking a diuretic to reduce urine calcium for stone prevention, sodium loads will reduce the efficacy of the treatment and promote losses of potassium. People with heart disease may develop worsening heart failure. Always ask your physician before using sodium bicarbonate as an alkali.
Even so, sodium bicarbonate is not sodium chloride – table salt. For physicians I have reviewed a few papers on the subject. If I sound ambivalent, I am. We may need a few more trials on this subject. In the mean time, all of my reservations hold sway. Use sodium bicarbonate sparingly.
How To Put It All Together
Compromise is the best policy, and I offer a general scheme which patients and physicians can use, if they wish, with their personal alterations. Be sure and check that your combinations provide the dosages your physician wants you to have.
Make a List of Equivalent Dosages
Each potassium citrate pill is 10 mEq; 2 are 20 mEq of alkali. Each liter of Crystal Light is just over 20 mEq of alkali. Each OTC 10 grain (650 mg) sodium bicarbonate tablet is 7.7 mEq of alkali so 3 make 23 mEq.
Make A Day’s Menu
Consider dividing the day’s alkali into 3 parts: Beverages; sodium bicarbonate; potassium citrate pills.
To Replace 2 Potassium Citrate Pills
If we only need 2 10 mEq potassium citrate pills (20 mEq), substitute 1 liter of Crystal Light (20 mEq). It is part of the day’s fluids, but also like a medication, so spread its use out over the day and, if possible, night.
To Replace 4 Potassium Citrate Pills
If we need 4 pills (40 mEq) consider 1 liter of Crystal Light and three sodium bicarbonate pills (20 mEq). The beverage and individual pills can be spread out through the day.
To Replace 6 Potassium Citrate Pills
If we need 6 pills (60 mEq), consider 2 liters of Crystal light (40 mEq) and three sodium bicarbonate pills (20 mEq) likewise spread out through the day. Reserve the potassium citrate pills for when you tire of the beverage or if the extra sodium is raising blood pressure or urine calcium.
Use Many Beverage Types But Keep the Dose of Alkali The Same
Crystal Light is convenient because of how much citrate it contains. But the chart shows many alternatives which can be used instead in larger volumes. Just remember to multiply so the total amount of alkali remains about the same. For example, you need 2 liters of Diet 7-Up to equal one liter of Crystal Light.
Be Inventive: Not All Days Need Be The Same
Mixing and matching is perfectly acceptable. Each day need not look like the one before so long as the correct amount of total alkali is used. The only drawback of a mix and match approach is confusion, so make lists and keep track. As a general rule, try to make the sodium component smaller than the beverage component. Keep the expensive potassium citrate pills as a convenience and source of variety. Obviously if sodium is contraindicated medically, and beverages are too tiresome as a source for all the alkali that is needed, potassium citrate pills can be used to replace sodium bicarbonate pills.
Not All Patients Need Potassium Citrate Or Any Other Alkali
This post is for those who have been told by their physicians to use alkali. Nothing I have written here should induce anyone to begin alkali unless their physician has prescribed or recommended it. Stone formation is complicated. Sometimes alkali can worsen stones, or even become a danger. Potassium can itself be dangerous if kidney function is below normal. Sodium loads are a problem for people with high blood pressure, heart disease, and other illnesses. Do not use sodium or potassium alkali or even high volumes of Crystal Light unless the physician who is treating your stones recommends you do so.
Stay Hopeful
Whatever caused the price rise, the changes in how insurers pay for this medication, or both, may be transitory. Millions of people have kidney stones in the US. Prices for 90 days of a standard treatment are so high that few can afford them without serious budgetary concerns. When so many people are affected, hopefully market or even political forces will countervail. In the meantime, between a few potassium citrate pills, a few liters of Crystal Light, and maybe some sodium bicarbonate, physicians can piece together an adequate regime of alkali for those patients who need it. Not every stone former does need alkali, of course.
ANOTHER AND FINAL DISCLAIMER
I have brought Crystal Light to your attention as an inexpensive substitute for some of the medicinal alkali your physicians may have prescribed. As in my initial ‘Loud Disclaimer’ I say here that I receive no financial or other benefits of any kind from the makers of this beverage, have not, in fact, ever tasted it, and do not currently plan to do so. My evidence for the value of Crystal Light comes from the work of Dr. John Asplin, and comparisons to the published work of Dr. Eisner and his colleagues.



My husband, Sam currently has a stint in and is on antibiotics before the doctor can blast an 18mm stone along with many smaller stones. His last bout was 7 years ago and those stones were calcium oxalate.
We are in search of an affordable source of potassium citrate; a mineral that should not require a prescription to obtain, but now apparently does. As we look, how many mg of Potassium Citrate is equal to 10 mEq?
Also, please evaluate the veterinary product below. 100 tablets cost $21.
http://www.allivet.com/p-5494-potassium-citrate-plus-cranberry-100-chewable-tablets.aspx?gclid=CMuTyKKk08YCFRCCaQodQF8Nqg
Active Ingredients (per chewable tablet):
Potassium Citrate (micro-encapsulated) 680.0 mg
Cranberry Extract 113.3 mg
Inactive Ingredients: Cellulose, Hydrolyzed Vegetable Protein, Iron Oxide, Liver Powder, Magnesium, Stearate, Silicone Dioxide, Stearic Acid, Sucrose and Whey.
How Potassium Citrate Plus Cranberry, 100 Chewable Tablets work?
Potassium citrate helps decrease the possibility of calcium oxalate stone formation and cranberry extract has been shown to enhance urinary tract health.
Cranberry Extract works by minimizing the bacterial colonization of the bladder mucosa.
Echinacea helps to support the pet’s bacterial resistance and boosts the animal’s immune system.
Oregon Grape Root contains the alkaloid berberine, known as an anti-bacterial and anti-inflammatory compound. Beneficial and effective anti-oxidant.
Sodium Ascorbate is beneficial in boosting the pet’s immune system to improve resistance (Vitamin C) to infection.
