 If the lives of the Tudor nobility were luxurious, they were dangerous in equal proportions, for the King who bestowed their riches could in a moment wipe them, and those who possessed them, out. So intimacy with the person of the King and with the whole Royal Family was prized and feared. They lived, these powerful and dangerous people, in their Royal Palaces, to which you must go or have no influence. Even worse, King and Consorts made Royal Progresses, staying here and there as guests of the high nobility. Imagine that, the King as your houseguest. A person, like any other, and yet not at all like any other: glamorous, dangerous, and involved with high concerns.
If the lives of the Tudor nobility were luxurious, they were dangerous in equal proportions, for the King who bestowed their riches could in a moment wipe them, and those who possessed them, out. So intimacy with the person of the King and with the whole Royal Family was prized and feared. They lived, these powerful and dangerous people, in their Royal Palaces, to which you must go or have no influence. Even worse, King and Consorts made Royal Progresses, staying here and there as guests of the high nobility. Imagine that, the King as your houseguest. A person, like any other, and yet not at all like any other: glamorous, dangerous, and involved with high concerns.
You could say this is a silly preface to my common discourse on citrate, but not so. I have written before about its powers in our little domain: It binds calcium, it inhibits crystals, giving it reduces stones. But I have not said how it gets into the urine.
It comes as a royal visitor to some Duke or Marquess, Earl, Viscount, or Baron.
For this molecule has high purposes. It is noble and powerful. What it does in urine is but a tiny fraction of its many actions and probably not one of the more important ones. But what we do when we take citrate calls into play a vast biology. For all our lives we eat a diet that imposes an acid load on our kidneys, our bones, and elsewhere. Our kidneys, especially, adapt to that acid load, so what we call our ‘normal’ state is actually at one extreme. The pills, being alkali, reverse this lifelong adaptation and thereby profoundly alter the physiology of the kidneys and bone. In general one might say the alterations are for the better.
This is a long article but one worth reading for those who prescribe or take potassium citrate pills.
I want to acknowledge the expert error checking of Dr Yangming Cao (UCSF – Fresno) in the section ‘Why are Potassium Citrate Pills an Alkali Load?’ He corrected a significant error in the original article.
A Picture of the Kidney
Many of you are physicians or scientists who know about the kidney, but a few reminders are always worthwhile. Others are neither and we need to have names in common. Human 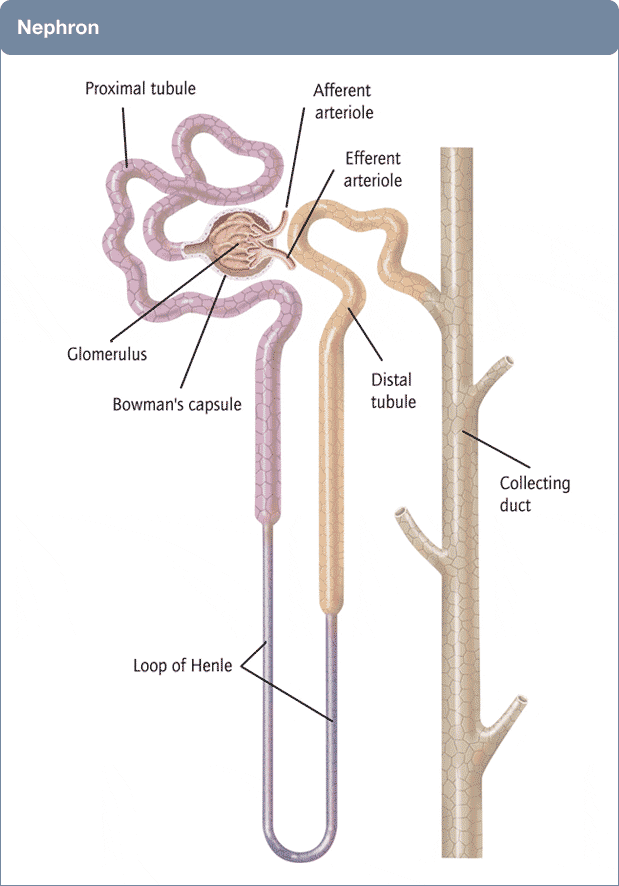 kidneys are made of about one million individual nephron units. The renal process begins with filtration of small molecules like citrate, or atoms like sodium and calcium, through the glomerulus, which is a complex of capillaries whose filtration pressure arises from the heart not a foot away.
kidneys are made of about one million individual nephron units. The renal process begins with filtration of small molecules like citrate, or atoms like sodium and calcium, through the glomerulus, which is a complex of capillaries whose filtration pressure arises from the heart not a foot away.
A majority of the filtered water, salts, and molecules is reabsorbed in the proximal tubule. The distal tubule (highly simplified here) performs tightly regulated absorption or secretion, so as to produce a final urine and maintain blood concentrations in their normal ranges.
These loops will come up again and again on this site so I should comment on the thin and thick portions. The long thin loops of Henle (Henle was the scientist who is credited with describing this part of the kidney) extract water specially well.The thick portions just below the ‘Distal tubule’ notation are called, appropriately enough, the Thick Ascending Limbs of the Loop of Henle. The thick limbs reabsorb NaCl, but not water, and in doing that entrain a marvelous system for – of all things – retaining water! In an article so long as this one, and concerned with citrate, I cannot pause longer here. But we will be back, someday.
Citrate is in the Blood
Kidneys Filter and Reabsorb Citrate
In one published study, concentration of citrate in blood is about 80 – 170 micromolar. A recent review places it at 120 micromoles/liter. If we use 120 micromoles/liter as a reasonable average, and a common value for glomerular filtration of 120 milliliters/minute, the filtration of citrate is about 21 millimoles a day. Of this about 1 – 4 millimoles appear in the urine, the rest being reabsorbed by the kidney cells. So the fraction of filtered citrate excreted is about 5 to 20%, and regulation of this fraction controls the amount of citrate in the urine.
Citrate in Blood Binds Calcium
The concentration in blood of calcium not bound with proteins is about 1 millimole/liter. Citrate concentration is about 0.12 mmol/liter, so in principle about 10% of non -protein bound – calcium can be bound by citrate. Because in calcium citrate crystals 2 citrate molecules can bind 3 calcium atoms, the the figure would seem to rise to to 15%. But in solutions like blood, other materials compete with calcium for a place on citrate – magnesium is one example. So the actual fraction is difficult to estimate. Normally blood citrate level is stable, so although significant, citrate binding of calcium is not likely to influence calcium metabolism by, for example, altering regulation of parathyroid hormone secretion.
Citrate has Signalling Roles
My purposes here are humble purposes, so all I wish to do is put here a tiny list of known effects of citrate on systems throughout the body without pursuing the details. Citrate concentration regulates lipid metabolism via malonyl-CoA. Citrate is sensed by the hypothalamus and thereby affects glucose intake and glucose metabolism by liver. To do these things citrate must enter the relevant cells, and it can do this only via a transporter that takes it across cell membranes.
The Citrate Transporters
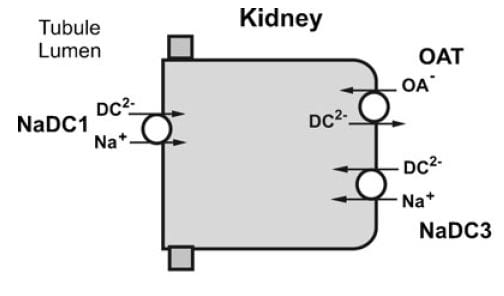
NaDC1 and NaDC3
NaDC1 is on the apical membranes of the proximal tubule cells of the kidney – the surface facing into the tubule fluid – and regulates the rate of reabsorption of the citrate that has been filtered. Its gene is named SLC13A2. This same transporter is on the food side of the small intestine cells and permits absorption of citrate from foods. The featured image for this article shows the structure of the transporter.
The citrate that enters the renal cells can be used for metabolism, or transported out the other side – called the basolateral side, facing the blood – via another transporter called the Organic Acid Transporter (OAT). Yet another transporter, NaDC3, permits citrate to enter kidney cells from blood. Because it appears to regulate urine citrate, my focus is on NaDC1.
The citrate transporter DC1 couples sodium and citrate movement. Since not everyone who reads this will know, let me mention an almost universal property of living cells: they pump sodium out of themselves and pump potassium in. Because they do this, sodium will tend to move into cells if given an opportunity – a hole. DC1 and DC3 can be thought of as sophisticated holes, or channels, through which sodium atoms can move if they have a citrate molecules with them. The actual proportions are 3 sodium atoms move with one citrate molecule, and the form of citrate which moves is one we have encountered before. Recall how citrate binds calcium because each molecule can have 2 or three negative charges on it. The doubly negative (divalent anionic) form of citrate is the one that traverse the channel.
They Transport More than Citrate
NaDC1 permits not only citrate to cross cell membranes but also succinate, alpha ketoglutarate, fumarate, malate, and a variety of less biologically relevant molecules. One might ask why, and I presume it is because the named molecules are all part of the citric acid cycle, which is the main engine of cell energy production. NaDC3 transports all of the same molecules as NaDC1, along with glutarate and a very long list of other molecules not in the citric acid cycle.
This cycle is at the center of that metabolism which uses oxygen to produce energy from food. The reference is to an excellent textbook review that is free online. Another chapter in that book finishes the story of how the cycle produces energy. The antiquity and centrality of the citric acid cycle will become apparent to you if you even browse these chapters. If you read them, you will encounter some of the most important aspects of living cells.
Why are Potassium Citrate Pills an Alkali Load?
In the citric acid cycle citrate is metabolized as citric acid, meaning that 3 protons are taken up from blood with each molecule. Removing protons is identical to adding alkali. Typical dosing is about 20 – 40 mEq of potassium salt daily, but the amount can vary widely.
Commercial potassium citrate contains 1080 mg of the compound in a 10 mEq pill. Typically the potassium citrate salts have a potassium on each of the three anion sites on the citrate molecule. The MW of citrate anion is 189.1. Urocit K, a common commercial version, is a crystalline monohydrate salt so it has a MW of 3×39 (for 3 potassium ions) + 189.1 (for citrate) + 18 for the one water molecule, or 324.1 in all. Given 324.1 for 3 mEq of base, the 10 mEq tablet contains 10/3 x 324.1 or 1080 mg.
The Flow of Citrate
In an earlier era organ physiology was popular and scientists often gathered together 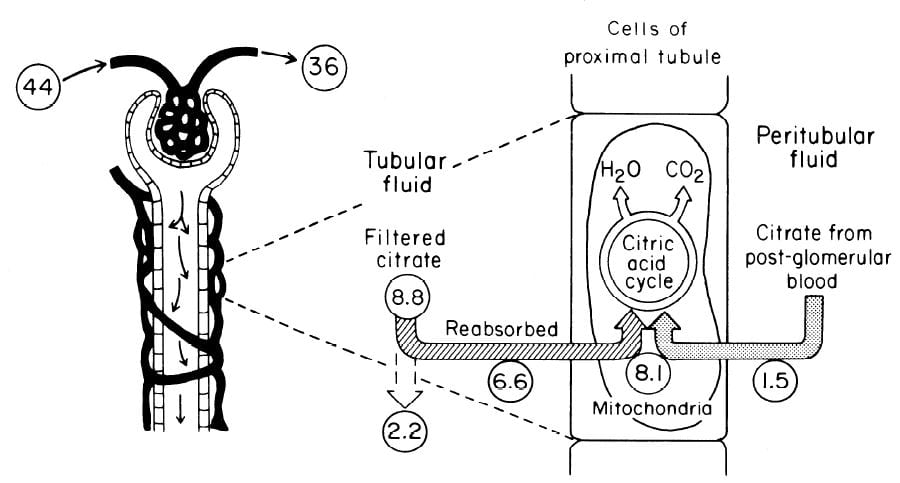 measurements to paint a picture of how things work overall. Here is such a picture from a wonderful review of renal citrate handling by Simpson. Values in small circles are micromoles (umol) per minute.
measurements to paint a picture of how things work overall. Here is such a picture from a wonderful review of renal citrate handling by Simpson. Values in small circles are micromoles (umol) per minute.
Citrate is presented to the glomerular filter at 44 umol/min, and 36 umol/min leaves the glomerulus (8.8 umol/min filtered) in blood what will pass by the blood side of the proximal tubules. From that 36 umol/min, 1.5 umol.min are taken up by renal proximal tubule cells and metabolized in the citric acid cycle. Of the 8.8 umol/min filtered, 6.6 umol/min are taken up on the urine side of the same cells making 8.1 umol/minute for metabolism. The remaining 2 umol/minute (3.17 mmol/day) are lost in the urine. NaDC1 and NaDC3 had not been cloned and sequenced at this early time, but physiologists knew the transporters were there and toted up what they did.
Urine Citrate Varies With Acid Base Status
Acid loads, such as high protein diets, will increase citrate uptake into the renal cells and thereby reduce urine citrate. Alkali loads such as diets high in fruits and vegetables or potassium alkali supplements reduce uptake and increase urine citrate.
Alkali
Clinical Response
In a trial, calcium stone formers with low urine citrate excretion eating a constant diet were given sodium bicarbonate or  potassium citrate, 20 mEq three times a day. Urine citrate rose with both treatments, as did the urine pH. Not relevant here, but in later articles, the sodium alkali did not change urine calcium, but the potassium alkali lowered urine calcium. Alkali itself lowers urine calcium, sodium raises it, and their antagonism is the reason for the differences.
potassium citrate, 20 mEq three times a day. Urine citrate rose with both treatments, as did the urine pH. Not relevant here, but in later articles, the sodium alkali did not change urine calcium, but the potassium alkali lowered urine calcium. Alkali itself lowers urine calcium, sodium raises it, and their antagonism is the reason for the differences.
Mechanism May be Increase of pH
If the citrate transporter is placed into test cells, the movement of citrate can be studied, and such a study shows how powerful is the effect of pH.
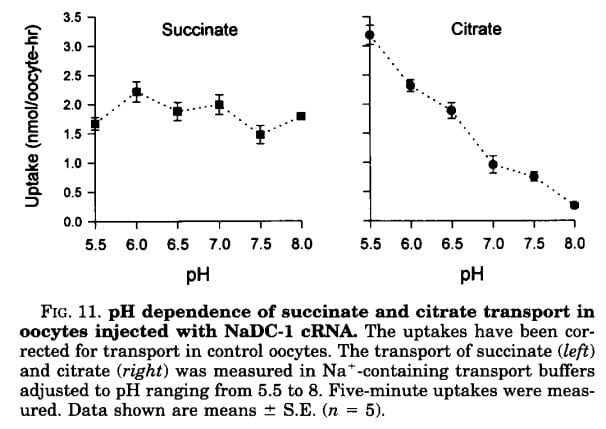 Succinate is a citric acid cycle intermediate like citrate, but its uptake by the citrate transporter is not affected by the acidity or alkalinity of the medium (pH). Citrate uptake is powerfully affected.
Succinate is a citric acid cycle intermediate like citrate, but its uptake by the citrate transporter is not affected by the acidity or alkalinity of the medium (pH). Citrate uptake is powerfully affected.
We have encountered pH before and remind ourselves here that urine values vary from about 4.5 to just below 8. Likewise, citrate has three sites that can accept protons, the acid component of water systems. As I mentioned in the paragraphs just above this point, the charge on the citrate molecule rises with pH as protons are progressively removed, and the sequence of pH values (the pKa values for the dissociating sites for those of you who know about such matters) are 3.13, 4.76, and 6.40. Obviously, in urine, the divalent (2 open negative sites) form will predominate until urine pH rises above 6 and will fall to about 1/2 of the total at 6.4. At about 6.4 transport of citrate was indeed just about half of that at the lowest pH.
pH in the Proximal Tubule
But it is not urine pH which affects citrate transport, it is the pH of filtrate in the proximal tubule of the kidneys, and that pH is not the same as that of the urine. At the end of the proximal tubule, the pH is about 6.7 to 6.8, and at that pH more than half of citrate is in the trivalent form and not available for transport. With alkali loads, as in the experiment in the table, the pH will rise, and citrate transport fall below normal, so citrate appears in the urine.
Problems with the pH Idea
Strangely, modern sources do not mention an older literature which raises questions about this mechanism. Simpson, in an important review from late antiquity (1983), mentions that the drug acetazolamide, which raises pH inside the proximal tubule and lowers pH inside the renal cells raises urine citrate only slightly and at first, but shortly after administration urine citrate falls despite a continuously alkaline urine and presumably tubule fluid. This suggests that even a high tubule fluid pH is not enough to counter the effects of changes in pH within cells or perhaps the blood. So it is not only tubule fluid pH that matters, but perhaps the pH inside the renal proximal tubule cell.
Acid Loads
Those unfamiliar with the matter may not realize that the diet we eat in the US and most of the other first world countries imposes an acid load that must be excreted daily in the urine. So the urine citrate excretion we find in our clinics and in experiments on ‘normal’ diets are those consistent with an acid load. When we give potassium citrate or other alkali we often do little more than neutralize this acid load, yet urine citrate usually rises. Experiments about acid loads add to the diet acid an extra amount of acid.
Tubule Fluid pH
As for alkali loads, a lower proximal tubule fluid pH will increase the fraction of filtered citrate in the divalent form which is transported by NaDC1. The pH of the tubule fluid will fall with acid loads for several reasons. Acid loads – for example a high protein meal – are buffered on blood bicarbonate which lowers the concentration of bicarbonate, and therefore the pH of the filtrate. LIkewise, the tubule cells are stimulated to increase their reabsorption of filtered bicarbonate which further lowers pH. All of this implies that kidneys sense the acidity or alkalinity of the blood, which they surely do.
Transport Adaptation
Over time – many hours to days – the NaDC1 transporter and its gene (SLC13A2) increase 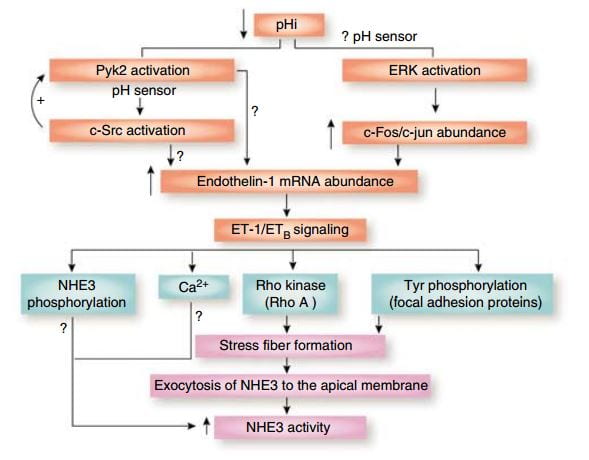 their abundances. This increase is mediated by endothelin – 1 (ET-1) through the endothelin B receptor (ETb).
their abundances. This increase is mediated by endothelin – 1 (ET-1) through the endothelin B receptor (ETb).
This figure from the above reference shows thinking about acid and endothelin as it was in 2007 and seems to be still. A fall in pH in proximal tubule cells can be sensed by a protein named Pyk2, which activates by adding a phosphate to one of its amino acids (tyrosine) and, interacting with another protein (c-Src), increases the abundance of the mRNA of ET – 1 which then signals through its ETb receptor to increase renal acid excretion – bicarbonate reabsorption – via NHE3, a transporter that reabsorbs sodium and secretes acid into the proximal tubule fluid.