Hi Again, Amy, I now know the stones were calcium oxalate. Veterinary products are not approved for human use and I cannot recommend that your husband use such products. I have summarized the evidence that potassium citrate does indeed help prevent calcium oxalate stones, but as for the other materials you mention I believe no real evidence exists. That does not mean they do not, but the probability they will be helpful is low. Regards, Fred Coe
Dear Dr. Coe,
As I previously mentioned, I have been taking magnesium citrate in addition to potassium citrate for my calcium oxalate stones. I did this because several studies had used this combination successfully. Could you comment on the use of this combination compared with potassium only? What is better? Thanks, Alan
One study of potassium citrate and one of a combination of magnesium and potassium citrate both showed efficacy in reducing calcium stones in patients whose 24 hour urine citrate excretion was below normal. The potassium citrate should be as effective as the combination. The trial results apply to the patients with reduced urine citrate. A third trial in which citrate dose was adjusted to maintain a specific urine pH was negative: The drug did not work. The link is to an article which has all the numbers from the trials. Best, Fred
Dear Dr. Coe,
As I previously mentioned, I have been taking magnesium citrate in addition to potassium citrate for my calcium oxalate stones. I did this because several studies had used this combination successfully. Could you comment on the use of this combination compared with potassium only? What is better? Thanks, Alan
I through in the towel several years ago and have been ordering my Urocit-K form northwestpharmacy.com; a canadian company. The cost for (100) 10meq pills is $75. If you order generic it’s a little cheaper but since it’s coming from outside the U.S. I order the brand name.
Thanks, Sherry; perhaps other patients might want to know about this. Fred Coe
I through in the towel several years ago and have been ordering my Urocit-K form northwestpharmacy.com; a canadian company. The cost for (100) 10meq pills is $75. If you order generic it’s a little cheaper but since it’s coming from outside the U.S. I order the brand name.
Thanks, Sherry; perhaps other patients might want to know about this. Fred Coe
I have seen steep price increases for Pot Citrate ER 100’s 10MEQ since 2014. I get 4 tabs/90 day supply (360 tabs) and these have been the prices through Express Scripts (I’m required to do the mailorder pharmacy for maintenance drugs): January 2014: $315.84; June 2014: $361.71; October 2014: $414.11; August 2015: $542.72. I only take 2 tabs a day, so my 90 day supply lasts 180 days. But with my high deductible medical plan, I’m 100% out of pocket and $542.72 is rather pricey to me!
Hi Jackie, Oh My! This is a widespread problem. The most imaginative ideas are in the many comments for this article, some of which seem practical to me. One person buys food grade potassium citrate and weighs out what he needs. Others have found sources. I suggest you read their ideas and try which ones seem to fit. Here is an area where the ideas of those I have written for far outshine my own. Certainly the small number of manufacturers who produce this product have chosen to take advantage of things. They are not kindly to those who buy their products and will eventually suffer the consequences. Good hunting, Fred Coe
The price of Potassium Citrate was too much for me, if I remember right, the last script was for 2)600mg twice a day. I found potassium bicarbonate online for about $8/lb and it has kept my uric acid stones in check taking 1/4tsp twice a day. I haven’t heard much about potassium bicarbonate only that it’s better than sodium bicarb. Any thoughts?
Hi Karl, How do you measure out a proper dose??? Regards, Fred Coe
The price of Potassium Citrate was too much for me, if I remember right, the last script was for 2)600mg twice a day. I found potassium bicarbonate online for about $8/lb and it has kept my uric acid stones in check taking 1/4tsp twice a day. I haven’t heard much about potassium bicarbonate only that it’s better than sodium bicarb. Any thoughts?
Hi Karl, How do you measure out a proper dose??? Regards, Fred Coe
I am in the same boat as the others here. I saw this article some time ago and thought it might be an option. I take 3 tabs daily and just 4 years ago I paid $20.00 for a 90 day supply. When the insurance for the big companies started to change a few years back it left me paying full price until my deductible was met. In 2015 for a 90 day supply of Urocit K went to $657.00. Not something I can afford.
I spoke with my urologist and she said she was fine that I try alternate in form of a liquid drink. I am getting started immediately.
Hi Alan, Lots alternatives have been proposed by other patients, so I would read them, too. I have commented on all of them, and some seem really excellent. Regards, Fred Coe
I am in the same boat as the others here. I saw this article some time ago and thought it might be an option. I take 3 tabs daily and just 4 years ago I paid $20.00 for a 90 day supply. When the insurance for the big companies started to change a few years back it left me paying full price until my deductible was met. In 2015 for a 90 day supply of Urocit K went to $657.00. Not something I can afford.
I spoke with my urologist and she said she was fine that I try alternate in form of a liquid drink. I am getting started immediately.
Hi Alan, Lots alternatives have been proposed by other patients, so I would read them, too. I have commented on all of them, and some seem really excellent. Regards, Fred Coe
A kilogram, 2.2 lbs, is $30 on Amazon.
I presume this is food grade potassium citrate. Please confirm. Fred Coe
A kilogram, 2.2 lbs, is $30 on Amazon.
I presume this is food grade potassium citrate. Please confirm. Fred Coe
I have been taking Urocite-K 10meq 3 times per day for over 2 years. No change in urine PH, so the doctor raised the prescription to 6x per day. Still no change in urine PH. I discovered a few weeks ago those pills were passing right threw me!!
I changed to Crystal Potassium Citrate and now my PH is now normal. Costs went from 0.84 per dose to 0.11 dose..
Hi Jim, often what you see passing through is just the wax matrix from which the active material has been eluted. But in your case it may be some fault in the release of the alkali. Could you tell if the urine ammonia – on the usual 24 hour urine panel – fell with the urocit K? That is the best indicator of effectiveness. Regards, Fred Coe
I have been taking Urocite-K 10meq 3 times per day for over 2 years. No change in urine PH, so the doctor raised the prescription to 6x per day. Still no change in urine PH. I discovered a few weeks ago those pills were passing right threw me!!
I changed to Crystal Potassium Citrate and now my PH is now normal. Costs went from 0.84 per dose to 0.11 dose..
Hi Jim, often what you see passing through is just the wax matrix from which the active material has been eluted. But in your case it may be some fault in the release of the alkali. Could you tell if the urine ammonia – on the usual 24 hour urine panel – fell with the urocit K? That is the best indicator of effectiveness. Regards, Fred Coe
Is there a difference between the Crystal Light Pure Lemonade (made with Truvia) and Crystal Light Lemonade Sugar Free with regards to the amount of citrate? I noticed that Pure does not list potassium citrate but does have sodium citrate, but the normal sugar free kind has both. I am low on citrate, so I was prescribed 15mg potassium citrate tablets once per day. After reading this article, I am going to try substituting Crystal Light, and would prefer the Pure if possible. However, I want to make sure that I am getting what I believe I am (21.7 mEq per liter as stated in the above article). Any help you could provide would be greatly appreciated.
Is there a difference between the Crystal Light Pure Lemonade (made with Truvia) and Crystal Light Lemonade Sugar Free with regards to the amount of citrate? I noticed that Pure does not list potassium citrate but does have sodium citrate, but the normal sugar free kind has both. I am low on citrate, so I was prescribed 15mg potassium citrate tablets once per day. After reading this article, I am going to try substituting Crystal Light, and would prefer the Pure if possible. However, I want to make sure that I am getting what I believe I am (21.7 mEq per liter as stated in the above article). Any help you could provide would be greatly appreciated.
Dr, Coe Thank you for your great info. I have had stones for many years and am currently trying cytra-k oral solution very very costly to try to dissolve my current stone condition. Would crystal light work as well?
Anthony
Hi Anthony, If potassium citrate is indeed the best treatment for your stones, crystal light will provide 20 mEq per liter as noted in the article, provided the manufacturer has not changed the preparation. Of the stone types, only uric acid stones will dissolve with such treatment. Calcium oxalate stones will not dissolve, but under the right circumstances citrate will reduce their recurrence. Regards, Fred Coe
I will continue taking Cytra-k solution at $89 per bottle 4 times a day a bottle a week, as directed, and not paid for by insurance, until my next Doctors visit, and hoping my stones have dissolved by then. My question is I guess another ct scan is the only way to know if this is so?
Anthony
Hi Anthony, No stones dissolve except uric acid and cystine. Calcium stones do not dissolve. The citrate can reduce new calcium stone formation. I would not expect you will find stones disappearing. IF they do, they probably are one of the two I mentioned. Regards, Fred Coe
Dr. Coe Thanks again for the info I have had both uric and calcium, was in the hospital last summer and stone was uric, I am praying the stones are uric and the cytra -k works. The pain I was having seems to have subsided.
Dr, Coe Thank you for your great info. I have had stones for many years and am currently trying cytra-k oral solution very very costly to try to dissolve my current stone condition. Would crystal light work as well?
Anthony
Hi Anthony, If potassium citrate is indeed the best treatment for your stones, crystal light will provide 20 mEq per liter as noted in the article, provided the manufacturer has not changed the preparation. Of the stone types, only uric acid stones will dissolve with such treatment. Calcium oxalate stones will not dissolve, but under the right circumstances citrate will reduce their recurrence. Regards, Fred Coe
I will continue taking Cytra-k solution at $89 per bottle 4 times a day a bottle a week, as directed, and not paid for by insurance, until my next Doctors visit, and hoping my stones have dissolved by then. My question is I guess another ct scan is the only way to know if this is so?
Anthony
Hi Anthony, No stones dissolve except uric acid and cystine. Calcium stones do not dissolve. The citrate can reduce new calcium stone formation. I would not expect you will find stones disappearing. IF they do, they probably are one of the two I mentioned. Regards, Fred Coe
I have had one calcium kidney stone. I had this last year at age 65. My uric acid count is 5 and doctor prescribed potassium citrate. The cost was $135 for 30 pills…a month supply. I am not taking them due to the cost. Potassium citrate was $131 for a month supply. There is OTC potassium citrate (99 mg / pill) but I would have to take 10 pills a day. The OTC has 180 pills for around $10.00. I have decided to drink water with lemon juice concentrate and 2-4 OTC pills. Time will tell!
Hi Kathy, The problem of cost for this simple compound has vexed everyone and a few of our commenters have offered ingenious workarounds. I cannot comment on the wisdom of your choice, but given only one stone I would hope that you maintain a high urine volume by staying well hydrated. The lemon juice is of dubious benefit, but Crystal Light does have considerable potassium citrate in it and one liter will provide 20 mEq (equivalent to two full size prescription pills). The level of blood uric acid – I am guessing the ‘5’ is that level has no bearing on calcium kidney stones. At the very least be sure and get proper 24 hour urine testing to identify your key risk factors. First stones at 65 are uncommon but not rare. Being uncommon it is always best to know why. Regards, Fred Coe
Would it be possible for you to share how you calculate the citrate in the Crystal Lite ? I want to measure how much I’m getting from other sources and I think it would be instructive to see how this calculation is done. TIA.
Hi Peter, There is no secret. My colleague Dr John Asplin measured total citrate conventionally along with pH and calculated the molar concentration of citrate – unprotonated citric acid. If you are a lab person, I can put you in touch with John who would be pleased to share technical details of handling the food product. The key is to allow for pH: Citric acid will not help in raising urine citrate as it does not consume a proton when metabolized. Best, Fred Coe
Would it be possible for you to share how you calculate the citrate in the Crystal Lite ? I want to measure how much I’m getting from other sources and I think it would be instructive to see how this calculation is done. TIA.