This same ET -1 and its ETb receptor also signal increase of NaDC1 transport. Here, mice engineered to have (ETb+/+)or have not (ETb-/-) the receptor were challenged with an acid load. 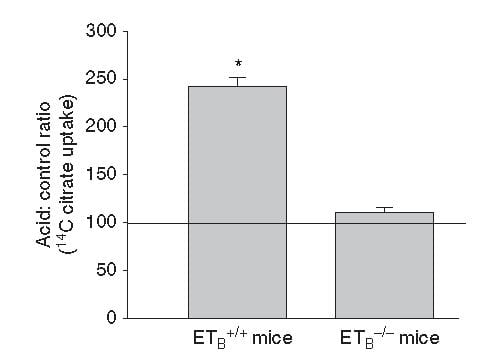 Citrate uptake by isolated NaDC1 transporters in the deficient mice do not respond to acid.
Citrate uptake by isolated NaDC1 transporters in the deficient mice do not respond to acid.
So one and the same effect, acid sensing and endothelin – 1 signalling increases acid excretion and citrate conservation.
But, you may ask, why am I grouping these two together?
It is because both concern acid base balance.
Citrate is metabolized as citric acid, taking up 3 protons per molecule metabolized, which is the same as saying it provides 3 molecules of alkali – like bicarbonate. Loss of citrate is therefore loss of potential alkali. NHE3 is a main driver of acid – protons – out of blood into proximal tubule fluid which reclaims filtered bicarbonate – conserving alkali.
So urine citrate, which we are interested in because it binds calcium and inhibits crystals, has a much larger role to play – part of the grand system which maintains a constant blood pH against the acid or base loads of diet.
Which pH?
I have spoken about pH of the proximal tubule fluid, of the blood, of the urine, but the one that is central to regulation of NaDC1 is the pH inside the proximal tubule cells. That pH appears to respond to acid or alkali loads, but the manner of its response is not simple. The signalling is through the Pyk-2 sensor already discussed and a parallel pathway via ERK (same diagram, above) which I did not discuss. But how sensing works, what is sensed, this remains very much an open research issues, and I will leave off here as this article was about urine citrate and the conversation has already taken us through many byways, beautiful if exhausting to follow.
Potassium
But – that awful word – one important fact remains to be uttered. Depletion of potassium lowers the pH inside kidney cells and lowers urine citrate. I will not pursue the details of this well worn story, except to point out its extreme clinical relevance. Diuretics that are used in stone prevention, or for hypertension, deplete cell potassium stores. It is the potassium citrate we give to patients.
Ammonium, and the Rest of the Story
How can I leave off without filling out the details of how kidney cells respond to acid challenge with production of ammonia that balances acid load with acid excretion?
Bicarbonate
A Better Buffer than Most
A buffer keeps pH relatively constant by taking up protons when they enter a solution and giving them up when alkali enters. It is a kind of shock absorber.
At the beginning, evolution favored bicarbonate. It is a buffer of considerable virtue in that it can take up protons or release them, like common buffers do, but has a special trait.
Bicarbonate is forever in equilibrium with carbon dioxide gas (CO2). When bicarbonate takes on a proton to become carbonic acid, much of that acid becomes carbon dioxide gas. When protons are taken out of blood, CO2 gas forms new carbonic acid which donates a new proton to the solution, and essentially bicarbonate appears in solution ‘out of thin air’. That it flows from solution into thin air and back makes bicarbonate a more stable buffer than those which live only in solution so it was an excellent choice.
What Kidneys do with Bicarbonate
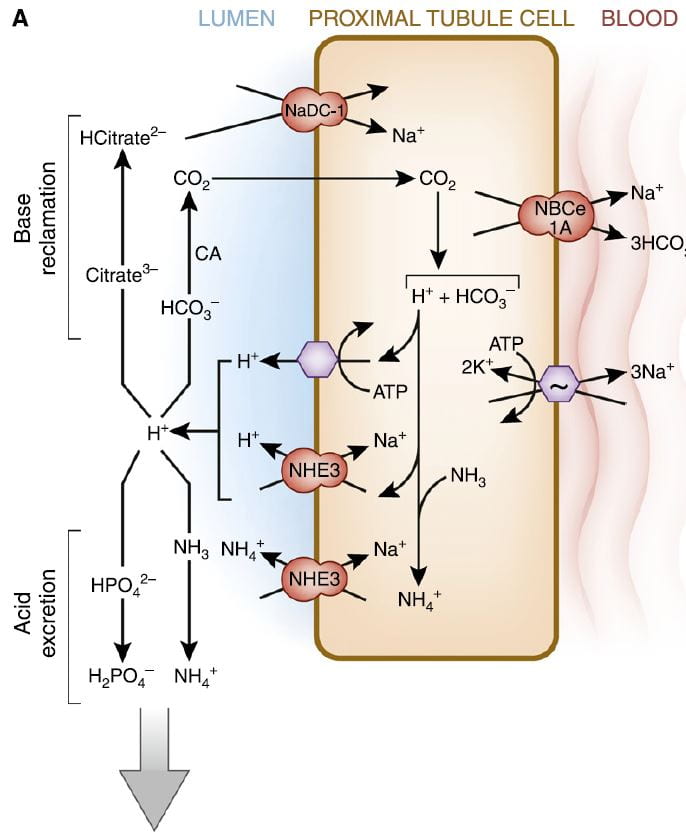 It is this very molecule, bicarbonate, which the kidneys traffic in when they respond to alkali or acid loads, and it is, of course, CO2 the lungs regulate in blood under the control of the brain.
It is this very molecule, bicarbonate, which the kidneys traffic in when they respond to alkali or acid loads, and it is, of course, CO2 the lungs regulate in blood under the control of the brain.
The figure is from the ‘A’ panel of a lovely drawing in a lively and engaging review. Being small, bicarbonate is filtered, and being the main buffer of the blood almost all of what is filtered must be reclaimed. So the proximal tubule cells, which do most of that reclamation, busy themselves forever with that task.
The way they do it is the simplest way. They add protons (H+) to bicarbonate in the tubule fluid, which becomes, as I have said, carbonic acid that transforms into carbon dioxide (CO2), which gas passes through the cell walls into the interior. Note, ‘CA’ is carbonic anhydrase an enzyme which speeds up the process of the transformation. In the cell, the CO2 becomes carbonic acid. Because protons are being pumped into the tubule fluid, protons are stripped off the carbonic acid so it becomes bicarbonate. The bicarbonate enters the blood with Na via the NBCe1A transporter.
There are two proton pumps. One uses ATP for energy to move the protons. The other (NHE3) uses the low Na in the cell as a gradient; sodium moves in through a channel like a revolving door, which makes one proton go out for every Na that moves in. At the blood side of the cell, the ancient ‘Great’ ATPase pumps Na out and potassium in, as it does in most cells that live on Earth. NHE3, the exchanger, is the molecule we met a few paragraphs above. It is increased by Endothelin 1 via the ET1b receptor.
At the top of the left side of the picture is citrate, our little slice of this massive structure. A few scraps of proton add to citrate so it has 2, not 3 negative sites, and can be reabsorbed. Its gene is regulated by endothelin 1 so when NHE3 is increased so is NaDC1.
Phosphate
Reclaiming bicarbonate is Sisyphean work. Nothing happens to get rid of acid loads from meals. But more protons are secreted than are needed to reclaim bicarbonate. Some are buffered on phosphate. But all the protons buffered on phosphate produce bicarbonate from carbonic acid inside the cell, and that bicarbonate enters the blood via NBCe1A.
Ammonium Ion
Ammonia is produced in the proximal tubule by removal of nitrogen from glutamine, pictured at left. As always, 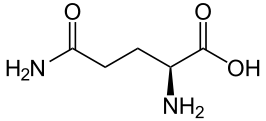 kinks are carbon atoms in this kind of drawing. The first one on the left has an oxygen and NH2 group, and a bond to the next carbon. Carbons typically form 4 bonds each. The next 2 carbons are merely linked to one another. The fourth has another NH2 and the final one at the right 2 oxygens. The left hand one is removed by an enzyme to produce NH3 and glutamic acid. The second one is removed to produce α-Ketogluteric acid which lacks any NH3. The 5 carbon skeleton remains unchanged.
kinks are carbon atoms in this kind of drawing. The first one on the left has an oxygen and NH2 group, and a bond to the next carbon. Carbons typically form 4 bonds each. The next 2 carbons are merely linked to one another. The fourth has another NH2 and the final one at the right 2 oxygens. The left hand one is removed by an enzyme to produce NH3 and glutamic acid. The second one is removed to produce α-Ketogluteric acid which lacks any NH3. The 5 carbon skeleton remains unchanged.
Ammonia (NH3) can tale up a proton to form NH4+, ammonium ion, which has a pKa of 9.3 meaning that at the pH of proximal tubules and cells, it is fully protonated. Loss of this ammonium ion in urine represents net acid excretion because the protons that were taken up came from carbonic acid which is converted to bicarbonate and transported into blood. Unlike titration of phosphate, excretion of ammonium ion does not increase urine pH because the pK is far above the pH of urine.
Under normal meal conditions, about 40 – 60 mmol/day of acid are excreted, of which about 2/3 is ammonium. Large acid loads, as for example, a ketogenic diet for weight loss, would induce a large increase in ammonia production so acid excretion can keep pace with acid production.
α-Ketogluterate
One might think this byproduct of glutamine metabolism, the 5 carbon skeleton, might be metabolized and done with, but no. A significant amount is metabolized. But some is not.