Hi Peter, There is no secret. My colleague Dr John Asplin measured total citrate conventionally along with pH and calculated the molar concentration of citrate – unprotonated citric acid. If you are a lab person, I can put you in touch with John who would be pleased to share technical details of handling the food product. The key is to allow for pH: Citric acid will not help in raising urine citrate as it does not consume a proton when metabolized. Best, Fred Coe
What about drinking limited amounts of alkali water (ph 9.5)? There is no sodium. Would this help?
Hi James, It is hard to predict what such water will do. Mostly water has so low a buffering capacity that whatever its pH it imposes a very slight alkali load on the body and is not likely to alter urine pH or urine citrate. So I think the result will be that of drinking water. If the water had a greater alkali concentration that I have encountered before, and you used a lot of it, it could raise the urine pH just like potassium citrate does. If your stones were calcium phosphate this might make stone formation worse. So the answer is as I said: Mostly nothing will happen apart from the benefits of water. Regards, Fred Coe
What about drinking limited amounts of alkali water (ph 9.5)? There is no sodium. Would this help?
Hi James, It is hard to predict what such water will do. Mostly water has so low a buffering capacity that whatever its pH it imposes a very slight alkali load on the body and is not likely to alter urine pH or urine citrate. So I think the result will be that of drinking water. If the water had a greater alkali concentration that I have encountered before, and you used a lot of it, it could raise the urine pH just like potassium citrate does. If your stones were calcium phosphate this might make stone formation worse. So the answer is as I said: Mostly nothing will happen apart from the benefits of water. Regards, Fred Coe
Maybe this is addressed in the older comments.. sorry if it is a repeat question. I can buy pharmaceutical grade Potassium Citrate for about $15 / 500g. I have a .01 gram Ohas scale, so I can get a consistent weight. Is there any reason I can not just get my citrate this way? I ‘m on a 4 x 10meq pills per day. Would you have a recommendation of how to take the pure form? Instructions were posted on one site that said mix with 4 oz water/juice and drink slowly over a 5 – 10 min period. Take within 30 min of a meal and do not lie down for 30 min…
Thank you – Tom
Hi Tom, yes this has been on the site but why not again. A 10 mEq tablet – standard dose – of potassium citrate is 1080 mg of the salt so you need 1.080 grams. Two pills worth is an easy weigh for your scale. I would indeed dissolve the stuff as the pills are slow release and I would drink the potion with meals. My taste in volume would he higher – about 8 ounces of water or juice and drink it over 10 to 20 minutes. Be sure your physician knows and that your kidney function is adequate with respect to potassium because you are receiving the material without any slow release provision. Ultimately your physician needs to be in the loop and responsible. Regards, Fred
I think it might help to explain what mEq means to your readers. How was it determined that there are 10 mEq in 1080 mg of the potassium citrate salt?
If one tablet is 10 mEq then it contains 391 mg elemental potassium, which is about 12% RDA.
Knowing how to do this conversion would be helpful in determining what supplier is giving you the best bang for your buck and I would think for determining best dosage based on kidney function status, pregnancy status, cardiac arrhythmia risk etc.
Potassium citrate molecular weight = 306.4 g/mol
Potassium RDA = 4700 mg
Potassium atomic weight = 39.1
Reference: http://www.pkdiet.com/pages/alkalizers/potassiumcitratefaq.htm
Hi Jason, Usually when concerned with kidney function, pregnancy and arrhythmia mEq/liter is all one uses. So conversion to mh is not an issue. The 1080 mg contains 1080/306.4 = 3.52 millimoles of potassium citrate. Citrate has more than one valence – open charged sites binding potassium, which depends upon the pH at which it is made – it is a weak acid. In the product, the valence needed to give 10 milliequivalents is 10/3.52 = 2.84. To make all three valence sites (oxygen atoms in the structure) available to bind potassium, which means removing all protons from all three sites, would require a very high pH in production. Because most protons have been removed, and it is metabolized as citric acid – all three sites occupied with protons, when this product is metabolized in the liver to bicarbonate – in the Krebs cycle – you get 2.84 mEq of base – bicarbonate – per millimole of the citrate. If it were fully protonated and just citric acid you would get no bicarbonate and it would not be an alkali agent. Regards, Fred Coe
Where you said “protons” in your explanation I believe you meant “electrons.” Atoms have electrons in their valence orbitals, while the protons are contained in their nuclei. Chemical reactions, the binding of atoms into molecules, involve the exchange of electrons, not protons. The “exchange” of protons would be a nuclear reaction, usually involving radioactive decay, nuclear fission, or particle accelerators. Additionally, if you do indeed remove one or more protons from an atom it becomes a completely different element altogether.
By the way, while it was an interesting and informative reply, you never actually answered the basic request to explain just what an “mEq” is; i.e. what is the definition of an mEq. Thanks. 🙂
Hi Dave, Thanks for your comment. I meant protons – which non covalently bind with anion sites of weak acids like uric acid, phosphoric acid, or oxalic acid. I am not speaking of covalent binding but of acid base chemistry. I had thought I did mEq but I certainly will look and do it if I did not. I think I may need to edit the article to make myself clearer and therefore I appreciate your comment a lot! Thanks, Fred
Dr. Fredric Coe,
I was asked to take 10 mEq tablet or Potassium Citrate (K-Citra brand). I choked on the pill when I tried to swallow it. I thought about breaking/cutting it in half but the bottle clearly states to not “break” the pill.
Do they come in smaller sizes? If there is a smaller dosage size, could I take two? Would it have the same efficacy?
Thank-you,
Hi Robert, They do come in a 5 mEq size – 1/2 the dose. Your physician can prescribe them and they are smaller. You will need twice as many and price wise this could be a problem. I have had one patient warn me that these smaller pills are coated and might not deliver the medication well. I have no way to know if this is true. The manufacturer has not indicated a problem with the smaller pill. All the best, Fred Coe
Dr. Fredric Coe,
I was asked to take 10 mEq tablet or Potassium Citrate (K-Citra brand). I choked on the pill when I tried to swallow it. I thought about breaking/cutting it in half but the bottle clearly states to not “break” the pill.
Do they come in smaller sizes? If there is a smaller dosage size, could I take two? Would it have the same efficacy?
Thank-you,
Hi Robert, They do come in a 5 mEq size – 1/2 the dose. Your physician can prescribe them and they are smaller. You will need twice as many and price wise this could be a problem. I have had one patient warn me that these smaller pills are coated and might not deliver the medication well. I have no way to know if this is true. The manufacturer has not indicated a problem with the smaller pill. All the best, Fred Coe
I am a stone former (calcium oxalate) of decades’ standing. Current urologist Rxs K-citrate, and even w/ insurance coverage it is way too expensive (why so much for a simple salt?). What I have gleaned from reading here is the marvelous complexity of the kidney and its functions and the interrelated metabolic pathways, but cannot follow the evidence and logic well enough to keep from asking this Q: why not use Ca-citrate, as it is much cheaper than the K-salt, and available OTC?
Hi Robert, It is the calcium. In order to get 40 mmol of potassium you would need that much calcium, and it is a lot of calcium – 40 mg/mmol or 1,600 mg of calcium a day. That is too much altogether. The comments from others give a lot of workarounds, however, some of them rather good. Regards, Fred Coe
I am a stone former (calcium oxalate) of decades’ standing. Current urologist Rxs K-citrate, and even w/ insurance coverage it is way too expensive (why so much for a simple salt?). What I have gleaned from reading here is the marvelous complexity of the kidney and its functions and the interrelated metabolic pathways, but cannot follow the evidence and logic well enough to keep from asking this Q: why not use Ca-citrate, as it is much cheaper than the K-salt, and available OTC?
Hi Robert, It is the calcium. In order to get 40 mmol of potassium you would need that much calcium, and it is a lot of calcium – 40 mg/mmol or 1,600 mg of calcium a day. That is too much altogether. The comments from others give a lot of workarounds, however, some of them rather good. Regards, Fred Coe
OK; thanks. i knew I was missing something simple. Found the same Crystal Light pouches at local Sam’s Club tonight = $6/pouch of 16 packets, each of which makes 2qts. So 3 such pouches = same as the Amazon offering, at $18 instead of $26. And I can quit making lemon Kool-Aid daily, w/ Sweet-n-Low…..triple-whammy savings for me…..thanks for the research!
Hi Robert, I am glad you found such a good price. Others can do the same! Best, Fred Coe
Is it not possible to purchase bulk Potassium Citrate and Citric Acid and duplicate the quantities present in Cytra-K Crystals?
Hi Dennis, people have purchased food grade potassium citrate and measured out the correct doses. Check out the comments to this article and you will find it. Chemical grade is not appropriate as it is not approved as a human food. Fred Coe
Is it not possible to purchase bulk Potassium Citrate and Citric Acid and duplicate the quantities present in Cytra-K Crystals?
Hi Dennis, people have purchased food grade potassium citrate and measured out the correct doses. Check out the comments to this article and you will find it. Chemical grade is not appropriate as it is not approved as a human food. Fred Coe
Dr. Coe,
It seems to me that the price increase of the Potassium Citrate might coincide with the inclusion of the timed release
technology. What was the typical pill size and accompanying dosing instructions before these larger timed release pills
became available?
Hi Michael, I was not aware of this correlation, as the Urocit K tabs have usually been slow release to my knowledge. Fred Coe
Dr. Coe,
It seems to me that the price increase of the Potassium Citrate might coincide with the inclusion of the timed release
technology. What was the typical pill size and accompanying dosing instructions before these larger timed release pills
became available?
Not being in North America, I am a little confused. I buy alkasolve (300 mg KCitrate and 300 mg NaCitrate per tablet) 180 tablets for 16$ (actually about half of that from my HMO) which is about .09 dollars/tablet. The recommended dosage is 4 tablets/two times/day. (4 tablets is a little hard to sqallow but 3 tablets is no problem) That doesn’t even come close the prices you r quoting.
Hi Laurence, the 300 mg size requires 3 -4 tabs for a standard 10 mEq dose – approximately so for a day the minimum would be 6 -8 2 times a day. That is not unreasonable. In the US we do not have the 300 mg size available, so the OTC pills, being a low smaller, would be unworkable. You are fortunate in not sharing the price inflation here, which is rather recent and a combination of increased MFR prices and a decision by insurance carriers to reduce payment for the drug. Regards, Fred Coe
Not being in North America, I am a little confused. I buy alkasolve (300 mg KCitrate and 300 mg NaCitrate per tablet) 180 tablets for 16$ (actually about half of that from my HMO) which is about .09 dollars/tablet. The recommended dosage is 4 tablets/two times/day. (4 tablets is a little hard to sqallow but 3 tablets is no problem) That doesn’t even come close the prices you r quoting.
Hi Laurence, the 300 mg size requires 3 -4 tabs for a standard 10 mEq dose – approximately so for a day the minimum would be 6 -8 2 times a day. That is not unreasonable. In the US we do not have the 300 mg size available, so the OTC pills, being a low smaller, would be unworkable. You are fortunate in not sharing the price inflation here, which is rather recent and a combination of increased MFR prices and a decision by insurance carriers to reduce payment for the drug. Regards, Fred Coe
I have been prescribed potassium citrate pills for stones associated with an incomplete distal RTA. However, I also have gastroparesis and have not been able to tolerate the potassium citrate – it makes my gastroparesis worse. Are there some options that I may be more likely to tolerate?