What is not metabolized traverses the kidney to cells in the later nephron, the intercalated cells in the collecting ducts, which usually pump protons into the tubule fluid to create the final urine p H which is critical to supersaturation and stone formation. But these same cells can reverse themselves and pump bicarbonate into the tubule fluid and protons into the blood, and they do this when confronted by an alkali load.
H which is critical to supersaturation and stone formation. But these same cells can reverse themselves and pump bicarbonate into the tubule fluid and protons into the blood, and they do this when confronted by an alkali load.
It turns out that α-Ketogluterate is itself filtered and reabsorbed in proximal tubule, and its reabsorption is profoundly reduced under alkali conditions so that more is delivered distally to a receptor (Oxgr1). When occupied by α-Ketogluterate this receptor signals the reversed intercalated cells (B and non-A cells) to increase their secretion of bicarbonate. The transporter for α-Ketogluterate is NaDC1. The net effect is to enhance bicarbonate – alkali – loss which offsets alkali loads.
The same receptor signalling stimulates pendrin, a complex exchanger which moves bicarbonate and Na together with chloride to effect NaCl and NaHCO3 reabsorption. Because acute acid challenge increases and acute base loading reduces proximal tubule NaCl reabsorption, this action would tend to maintain salt balance in that the intercalated cells would increase salt reabsorption as proximal tubule reduces salt reabsorption. Of note, although chronic acid challenge increases NHE3 abundance and activity, it reduces NaCl reabsorption via effects on other transporters. For these reasons the α-Ketogluterate – pendrin link is probably more important in minute to minute or hour to hour regulation than in adaptation to acid or base loading diets or treatments.
Citrate and Oxalate
You would think I had exhausted the topic by now, but no. NaDC1 and slc26a6, the citrate transporter and the anion 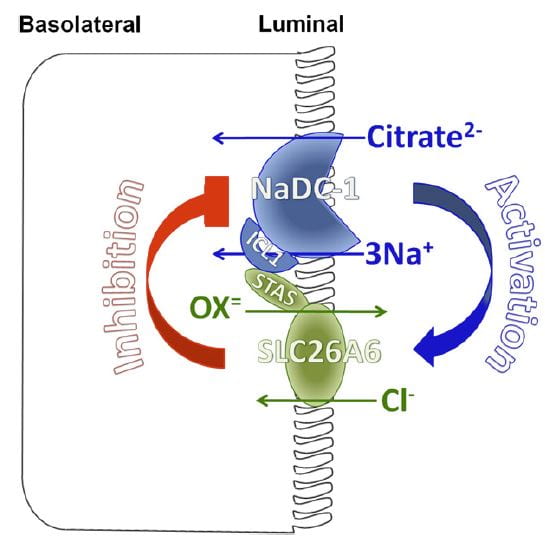 transporter (oxalate is an anion it can transport) which disengages NaCl transport from NHE3, themselves interact in relation to kidney stone formation.
transporter (oxalate is an anion it can transport) which disengages NaCl transport from NHE3, themselves interact in relation to kidney stone formation.
At least in animals and in cell experiments, the two transporters – which are present in a complex within the renal cell membrane – interact as in the figure. Slc26a6 inhibits NaDC1, so that when actively transporting oxalate into tubule fluid citrate reabsorption is reduced, urine citrate rises, and binds urine calcium to reduce risk of calcium oxalate stones. When oxalate secretion is minimal, NaDC1 increases to salvage citrate.
These animal and cell experiments imply that in human urine citrate and oxalate excretions should show parallel changes; this has not been tested.
Putting it All Together
Just Diet
Our urine citrate is an outcome of our biologies, which are variable, and our diets. Most of us eat a diet that imposes a net acid load, so our kidneys tend to conserve citrate and α-Ketogluterate, our intercalated cells pump protons not bicarbonate in to the final urine, our proximal tubules produce considerable ammonia and our urine pH is about 5 – 6.
Some of us, vegetarians whose diets do not have a proper balance of protein, very massive fruit eaters, as examples, have low citrate reabsorptions and high distal deliveries of α-Ketogluterate; our intercalated cells are reversed and stimulated to put bicarbonate into the final urine, our proximal tubules do not make much ammonia.
But the words ‘most’ and ‘some’ are misleading. In the US, certainly, chronic acid loading is the overwhelming rule, and the same throughout Europe and considerable parts of urban Asia. So our ‘normal’ poise centers on adaptations to acid load. It is not that we live in a neutral acid base condition, demanding from our kidneys little excretion of acid or of alkali. Life long we demand acid excretion. That is where we start. It is to that task our kidneys – and our bones, as I shall someday speak about – apply themselves all the days of our lives. However it is, for good or for evil, that lifelong adaptation to acid load affects us, that is our state, our permanent condition.
What Does Normal Mean?
When we give potassium citrate or any other alkali in doses of 40 to 60 mmol/day we neutralize a large fraction of diet acid. This is best considered not so much as an ‘alkali load’ as it is the removal of that acid load to which we have long been adapted.
Of course, urine citrate rises. Because we give alkali over months or even years, renal cells will adapt fully to the changes. But by ‘adapt’ I mean they give up the adaptations to acid loading. In the case where the dose of alkali just matches acid production one might best say the kidneys are relieved of their burdens in either direction, and reveal the way they would function if not driven to either extreme.
Like the small sailor plying the simple waters of a bay fills its sails sometimes southerly, sometimes northerly, making little way, dancing before a playful breeze, the cells shift their powerful machinery a bit here or there as one meal gives way to another. What shall I call this state of freedom? Why is this not the ‘normal’ from which point we register the responses to extra alkali or acid?
I have read where it was in Eden a condition of fruit, as the animals were not for them to eat. Perhaps I am wrong, and if Eden was as it says in our books the ‘normal’ state was alkali load. Perhaps Milton is wrong. After all he was not there, merely a poet making into life what he read in a holy book.
Potassium Citrate Pills
Raise Urine Citrate and pH
The expected changes are a decrease of proximal tubule reabsorption through reversal of the effects of chronic acid load. ET-1 signalling must fall, citrate reabsorption must fall because NaDC1 is no longer stimulated by ET-1 and because proximal tubule fluid pH will rise and with it the fraction of trivalent negative citrate.
Urine bicarbonate and urine pH will also rise. Partly, blood bicarbonate will rise and with it filtrate bicarbonate concentration and pH. NHE3 transport will be decreased vs. chronic diet acid loading, the baseline in the first world countries, and much of the proton secretion will be used in reclamation of bicarbonate. Naturally, NH3 production will be greatly reduced because the Pyk-2 sensing system will be signalling a higher pH.
Increases in Citrate and pH Vary Among People
But the biology is complex enough that in some people the main response will be citrate, and in other bicarbonate. Given all of the regulatory steps and signalling pathways involved a variety of responses is inevitable. Clinically this means one must measure and determine if the main effect is mainly increase of citrate excretion or of pH and therefore of CaP SS.
What is the Ideal Dose?
A nimble answer is enough to match net acid production – urine sulfate excretion is a decent index. I suspect that answer because of the problem of high urine pH in some people, and because as a clinician I never find it perfectly suits most patients. Yet it is a good starting dose because it aims at neutral acid base balance.
A Simple Pill with Powerful Effects
Physicians who treat kidney stones may well be the main ones who prescribe alkali loads to people with normal kidney function over months or even years or decades of life. This is indeed a remarkable physiological and clinical experiment, and that we do it makes the physiology and cell biology of acid base balance a central topic in clinical practice of stone prevention.
Likewise patients who take this humble medicine undergo what amounts to a reversal of cultural norm, which is a condition of chronic acid loading.
Thence, and for this reason, I have written a very long article about the topic, for physicians and their patients, and especially for scientists who know more about this topic than I do but may not see things from exactly the same view point.

My husband passed your article on to me to decipher what it could mean to him because he has kidney stones. I’m sorry to say this article is far too technical for me so I have no answer for him. I may try looking up potassium citrate on line and see if I can find a simpler explanation because citrate may have some indications for my husband.
Dear Mrs Jusick,
Thank you so much for your thoughtful comment. In it you sum up the dilemma of medicine on the web and point to where a possible solution might be found. The dilemma is the unavoidable tension between between the story and the details. Without a ruling story the details become unusable; without its details the story becomes untrue. It is precisely here we find the reason why physicians need so much education as they do, and why the problem of time haunts the practice of medicine right now.
One way I can help you is to point out the other articles on the site. The only evidence potassium citrate works is from the trials. Take a look: This is really all anyone has to go on. The pills work exactly for those they were tried on. The pills can be costly, and there are alternatives to the expensive forms of this supplement. Some alternatives are simple beverages.
Other articles fill in around what citrate does. It binds calcium in the urine, which helps prevent calcium stones. It inhibits stone crystals from forming and growing. Throughout is the problem of what kind of stones we want to prevent. Uric acid stones are completely prevented by citrate, calcium oxalate stones are main ones in the trials, and are reduced. Calcium phosphate stones are an open issue, as the drug may be good or bad. The need for stone analysis is therefore paramount.
The big article that has rightly set you wondering where to find useful information takes us into the reality of the drug, and is meant as the end of the story, not where one would start. So you have shown me a need to add something on this site – a guide to wayfarers, an overview, and there is now so much about citrate already here I should have written one before.
Take a look at the links I have put out for you, and see if your husband seems a proper candidate. But – awful word – physicians do have a key role. Wise patients educate themselves; the wisest of all then seek counsel. Regards, Fred Coe
IF you do not have any reason for magnesium deficiency – for example some drugs cause it – there is no special proven value to magnesium supplements. Brief trials have given ambiguous results so far. The generic pill should do as well as the name brand as best we know.
Dr. Coe,
Thank you so much for this. I have had dRTA for 50 years in March or April. I have had these pills prescribed and have trouble being compliant… I have to force myself . I have a better understanding of the nephrons and how the kidney works. Since my stones are not primarily calcium oxalate, I wonder how much good the pills do. I know I have to have a 24 urine test and other lab work to determine this. I know that these pills are the best science has to offer for my disease. I trust my doctors and will have to become compliant.