Hi Susan, I suspect the finding in your case is a low urine citrate level with perhaps a more alkaline urine pH than normal and calcium phosphate stones – that is the usual basis for this diagnosis. If you cannot tolerate the potassium citrate, ask your physician for a work around. For example, perhaps your urine calcium is higher than 200 mg daily and can be lowered with reduced salt intake and possibly a thiazide diuretic; perhaps your urine oxalate is high and your stones are calcium oxalate. Gastroparesis is often from diabetes; what is yours from? In other words, your problem is complex and perhaps this one pill is not the ideal. Another alternative is a liquid alkali form: SImple crystal light lemonade has 20 mEq of potassium citrate in a liter, and that might serve instead of the pills. There are liquid medicinal forms of potassium citrate. Regards, Fred Coe
Thanks very much for your reply. The etiology of my gastroparesis is uncertain, although I do have a diagnosis of lupus with possible Sjogrens – currently the thought is that that is likely the explanation for the incomplete distal RTA and the gastroparesis. The next thing we are going to try is potassium bicarbonate citrate, which will dissolve in liquid, and see how that goes. Thanks again.
Dear Susan, It should work. Incidentally, the RTA of Sjogren syndrome is often complete indeed. Be sure the urine calcium, if high, falls with the alkali treatment. If it does not, then you need additional measures to lower it. High urine calcium from RTA does not always fall when the RTA is treated. Regards, Fred Coe
I am currently facing the loss of insurance coverage for potassium citrate. My prescribe does is 3000mg three times daily. The best cash price is Walgreens at $100 per month–this is for potassium citrate-citric acid crystals, delivering the above potassium citrate plus 1000mg citric acid. A bottled/mixed liquid version is twice that price. I suppose I could measure 3000mg doses of pot citrate bulk and dissolve in 8-12 oz of some beverage. That would be the only practical option for me. I would have to drink 4 plus liters of Crystal Light which is far too much. By the way, the piil form never worked for me, never raised the ph. The above dosage raises my urine ph from 5.0 to 6.5. My low ph is from Type II diabetes. My urologist says uric acid stones will not form above 5.5.
Hi Michael, Let me move backward in your comment. It is true that at a pH above 6 uric acid stones will not form; 5.5 is right on the borderline. Your dose of potassium citrate is 30 mEq 3 times daily, or 90 mEq, very high, but in diabetes I do sometimes see such requirements. The Walgreen preparation seems alright – why not use it. Perhaps some Crystal Light can be added to fill in for some of the crystals. Being diabetic, potassium handling might be askew so have your physician check serum potassium from time to time. The weighing out of bulk potassium citrate worries me because most people do not have accurate enough balances and potassium is not entirely safe when in excess. Regards, Fred Coe
I have been prescribed potassium citrate pills for stones associated with an incomplete distal RTA. However, I also have gastroparesis and have not been able to tolerate the potassium citrate – it makes my gastroparesis worse. Are there some options that I may be more likely to tolerate?
Hi Susan, I suspect the finding in your case is a low urine citrate level with perhaps a more alkaline urine pH than normal and calcium phosphate stones – that is the usual basis for this diagnosis. If you cannot tolerate the potassium citrate, ask your physician for a work around. For example, perhaps your urine calcium is higher than 200 mg daily and can be lowered with reduced salt intake and possibly a thiazide diuretic; perhaps your urine oxalate is high and your stones are calcium oxalate. Gastroparesis is often from diabetes; what is yours from? In other words, your problem is complex and perhaps this one pill is not the ideal. Another alternative is a liquid alkali form: SImple crystal light lemonade has 20 mEq of potassium citrate in a liter, and that might serve instead of the pills. There are liquid medicinal forms of potassium citrate. Regards, Fred Coe
Thanks very much for your reply. The etiology of my gastroparesis is uncertain, although I do have a diagnosis of lupus with possible Sjogrens – currently the thought is that that is likely the explanation for the incomplete distal RTA and the gastroparesis. The next thing we are going to try is potassium bicarbonate citrate, which will dissolve in liquid, and see how that goes. Thanks again.
Dear Susan, It should work. Incidentally, the RTA of Sjogren syndrome is often complete indeed. Be sure the urine calcium, if high, falls with the alkali treatment. If it does not, then you need additional measures to lower it. High urine calcium from RTA does not always fall when the RTA is treated. Regards, Fred Coe
I am currently facing the loss of insurance coverage for potassium citrate. My prescribe does is 3000mg three times daily. The best cash price is Walgreens at $100 per month–this is for potassium citrate-citric acid crystals, delivering the above potassium citrate plus 1000mg citric acid. A bottled/mixed liquid version is twice that price. I suppose I could measure 3000mg doses of pot citrate bulk and dissolve in 8-12 oz of some beverage. That would be the only practical option for me. I would have to drink 4 plus liters of Crystal Light which is far too much. By the way, the piil form never worked for me, never raised the ph. The above dosage raises my urine ph from 5.0 to 6.5. My low ph is from Type II diabetes. My urologist says uric acid stones will not form above 5.5.
Hi Michael, Let me move backward in your comment. It is true that at a pH above 6 uric acid stones will not form; 5.5 is right on the borderline. Your dose of potassium citrate is 30 mEq 3 times daily, or 90 mEq, very high, but in diabetes I do sometimes see such requirements. The Walgreen preparation seems alright – why not use it. Perhaps some Crystal Light can be added to fill in for some of the crystals. Being diabetic, potassium handling might be askew so have your physician check serum potassium from time to time. The weighing out of bulk potassium citrate worries me because most people do not have accurate enough balances and potassium is not entirely safe when in excess. Regards, Fred Coe
For people who want to just take pills or capsules, there are capsule filling “machines” avail usually on Amazon for $15-$20 or so (not currently, check Swanson) and then a bag of 1000 empty “00” or “0” size capsules will run just over $10-$12 dlv. A scale to weigh the potassium citrate powder will cost abt $10-$15 on Amazon (check the ratings) and finally the powder will cost $30/kg from Bulk Supplements of Pure Bulk etc. For $50 out of pocket just one time plus $30 for the 1 kg powder, you will have potassium citrate for years.
also, my local Dollar Tree has 32 ounce bottles of lemon juice from concentrate for $1.
dont let the health “care” industry rip you off.
Wow, Joe, this is the US enterprize spirit in full bloom. But I would not count on the lemon juice to raise urine pH unless you check out how much is needed. Thanks, Fred Coe
Yes, you’re prob right on the lemon juice but i thought someone else mentioned it as a source of citrate.
Also, i hope that you might consider editing the article to recommend that people purchase the bulk powder. If the pills really do cost around $144 every 3 months then one cannot in good conscience just say “shop around for the lowest price of the pills”
The supplement industry will put these clowns out of business.
Hi Joe, let me respond to both of your comments. Bulk potassium citrate is certainly available. The problem is to weigh out the right amount per dose. Potassium salts are dangerous if not given in the right dosages, and many people may not be as equipped as you are to undertake the needed measurements. For that reason I cannot medically endorse bulk products and home weighing out of doses. One of the many commentators on this article has identified a proper balance scale and he seems able to use it. I imagine you are the same. But I caution you and everyone that weighting out 1080 mg is not easy and mistakes can be dangerous and even fatal. Another issue is suitability of a particular bulk product for human use. I imagine the products you are buying are indeed suitable, but not everyone will know as much as you do about making sure. Regards, Fred Coe
btw, for those concerned about Sodium Bicarb i also see Potassium Bicarbonate avail in bulk for $6.50/lb in 5 lb lots (USP grade) and there is also Magnesum Citrate avail if its only the citrate portion thats needed. Good quality Arm + Hammer baking soda is only $1/lb or less. looks like the manufacturer of the pills is charging 80 cents per gram for the potassium citrate while the Amazon bulk sellers charge abt 3 cents per gram. I see one website that sells it for $4.50/lb plus ship in 100 lb lots (USP grade) – 1 cent per gram so potassium citrate is very cheap. no reason for 8000% markup
Well you didn’t try too hard to find out what the cost of this drug is. All it takes is to contact some local pharmacies. I was shocked. This is a very simple formulary. But, I found that at 3 local pharmacies this was going to cost me $80 a week. This appears to be another drug made by only a few companies which jacked up the price “because they could”.
Hi Dennis, I did work pretty hard at this and came up with high prices. The purpose of the article was to find low ones, or ways to lower the costs. You are right; prices are indeed high, the drug is made by only a few companies, and they raised the price because they could. Many comments offer workarounds, as does the article. Regards, Fred Coe
Well you didn’t try too hard to find out what the cost of this drug is. All it takes is to contact some local pharmacies. I was shocked. This is a very simple formulary. But, I found that at 3 local pharmacies this was going to cost me $80 a week. This appears to be another drug made by only a few companies which jacked up the price “because they could”.
Hi Dennis, I did work pretty hard at this and came up with high prices. The purpose of the article was to find low ones, or ways to lower the costs. You are right; prices are indeed high, the drug is made by only a few companies, and they raised the price because they could. Many comments offer workarounds, as does the article. Regards, Fred Coe
My urologist told me potassium supplements from the store will not help as the stomach acid eats it up before it can be absorbed. I’ve been prescribed potassium citrate ER as it is coated with wax to prevent it from being dissolved before it can be absorbed. Sound right to you?
Potassium citrate is potassium citrate and so long as it is in the right dose and approved for human consumption will do what it is intended to do. The coatings delay absorption, but citrate needs to be metabolized so its action is not immediate. Given the high pricing if you can use cheaper materials – with the two provisos I have just mentioned, I can see no reason why not. Fred Coe
My urologist told me potassium supplements from the store will not help as the stomach acid eats it up before it can be absorbed. I’ve been prescribed potassium citrate ER as it is coated with wax to prevent it from being dissolved before it can be absorbed. Sound right to you?
I was just prescribed 10 meq of potassium citrate it was $50 for a months supply. ( two pills a day)
Im suppose to take 2 pills a day..Amazon has it in a powder form in 17.6 oz for $20.
its called Pure potassium citrate powder what would the dose be? Its made by Bulk Supplements
thanks
One other thing,,I take 300mg of Allupurinal for gout, Im wondering if the potassium citrate could replace the gout med, I also take Hydrochlorothiazide 25 mg and 100mg of Losartan Potassium for Blood Psi..any conflicts??
Thanks again
Hi Again Wally, Allopurinol will lower serum uric acid and is used for treatment of gout. Potassium citrate cannot substitute for allopurinol in treating gout. The diuretic and losartan are a good pairing for hypertension as losartan lowers serum uric acid. Regards, Fred Coe
Hi Wally, A lot of people have commented on powder form. The problem is weighing out the correct dose. Check out the comments for this post because several people have offered proper equipment and techniques for doing so. Warning: Potassium is dangerous. Weighing out your own doses is not advised by anyone unless you know what you are doing. An overdose could be lethal. Be careful!!! Fred Coe
I was just prescribed 10 meq of potassium citrate it was $50 for a months supply. ( two pills a day)
Im suppose to take 2 pills a day..Amazon has it in a powder form in 17.6 oz for $20.
its called Pure potassium citrate powder what would the dose be? Its made by Bulk Supplements
thanks
One other thing,,I take 300mg of Allupurinal for gout, Im wondering if the potassium citrate could replace the gout med, I also take Hydrochlorothiazide 25 mg and 100mg of Losartan Potassium for Blood Psi..any conflicts??
Thanks again
Hi Again Wally, Allopurinol will lower serum uric acid and is used for treatment of gout. Potassium citrate cannot substitute for allopurinol in treating gout. The diuretic and losartan are a good pairing for hypertension as losartan lowers serum uric acid. Regards, Fred Coe
Hi Wally, A lot of people have commented on powder form. The problem is weighing out the correct dose. Check out the comments for this post because several people have offered proper equipment and techniques for doing so. Warning: Potassium is dangerous. Weighing out your own doses is not advised by anyone unless you know what you are doing. An overdose could be lethal. Be careful!!! Fred Coe
I found this page while searching for a way to get potassium citrate at a lower price. Out of pocket cost for a one month 90 pill supply is approximately $224 from my pharmacy. I just went on Cobra health insurance and the co-pay fee for this med went up to approx. $89 a month. That’s over $1000 a year! Are there no alternatives for me? This seems outrageous.