I probably will have some stones removed as a preventative treatment to improve kidney function later this year. A doctor called this debulking my kidneys. I know not all urologists believe in this. I also found out the hard way that my stones are called “difficult stones” and urologists who have the skill and experience to treat me are few and far between. I had help from some other medical professionals who taught me things I never needed to know until 2007.
I enjoy this web site and look foreword to reading everything I can. I wish I had taken more science classes in college so this is easier to figure out. I know you are writing this for lay people, and not other doctors, residents and medical professionals. Thanks
Trish
Dear Trish, Since you have distal renal tubular acidosis, your stones are most likely calcium phosphate. This disease reduces the ability of the kidneys to remove acid loads from diet, so potassium citrate is ideal. It neutralizes the diet acid load and corrects the acidic condition of the blood. I would hope it has reduced stone formation, as well. If it has not done so, perhaps your physicians might want to consider additional measures. Best, Fred Coe
Dear Dr Unikowski, thank you for these excellent comments and questions.
You are correct that each 10 mEq tablet contains 10 mEq of potassium and therefore there are 10 mEq of base equivalents.
It works this way.
Citrate is a trivalent anion molecule. When taken, the citrate enters the enteric venous circulation and is metabolized in the liver as citric acid. In order to take up the citrate as citric acid, protons must come from the hepatic blood thereby producing bicarbonate: For each proton removed from the bicarbonate buffer system of the blood, a new bicarbonate is produced via simple shifts of equilibrium, from carbonic acid and ultimately from dissolved carbon dioxide gas. The citric acid is metabolized so that none of its acidic metabolites re-enters the blood, with the result that one obtains 3 bicarbonate molecules from a single molecule of trivalent citrate. The pills give the mole equivalents, and one bicarbonate is produced for each equivalent.
You are perceptive about the citric acid cycle. Protons are produced and used to create a proton gradient which transfers energy to produce ATP. The protons do not get out of mitochondria to affect blood pH.
Warm regards, Fred Coe
Dr. Coe, I thoroughly enjoyed your exemplary article. I too have DRTA. Potassium citrate was not helpful. After my trial with it, we did another 24 hours urine collection and just as my team suspected, my citrate levels were “in the toilet.” My nephrologist has moved on and no one else seems to know what to do. I continue to deteriorate…well…only in a physical sense…doing just great otherwise. What next? Be well. Peace, Jane R.
Dear Jane, I presume you are making more stones, and I guess they must be calcium phosphate stones. While you may have low urine citrate and benefit from potassium citrate to raise the urine citrate, basic principles of treatment are the same for you as for everyone else. Your urine volume should be as high as possible, certainly 3 liters a day – if you are indeed making new stones on a regular basis. Your urine calcium excretion should be below 200 mg daily. I suspect what you meant by the potassium citrate being not helpful is that stones continued; with enough water and attention to urine calcium stones should stop forming at a high rate; whether potassium citrate is ideal or not depends on other issues like the pH of your urine and exactly what your stones have been made of. Regards, Fred Coe
Dr. Coe, Thanks a lot for this very interesting article. I’ve calcium oxalate stones since 7 years, gout, hypertension and higher level of blood uric acid. From my research I’ve realized that all these issues are caused by my diet/blood acidity. I don’t really eat too much of meat and purines, however I’m not a vegetarian either and getting gout attack a few times a year. I’m very interested in starting with potassium citrate (already did) in proper quantities: 1 gram 3 x day, so perhaps I will be able to neutralize my diet’s acidity. However I’m not sure if I understand that correctly: is using potassium citrate going to lower my blood uric acid levels? or perhaps potassium citrate affects only urine PH, and citrate levels. Do you think that potassium citrate can be effective in decreasing my blood uric acid levels? Thanks again, J.G.
Hi Jake, I guess your stones are calcium oxalate, and do not contain uric acid. Potassium citrate will increase your urine pH and in that way prevent uric acid stones. It will increase urine citrate and citrate can help prevent calcium oxalate stones by binding calcium and by inhibiting calcium crystal formation. The drug will not alter serum uric acid. That you have gout and high blood pressure gets me to ask if your blood glucose is always normal or a bit high, and likewise in your 24 hour urines is your citrate really low or high; likewise, what is your 24 hour urine calcium? All these factors make a difference to answering your question. Regards, Fred Coe
Dr. Coe, Thank you very much for your explanation. My recent 24 hour urine (with acid) and with no acid tests came out in range (around 2.5 L in volume). The only result that is above is serum uric acid. In the recent past I’ve had a little of triglyceride increase above normal range, that’s all. MRI of abdomen is all good except kidney simple cists, one larger 5cm (Bosniak II).
I’ve observed that my hypertension fluctuates seemingly without any reason, sometimes normal other times high. Perhaps it is relevant to fluctuation of my serum uric acid that can affect that. I was told that changes in my diet (fairly difficult to achieve since I already eat (almost) only “good stuff”) and increased water intake would help with serum uric acid. Hence my question, rather then having “mostly greens” diet, how can I affect my serum uric acid (without allopurinol), by way of supplements? Is there anything else that can be added to a diet for that very reason or genetics and diet only can regulate that? Cheers, J.G.
Hi Jake, You are really speaking about the management of gout as the serum uric acid level itself is not the problem but that high levels can result in crystallization of urate salts in joint fluid with acute arthritis. How your gout is managed is separate from stones, and really needs to be overseen by your personal physician. Likewise, high blood pressure is a serious issue and is treated with weight loss, aerobic exercise, reduced sodium intake and medications as needed. That, too, is the work of your personal physicians. No amount of water will itself lower serum uric acid that I know about, although reducing purine intake – purines come from the breakdown of DNA and RNA so foods with cell nuclei provide the substrate for uric acid (meats, some bean products) – is of modest help. Regards Fred Coe
Hi Fredric, it’s me again! Obviously all the chemistry involved in kidney function, absorption, excretion and reabsorption is diffucult for me to understand. although I do understand the importance of the effects potassium citrate has on increasing alkaline urine, preventing cystalization thus stone formations, but I have just one question. One of our members today posted about just recently starting potassium citrate treatment, and is now noticing white shards, gravel in the urine. In your opinion, besides increasing urine pH, binding with calcium, decreasing crystalization thus preventing stones, could it possibly help to dissolve calcifications? In nephrocalinosis and MSK for example? Thank you!
Hi Celia. I believe the urine pH has risen, urine supersaturation with respect to calcium phosphate has risen with the pH, and she is forming de novo crystals of calcium phosphate. Potassium citrate is a fraught treatment for calcium phosphate stone formers, especially those with hypercalciuria. It is a race between two opposing forces: High pH and high CaP SS against the effects of the supplement to lower urine calcium, bind calcium, and inhibit crystal formation. I think this person lost: Crystals mean the balance is not right. Higher fluids, perhaps a lower dose, perhaps attention to her urine calcium. I would not rest content with the soothing but unlikely idea that stones are dissolving. The key response is to let the prescribing physician know, who will be in the best position to decide what is the best course of action here. I may have some expertise, but only the responsible physician can really be sure to take the right action. Warmest regards, Fred Coe
Thank you, I’ll let her know.
9 month’s ago had a kidney stone as big a nichel they litho broke up the stone having stones every day for 9 month’s taking potassium citrate pills stop my right kidney. Please make them stop
Hi Lisa, I am not clear about all that has happened. Certainly your physicians have analysed that stone, and I imagine they are trying to prevent more. If the potassium citrate is not working, let them know so they can find an alternative for you. Above all drink a lot, at least three liters a day of fluids. Regards, Fred Coe
I was wondering about the citrate affecting glucose intake and metabolism. Does it in any way lead to problems with diabetes? I am hesitant to take it on a regular basis … possibly the rest of my life, however your article gives me some hope that it is not all bad for my body. I have MSK and we are trying to decrease my stone formation.
Hi Laurie, I know of no reason why potassium citrate should worsen glucose tolerance or risk of diabetes. Potassium itself is somewhat low in our present diets compared with what we appear to have eaten during evolutionary time. Citrate is metabolized to bicarbonate in the liver and kidney and seems to simply neutralize the daily acid load from foods. Although no one ever knows for sure in medicine, this particular agent should be pretty safe. Regards, Fred Coe
A most interesting article, Dr. Coe. I researched why I was getting huge oxalate stones, several times a year, & seven years ago, began taking 10 MEQ KCit three times a day, & have not had an oxalate stone since. However, I had an emergency cholecystectomy in 2013, & have started having gout episodes, including tophi. My bloodwork indicates borderline hyperuricemia (8.0-8.4 mg/dL), & I have been taking 0.6 mg Colcrys QAD, which leaves me symptom-free, except for tophi. I have no signs of RA (I’m a martial arts instructor), & I am wondering why my KCit has not mitigated the gout. Of course my loss of one kidney to a papilloma 6 years ago does confuse the issue a bit. My PCP & Rheumatologist are scratching their heads. Any ideas or suggestions?
Hi Ken, I am not surprized that K Cit has prevented CaOx stones. The two reasonable trials were pretty convincing. Your gout is from NaHUrate crystals, and I would not have expected K Cit to be of much help there. I have had several patients tell me K Cit was associated with worse gout attacks and I cannot imagine a reason for that, either. Loss of the one kidney will surely worsen risk of gout because of a reduction of urate clearance. Is everyone sure it is gout – not calcium pyrophosphate crystals – pseudogout? Regards, Fred
My wife has been battling recurring stones for the better part of 15 years, this time they are so large, her Dr told me that he has never seen a case so bad. I started researching natural help and stumbled upon calcium citrate. Why has her Dr not brought up this option before? Why did we have to find this out on our own? I’m rather upset about this. She has endured at least a dozen surgeries and/or lithotripsy procedures so far.