Hi Ron, You have joined the club. There are 129 others so far who have offered comments and ideas. The article offers alternatives that are a lot less expensive. Take a look at both. The pricing is outrageous and the manufacturers should be ashamed of themselves. Regards, Fred Coe
Dr. Coe,
I am a fellow researcher and would like to look into citrate content as alkali in other beverages. Would you be able to shed light on how Dr. Eisner and Dr. Asplin were able to ascertain the citrate as alkali content?
Reading their papers it seems this required knowledge of the pKa of citrate and malate, as well as the concentrations of citrate and malate in their respective solutions. Also required was the pH of solution. If I have all of this data, how do I plug it all in? Looking back on my chemistry days it seems related to the henderson hasselbalch equation.
As a generality, however, I take it that the higher the pH of a solution, the more the citrate and/or malate will exist as base (the good kind for stone prevention)
Cheers
Ryan
Hi Ryan, Yes, one needs to measure the total citrate and malate contents and the pH. Values for the dissociation constants of malic and citric acid are well established and do not need to be measured. The concentration of the salt forms is calculated directly from the measured total concentrations of the species in question, the dissociation constants and the pH. The salt forms are metabolized as the acids with uptake of protons making them base equivalents. It is John Asplin who did the measurements; Dr Eisner is a urologist. You can reach John at Litholink. He is a close friend of mine and would certainly welcome company in this arcane business of beverage analysis. If you get data be sure and publish it so I can post the results here. Best, Fred Coe
Regarding the cost of Uriocit K 1000 – I had been paying $15 for 90 caps until about 2 years ago when the price suddenly shot up to well over $100 for the same amount. Today the price is $152.32 for the same amount of pills. When I asked the pharmacist why the price was so high he said there was only one drug company making it for pharmaceutical distribution and they decided they could squeeze that much money out of patients, so they did. This is not a complex salt – there must be sources that can make money and still charge something in the range of the old price of $15/90 tabs of 1000 each.
Hi Kirk, You and your pharmacist have it about right. It is called gauging. You might benefit from the many comments about alternatives and the few in the article. Regards Fred Coe
Regarding the cost of Uriocit K 1000 – I had been paying $15 for 90 caps until about 2 years ago when the price suddenly shot up to well over $100 for the same amount. Today the price is $152.32 for the same amount of pills. When I asked the pharmacist why the price was so high he said there was only one drug company making it for pharmaceutical distribution and they decided they could squeeze that much money out of patients, so they did. This is not a complex salt – there must be sources that can make money and still charge something in the range of the old price of $15/90 tabs of 1000 each.
Hi Kirk, You and your pharmacist have it about right. It is called gauging. You might benefit from the many comments about alternatives and the few in the article. Regards Fred Coe
THANK YOU for this valuable service in the true spirit of medicine.
I’ve been enrolled in ‘automatic refills’ through my insurance provider’s mail order pharmacy for years. Fortunately, I had set a ceiling on approved amounts. When the pharmacy called to get permission to renew my potassium citrate, good grief! Forget the citrate, what do you have for price-induced heart failure?? $535 for a three month supply. I declined without hesitation and started searching.
The information you’ve provided is terrific. Again, THANK YOU.
Hi Spencer, Join the unhappy parade. The article and comments add a lot of ideas, and one needs them because of the prices. Be sure you need this drug. Did your 24 hour urine tests mandate it? Your stone type? Good luck, Fred Coe
Yes, my tests mandate it. And today my company’s “discount pharmacy” informed me that a 30 day supply (120 generic tabs) now costs $4,930.17! That is AFTER my company’s share of more than three-thousand dollars. This in insane.
I notice that there are 99mg potassium citrate supplements available for about $5. How might those compare?
And thanks again for this valuable service.
Hi Spencer, the problem is their small size – about 1/10th of a single pill. The comments on this article give a lot of alternatives. The price you quote must be a world record. I get a price of $41.08 per pill! That is shocking and I suspect a mistake. If not, we are in a bad world when so simple a chemical as potassium citrate can go for $41 per 10 mEq; that is $4 per mEq and you can buy – no doubt – pounds of it for $40 – but not approved for human use. Sorry. Use one of the suggestions from the others who have written in. Regards, Fred Coe
THANK YOU for this valuable service in the true spirit of medicine.
I’ve been enrolled in ‘automatic refills’ through my insurance provider’s mail order pharmacy for years. Fortunately, I had set a ceiling on approved amounts. When the pharmacy called to get permission to renew my potassium citrate, good grief! Forget the citrate, what do you have for price-induced heart failure?? $535 for a three month supply. I declined without hesitation and started searching.
The information you’ve provided is terrific. Again, THANK YOU.
Hi Spencer, Join the unhappy parade. The article and comments add a lot of ideas, and one needs them because of the prices. Be sure you need this drug. Did your 24 hour urine tests mandate it? Your stone type? Good luck, Fred Coe
Yes, my tests mandate it. And today my company’s “discount pharmacy” informed me that a 30 day supply (120 generic tabs) now costs $4,930.17! That is AFTER my company’s share of more than three-thousand dollars. This in insane.
I notice that there are 99mg potassium citrate supplements available for about $5. How might those compare?
And thanks again for this valuable service.
Hi Spencer, the problem is their small size – about 1/10th of a single pill. The comments on this article give a lot of alternatives. The price you quote must be a world record. I get a price of $41.08 per pill! That is shocking and I suspect a mistake. If not, we are in a bad world when so simple a chemical as potassium citrate can go for $41 per 10 mEq; that is $4 per mEq and you can buy – no doubt – pounds of it for $40 – but not approved for human use. Sorry. Use one of the suggestions from the others who have written in. Regards, Fred Coe