Her latest bought is with calcium phosphate stones, but I believe earlier stones were calcium phosphate but I’m not 100% sure about that.
We had to bring up the 24 hour urine collection to her Dr as well. Again, why didn’t he tell us about this say, 5-10 occurrences ago? Hopefully this will give us a starting point.
What would be your recommendations in the 2-3 week interim here?
Thank you.
her earlier stones were oxalate, sorry about that and again I’m not 100% sure on that.
Hi Scot, Calcium phosphate stones are harder to prevent than calcium oxalate, more numerous, too. Calcium citrate is not a treatment for stone prevention; potassium citrate is the medication in the articles. For calcium phosphate stones, potassium citrate is as yet an unproven treatment. As the article points out this agent makes the urine more alkaline which would raise supersaturation for calcium phosphates. Given her 24 hour urine studies, you need to look for the cause of her stones. Stones are made of crystals, crystals follow the laws of physics, so there is always a cause in the urine chemistries. Usually the urine pH will be too high and the urine calcium high as well from idiopathic hypercalciuria or perhaps another cause of hypercalciuria. Prevention is always possible and usually a lot more simple than surgery. Take a look at the articles I linked for you. Fred Coe
Hi Dr. Coe,
I’ve had KSs for over forty years and they have been analyzed as oxalate. Four months ago my doctor at NYU put me on Four 1080 mg Potasium Citrate regimen. I currently don’t have any stone pain that i’m aware of. I take two pills at night after dinner with my other pills and two in the morning on rising, again with my regular pills. Later in the morning, approximately thfree hours after the pils wer taken, I had a bowel movement, and lo and ehold floating on top wer two of the pills. Yes, I picked them up and they seemed basically intact.
Any thought before I go back to my doctor, who meantime has moved on to Columbia Hosp.
Thank you in advance for any information you may be able to provide.
RW
Hi Rudy, Many people have observed this, and I understand that what you are seeing is simply the wax matrix that once held the potassium citrate and that the potassium citrate itself has been leached out and absorbed. Ask your physician to look at the changes in your urine chemistry with treatment, and be sure you are actually absorbing the Drug. Expected changes between before and during treatment: With treatment urine potassium and pH will rise, the difference between urine sulfate and ammonium ions will fall. Urine citrate may rise, but that is not so predictable. Regards, Fred Coe
Thank you very much for being so responsive Dr. Coe.
Hi Chris, I do not know; John Asplin analyzed the pure lemonade, and not the sugar free. From your note I would doubt they are the same. One liter of the pure lemonade is 20 mEq of potassium citrate, so it will be more than your tablets. Fred Coe
Dear Dr. Coe – thank you for your informative article. you mention re: potassium citrate – “The pills can be costly, and there are alternatives to the expensive forms of this supplement. Some alternatives are simple beverages.” what might those beverages be? thank you for your time.
dr Coe –
i read about drinking Crystal Lite! ugh. i drink no sodas. i do have a meyer lemon tree though. i drink coffee, tea (all varieties), some grapefruit and carrot juices and the occasional glass of champagne. drinking hard liquors seems to exacerbate my gout.
Hi Karen, I guess you read the article and found Crystal Lite. There are other beverages in the table as well. Lemons are too irregular to use as a medication. The juices are filled with sugar and add calories. In the article ‘A thirst for Variety’ Jill Harris goes through a lot more beverage classes. Between that article and the one on the cost of potassium citrate you know all I do. Regards, Fred Coe
Hi Karen, In the article on the price of potassium citrate we put in all we knew about alternatives. Subsequently many commentators have added valuable alternatives. Of the beverages, they are listed in the article and Crystal Lyte does win the prize. Regards, Fred Coe
thank you.
On September 30 I had surgery to remove a stone analyzed as a uric acid stone. At that time the urologist prescribed 15 mEq (1620mg) of potassium citrate twice daily. On October 19,2015, having been on the potassium citrate for 19 days a 24 hour urine collection test was performed . The results were oxalate urine 67 ( high), uric acid 851 (high) , sodium urate 4.77 (high) sodium urine 301 (high ) The urologist referred me to a nephrologist, and I’m awaiting the appointment date. I was not instructed to not eat or drink any food or beverages containing vitamin C 24 hours before the 24 hour urine collection test, which I have read as a direction for the test. My research seems to tell me that Vitamin C and potassium citrate are related (derivatives) My question is , would taking the 1650mg of potassium citrate twice a day, contributed to the high 67 metabolic level of oxalate, and if potassium citrate is supposed to help dissolve uric acid stones, would taking the potassium citrate helping to dissolve the stones have increased the uric acid urine resulting in the high 851 test result and the super saturation high reading of 4.77 ? Simply, could taking the potassium citrate for 19 days before the 24 hour urine collection test have contributed to the high level test results? I appreciate your response as I await my visit to the nephrologist.
Hi Emmett, Great questions! Let me presume the stone is pure uric acid. The potassium citrate is the ideal treatment. Although you do not give it, the urine pH controls uric acid crystallization to such an extent nothing else really matters. When you raise the pH, urine uric acid often rises, because it had been crystallizing before – when you were making stones – because the urine pH was too low. The sodium acid urate supersaturation can be ignored in your case as it has to do with other settings. The high urine sodium reflects your very high sodium intake – over three times the recommended upper limit for the US. Urine sodium is what we have eaten, no other sources. As for the oxalate, there is some in vitro conversion of vitamin C to oxalate especially when urine pH is high, so I suppose that could have happened. YOu may have a low calcium diet – a very bad idea – which will raise urine oxalate, or just eat a lot of oxalate. It is not mainly the potassium citrate. As I said, the supersaturation with respect to sodium acid urate is irrelevant to you. So, if the pH is above 6, the main supersaturation you care about, for uric acid, will be below 1; if not, higher urine volume and a bit more potassium citrate will do. It is a cure for uric acid stones. Regards, Fred Coe
Hi Dr Coe,
Can potassium citrate still break up a big stone (between 4-7mm) in my Ureter? I think it is up by the Pelvic Junction? I was given these some time ago by my doctor but I read alot of people get very sick with this pill and my stomach can just look at a pill and get sick !! Is there a way to counter act the sickness that would come from taking this pill?
Thanks much,
Renee
Hi Renee, Unless your stone is made of uric acid, it cannot be dissolved with medication. If you indeed have a stone lodged in your ureter, it is very important for your physician to be following its progress. Such stones can obstruct the kidney and damage it. So, it is not a pill matter once the stone is in the ureter. Pills are to prevent more stones. IN order to use them wisely your physicians will need to study your 24 hour urine and blood to determine the causes of your stones, and then take the proper steps to prevent them. Regards, Fred Coe
Dr Coe
What medications tend to dissolve uric acid stones/
Hi Richard, Only one medication can dissolve uric acid stones and that is potassium citrate – or another alkali – sufficient to raise the pH of the urine above 6. These stones form because of an acid urine and stop forming when the urine becomes alkaline. Treatment with alkali is near perfect. Now, dissolving uric acid stones is a bit tricky because such stones are not usually one thing but a cluster of smaller stones. So when you take alkali you make begin to pass the smaller fragments. Obviously you should not undertake treatment without your personal physician supervising. Potassium salts are not without risks, and assessing urine pH and the progress of stones requires medical supervision. But given that supervision the treatment should be excellent. Regards, Fred Coe
Can potasium citrate cause dizzyness, vertigo, or stomach aches and nausea? Or, could it be my minicycline causing these things? Please e-mail my answers. It’s very important. Sometimes I am dizzy for a week at a time and can’t find out why. Thank you for your reply.
Hi Linda, potassium citrate can certainly cause intestinal symptoms of all kinds. Minicycline is very likely to do this. Perhaps you might want to try one at a time – perhaps a week each – and see which it is that causes your symptoms. True vertigo – the room going around you – is not caused by either drug so far as I know. Dizziness is common with nausea, so I cannot be sure whether it is a primary symptom or not. Fred Coe
Hi Fred,
I am revising a book chapter on RTA and wanted to say something about how ingested preformed citrate alkalinizes the urine, since it seemed that citrate would be absorbed and metabolized to CO2 and H2O by enterocyte mitochondria. Anyway, your digression into the wiles of the Tudors was an unexpected intro to my exploration of citrate.
Best,
Ron
Hi Ron, A rather elegant thesis, and perhaps true. Is it original with you? Any mitochondria will do, I suppose, and why not there? If asked I would always point to the liver because it is so big and always doing things. The Tudors have a bad rep because so vicious, but there was Gloriana, and for all her ways, she kept England out of war, made the people a wealthy nation, and founded one of the great ages of English letters and music – Shakespeare, Dowland, Donne. All the best, Fred
Fred,
Your commentary above is illuminating about the wiles of citrate and its salubrious effects on human health, if not kidney health.
Assuming that ingested citrate is absorbed in an intestinal segment by an apical transporter (NDC1 ?) of an enterocyte, and that the citrate is metabolized therein (mitochondria) completely such that no citrate exits the enterocyte and flows northward in the portal stream, how then is the ingested citrate transduced into an increase in urinary citrate? Rather than clutter this blog with my musings, if you reply to my email, I can add to my thought process.
Best,
Ron
Hi Ron, Here is what must always be the case so far as I can tell. Citrates are used in the Krebs cycle as citric acid, and at the end of the cycle we have CO2 and water. So whether in the liver or enterocyte, protons have to be taken out of the blood as citrate is taken up into the cycle as citric acid. The removal of a proton from blood produces a bicarbonate molecule because of the CO2 buffer system being predominant and that new bicarbonate signals kidney cells as I reviewed in the article. Your point is really interesting – that intestinal cells could be the locale of citrate use. I would say it is testable in animals and a nice insight. As for cluttering the site, no you are not. A large number of physicians and scientists lurk on this site – I know that – and this kind of commentary may get a few to come out of the shadows and say something. Warmest regards, Fred
Dear Dr. Coe, I’ve enjoyed reading your article and responses to the comments made.
The paternal side of my family has a genetic condition called M.E.N.1. I’ve recently undergone my third parathyroid surgery in a final attempt to stop my production of kidney stones. My first surgery was in my early 20s, and my second surgery was 14 years ago during my second trimester with my second daughter to prevent her from developing rickets in utero. I experienced many years of lithotripsy, ‘scooping’, suffering the passings, and once, percutaneous nephrolithotomy removal of one stone nearly 2 cms. in size. The most recent renal calculus analysis reported major component Calcium Phosphate and minor component Calcium oxalate. Currently my blood calcium is within normal range, but my urine calcium 24h (8.4 memol/d) and PTH (13.5 pom/L) are both still high.
My endocrinologist advises against a fourth surgery of the known remaining small piece of the parathyroid gland that was relocated to my inside forearm in the last surgery. Instead, in conjunction with advice from my urologist, he’s prescribed a combination of 25mEq potassium citrate dissolving tablets x2/day, and 25mg Teva-Hydrochlorothiazide x1/day.
My question is, given your advice that although potassium citrate is as yet an unproven treatment against calcium phosphate stones, do you agree this is still the best course of treatment?
We have found, not rarely, persistent hypercalciuria after successful parathyroid surgery. So the treatment with a thiazide seems a good idea. I do not like hydrochlorothiazide once a day as that has never been tested in a trial only twice a day. A long acting thiazide type drug as mentioned in the linked article is better. Thiazide will not work well without careful diet sodium reduction to 65 – 80 mEq/day, which you should do. As for calcium phosphate stones you are right, there are no trials and I would shun it in favor of low sodium + thiazide. Of course, very high fluids are critical for you, over 2.5 liters daily and consistent. Regards, Fred Coe
Dr. Coe I am so happy to have found your site. And here I am, another stone sufferer seeking advice.
I make recurring Uric stones… They are generally small, but constant every few weeks. If I do allow myself to dehydrate, they tend to be bigger. I have drastically cut down on my protein, but it doesn’t seem to help much. I cannot take Urocit-K or any potassium pills because I also have PVC arrhythmias (controlled with Atenolol) and any time I’ve tried the pills, my arrhythmia goes wild. To add to the mix, I also take Byetta which has a desired affect of delayed stomach emptying, but that also affects other meds I take, they’re delayed too.
Do you think the Crystal Light “therapy” will help? Do you have any other advice you can offer?
Thank you so much in advance!
Hi Jennifer; as it happens I just finished my article on uric acid stones, not yet even fully cited in the pages. Any alkali at all will do more or less the same things, and you can read about the whole matter. The article on price of potassium citrate offers a lot of alternatives. You are forming more stones because your alkali intake is too low and your urine pH is also low. Check out the articles and come back if you are not cured. Uric acid stones never have to occur at all. Regards, Fred Coe
Dr. Coe, for the first time in years, I actually feel hope! Thank you.
I read your article on uric stones (sad that such a pretty crystal causes such havoc!) and much of all the others cited here.
I’m going to give Crystal Light a try. I’ve decided on 17 oz / 4x a day, with each meal and before bed. I’m hoping that will spread things out, and help keep an even level.
I have one more challenge ahead, I’m leaving for Tanzania on a 12 day Safari next week, where water is scarce and bathrooms are scarcer ; ) I will bring the travel sticks of powered Crystal Light and hopefully keep to my plan!
I would like to add for anyone trying Crystal Light as their main source, to use a straw when drinking and rinse your mouth out with water after you finish. All the citric acid can damage your tooth enamel.
Dear Jennifer, I am glad you read the article. Be sure your urine pH is high enough. I believe you can purchase urine pH paper at drug stores and check it from time to time yourself. I did not include that in the article but should have. Water will be scarce on your trip so pH is everything. Be careful. Crystal light has a high content of citrate meaning that the citric acid is not in its acid but in its salt form so it has a higher pH and should not disturb teeth. Best, Fred Coe
Hello,
I have a case of encrusted cystitis following mitomycin instillation to treat urothelial carcinoma, crystals composed of calcium phosphate. Did urine and bloodwork with a Nephrologist (largely unremarkable), urine PH normal, but the Nephrologist has prescribed a rather high dose of potassium citrate. Isn’t this backwards? Is it time to seek a second opinion?
Hi Kirt, The crystals are forming because the bladder lining cells have lost their normal coating and protections against them, so the problem is like kidney stones but not the same. Urine is generally supersaturated, so crystallization is prone to occur whenever surface defenses give way. The high dose of citrate will raise pH and therefore calcium phosphate stone forming propensity. If it raises urine citrate appreciably the citrate is a potent crystal poison, and can reduce crystallization. So the effects will offset each other. The best strategy is very high fluid volumes to dilute everything and the potassium citrate in hopes of an increase excretion and therefore concentration for the given volume. Since the amount of calcium excreted should be as low as possible, low sodium diet or even thiazide is ideal with a goal of a CaP supersaturation below 1 despite the high pH. Followup urines should be scrutinized for the final concentration of citrate vs. supersaturation with calcium phosphate, the higher that ratio the better. SOmetimes only the pH goes up, and the citrate is futile. Sometimes higher doses raise pH but not citrate so the increases above conventional dosages do no good. IN other words you need some trial and error to find your best balance. Regards, Fred Coe
This is an email dialogue between Drs Ronald Kallen, me, and Orson Moe that occurred at the end of December 2015. It alludes to some very interesting matters concerning citrate handling and both renal and intestinal cells. With their permission I have placed it here for general interest. Fred Coe
Hi Fred,
I am revising a book chapter on RTA and wanted to say something about how ingested preformed citrate alkalinizes the urine, since it seemed that citrate would be absorbed and metabolized to CO2 and H2O by enterocyte mitochondria. Anyway, your digression into the wiles of the Tudors was an unexpected intro to my exploration of citrate.
In reply to RON KALLEN.
Hi Ron, A rather elegant thesis, and perhaps true. Is it original with you? Any mitochondria will do, I suppose, and why not there? If asked I would always point to the liver because it is so big and always doing things. The Tudors have a bad rep because so vicious, but there was Gloriana, and for all her ways, she kept England out of war, made the people a wealthy nation, and founded one of the great ages of English letters and music – Shakespeare, Dowland, Donne. All the best, Fred
Fred,
Your commentary above is illuminating about the wiles of citrate and its salubrious effects on human health, if not kidney health.
Assuming that ingested citrate is absorbed in an intestinal segment by an apical transporter (NaDC1 ?) of an enterocyte, and that the citrate is metabolized therein (mitochondria) completely such that no citrate exits the enterocyte and flows northward in the portal stream, how then is the ingested citrate transduced into an increase in urinary citrate?
Best, Ron
Hi Ron, Here is what must always be the case so far as I can tell. Citrates are used in the Krebs cycle as citric acid, and at the end of the cycle we have CO2 and water. So whether in the liver or enterocyte, protons have to be taken out of the blood as citrate is taken up into the cycle as citric acid. The removal of a proton from blood produces a bicarbonate molecule because of the CO2 buffer system being predominant and that new bicarbonate signals kidney cells as I reviewed in the article. Your point is really interesting – that intestinal cells could be the locale of citrate use. I would say it is testable in animals and a nice insight. As for cluttering the site, no you are not. A large number of physicians and scientists lurk on this site – I know that – and this kind of commentary may get a few to come out of the shadows and say something. Warmest regards, Fred
I did not want to burden your blog and elegant review of the salubrious effect of K Citrate. Of course, I don’t mean ‘climate change’ as in the recent Paris meeting but the climate change of the internal environment that makes stones or breaks stones. I have been puzzled for a long time as to how orally administered citrate ‘transduces’ to increased urinary citrate. I have sketched a schematic that attempts to integrate some of this (which I can send separately). But one paradox that crops up is: if increased urinary citrate as a consequence of acidity sensing (Pyk-2 sensor, ET-1, etc cascade ?) after citrate administration dials down proximal tubule NaDC1 citrate transport, then it should do the same for the intestinal NaDC1, assuming that the enterocyte also senses a pH change somewhere (extracellular or intracellular). The paradox is that this would also dial down intestinal absorption of citrate via NaDC1 and thus vitiating the intended effect of the administered citrate (if I have this right or else I am totally bonkers), thus recapitulating the usual scenario of the Chicago Bears: third down, no gain, forced to punt.
All these considerations have come up as I revise a book chapter on RTA. Moreover, I have a stone-forming patient with presumed RTA, who I have followed for about 10 years. Despite K Citrate, the urinary citrate remains phenomenally low. He has had many measurements at Litholink and I have had an email exchange with John Asplin about this conundrum.
Dear Ron, these ideas are really interesting. I do not know if enterocytes are known to metabolize citrate taken up from the bowel lumen, nor if their transporter is regulated by blood pH or bicarbonate. I am copying Orson Moe who probably does know, as one expert can save us a lot of work.
From Orson Moe:
Fred, Ronald:
You raised some very important points. I will try to answer as bullets.
• The study of citrate transport in the gut is very sparse and somewhat due to technical hurdles. As a result, we know very little about the route and even less the regulation of intestinal citrate absorption. The presence of NaDC-1 transcript per se does not prove they mediate transport. No one has put the intestine on Ussing chambers or make intestinal BBM vesicles from the NaDC-1 null mouse to measure flux. We always assumed complete absorption because in normal individuals in steady state zero balance, the ingestion mEq is the same as the mEq in urine but mechanisms of transport is unknown.
• Whole animal studies of intestinal citrate are hard. Isotope flux is impossible to interpret because all 6 carbons on citrate will become CO2 and get exhaled as labeled CO2. Chemically, any unabsorbed citrate will get consumed by colonic bacteria so stool measurements are always very low and does not reflect absorption. The definite assays are Ussing chamber ex vivo and portal and hepatic vein sampling in vivo after small bowel luminal bolus. Even in these conditions, interpretation of flux is confounded by cellular metabolism.
• The mechanism of citraturia from oral citrate ingestion in my view is purely *at least mostly) due to sensing of a base load in the liver, which then signals the kidney to release base in the urine. Since one cannot afford bicarbonaturia on a regular basis due to risk of calcium phosphate precipitation, citraturia is the best way to excrete base in urine. Some have suggested years ago that some absorbed citrate “escapes” into the urine using citrate acid (acid-base neutral molecule) as a test molecule. I personally do not think that is true; or at least very low in magnitude. If ingested citrate does not undergo complete (or at least near complete) hepatic metabolism, it will be fatal. A nice bolus of breakfast orange juice in the morning will beget fatal hypocaclemia for the heart as the blood traverse the hepatic vein to the SVC and right heart. Only a massive oral dose (accidental poisoning) of citrate can overwhelm the liver and create systemic low ionized calcium. When we give it in a central vein in CRRT, we bypass the liver and we can often see low ionized calcium.
• I think there are generic pH sensing mechanisms that all cells use for cell pH defense. The renal epithelia is special as it defends systemic acid-base and not just the cell itself. The Preisig-Alpern cascades may not be operational in the intestine. i.e. I doubt acidosis will suppress NaDC in the gut. A good example is the Na/H exchange NHE3 which is present in the proximal tubule vs. the small and large intestine. This apical membrane transporter is identical but very few of the regulatory mechanisms are shared. Intestinal NHE3 has been studied in acidosis and there is no regulation. It will be easy to measure NaDC transport in BBMV, protein, and transcript in the gut with acid loading. I think it will be negative but has not been done to my knowledge.
• The existence of non-response to K Citrate in RTA patients is very real. We have collected quite a few of these patients in Dallas. Typically, their UKV will increase by 60 mEq/day (as prescribed) but the Ucitrate V increases by perhaps 10 mEq/Day. The increase in urinary anion appears to be a plethora of other anions including loss of chloride. We cannot measure stool citrate to interrogate lack of absorption. None of the rodent genetic models of RTA has this phenomenon. In the acquired “pan RTA” model induced by Topiramate, there is a discrepancy of delta UKV vs. delta UcitrateV but nowhere close to the non-responding patients we see; so I have not jumped on this model to investigate citrate non response. This can stem from a base deficit in the bone from chronic acidosis (absorbed from the gut but not end up in the urine; a form of “pay-back”) but quantitatively, we cannot account for the >60 mEq base gain/day by the bone for months or even years (not enough bone). We may push the topiramate rat model harder and do some portal and hepatic vein sampling (easy in a Sprague-Dawley) but I need some motivated individual. In short, our docs (many) have seen this over and over and it represents a physiologic conundrum and a real problem in clinical practice.
ORSON – WHY DOES CHLORIDE RISE WHEN THESE ODD PATIENTS GET K CITRATE?
Good point, Fred. Electrical neutrality mandates some form of anion. Chloride happens to be most abundant. If there an intestinal citrate absorption defect, perhaps the K is absorbed with Cl (e.g. K/H exchange in parallel with Cl/HCO3 exchange). I am just taking liberty to speculate. If the 60-90 mEq of prescribed citrate enters, the same quantity of base has to go the the bone or the urine.
• The existence of K Citrate non-responders is very real. We have collected quite a few of these patients in Dallas between the mineral clinic and urology. Typically, their UKV will increase by 90 mEq/day (as prescribed) but the Ucitrate V increases by perhaps 10 mEq/Day. The increase in urinary anion appears to be mostly Cl plus a plethora of other anions. As mentioned above, we cannot measure stool citrate to interrogate lack of absorption. None of the rodent genetic models of RTA has this phenomenon. In the acquired pan RTA model induced by Topiramate, there is a discrepancy of UKV vs. UcitrateV but nowhere close to the non-responding patients we see, so I have not jumped on this model to investigate citrate non response. We looked at whether this can stem from a base deficit in the bone (absorbed from the gut but not end up in the urine) from chronic acidosis but quantitatively, we cannot account for the 60-80 mEq base gain/day by the bone for months or even years (not enough bone). I ran this by the ultimate master of acid-base (in my opinion), Mitch Halperin, and Mitch has not been able to help me solve this problem. We may push the topiramate rat model harder and do some portal and hepatic vein sampling (easy in a Sprague-Dawley) but I need some motivated individual. In short, our docs (many) have seen this over and over and it represents a physiologic conundrum and a real problem in clinical practice.
After a 1 hour spin class I take 30 meq kcitrate and a alkaline smoothie and my urine oh still stays 5.5 ph
If I don’t workout it goes to 6.5
Is recovery that long?
Hi Dwight, I imagine you are seeing the well known effect of low urine flow to lower urine pH. If you are a stone former, consider that during your workout you might need additional fluids. Regards, Fred Coe
My doctor prescribed this:
http://medlibrary.org/lib/rx/meds/cytra-k-crystals/
It is a little cheaper than the pills. I have low urine citrate and low pH. Will it work as good as the pills? I noticed the cytra has alot of citric acid. Is that bad?
Hi Ron, It is the same as other K Citrat4, the citric acid is not a problem. Just watch the dose and be sure it conforms to what was prescribed. Potassium is safe so long as the dose is right. Fred Coe
Thank you
Do you still have an office in burr ridge
I would like a consult
What number should I call
Hi Dwight, I was never in Burr Ridge, the University of Chicago is my home and it is in Hyde Park, Chicago. I am not sure how the Burr Ridge address got on the web, perhaps it is that the University billing group is situated there. My secretary Kathleen is at 773 702 1475. Regards, Fred Coe
Hi, I’m going to be having my 5th ESWL done within the last 20 years. Fragments that are left continue to grow, so I’m not producing new stones. Calcium oxalate. I’ve had various work ups during the years and I have high urinary oxalate and low ph. I take Theralith xr but I also take Lisinopril and Hydrochlorothiazide. One depletes and the other stores potassium. So, the Theralith daily supplies 198 mg of potassium citrate along with magnesium and b6. I have recently started drinking lemon water, adapted a low sodium diet, cut down on protein and cut down on high oxalate foods. Should I consider taking prescription potassium citrate? Also, I read that IP6 is a good supplement to take for kidney stones. Do you do phone consults? I live right outside NYC.
Dear Michelle, If I may be forgiven for commenting from a distance, you may have had enough SWL procedures and benefit from ureteroscopy as an alternative. You might want to discuss this with your personal physicians. Growth of retained fragments is commonplace and fostered by the templating effects of crystals in fragments to support growth of more crystal mass. I wonder how high the urine oxalate is; commonly values for ‘high’ are 45 – 60. The potassium citrate dose is too low to influence potassium stores or affect stone formation. Inositol hexaphosphate is proposed for cancer and dementia and for all I know as a means for promoting world peace. The scientific foundations are not known to me and I am sceptical. For stones, no evidence. I can speak with you by phone but to make it worthwhile I would need to see your medical records. My secretary Kathleen is at 773 702 1475. Regards, Fred Coe
Hello, Dr. I’ve been taking Potassium Citrate for a week and a half now. My nephro advised me to drink 6 tablets per day, 2tabs each meal. Do you think the potassium citrate is already taking effect? I’m not feeling anything at all while taking this medicine.
Hi Afeena, The only way to tell is to repeat your 24 hour urine and see if the effects of potassium citrate are present. You cannot otherwise tell. It is worthwhile to do such testing exactly for that reason. Regards, Fred Coe
An alkaline pH favors the crystallization of calcium- and phosphate-containing stones, whereas and acidic urine pH promotes uric acid or cystine stones
http://www.ncbi.nlm.nih.gov/pubmed/21170875
So don’t take citrate for calcium oxalate stones?
Hi Jason, You are a thoughtful commentator. Calcium oxalate stones are indifferent to pH, but the increase of urine citrate from potassium citrate helps in prevention: Citrate binds calcium which lowers supersaturation, and citrate binds to crystal surfaces and reduces their growth. Thus far no one has linked potassium citrate use to calcium phosphate stones. Whether potassium citrate is a good treatment for calcium phosphate stones is unknown. It will reduce stone risk to the extent that urine citrate rises, but it will raise urine pH and calcium phosphate supersaturation, so I am not in favor of its use for calcium phosphate stones. For uric acid stones it is basically a cure. Regards, Fred Coe
I’m trying to understand all of this in better detail. 1st what is it that causes your body not to produce Potassium Citrate? And Magnesium? Other than producing additional kidney stones, what are the other real risk of not talking ir? Are there any further concerns with using the crystals through a feeding tube ? Any help and answers you are able to provide I would really appreciate.
Hi Kay, Low urine citrate has more than a few causes, and I am sorry I have not written an article on this matter as yet. The common reason may be heredity. Other reasons are bowel diseases and potassium depletion. Low urine magnesium is due to bowel disease, or other diseases and is not a part of common stone disease. Feeding tubes are never used for anything except when there is severe bowel disease or in hospitalized patients. In people with calcium oxalate stones citrate can reduce new stones. The reasons are a in the article. Regards, Fred Coe
I notice the Potasium Citrate pills in my stool several times a month from normal observation.
I suspect that is a small fraction of what gets passed. Is there something I can eat or drink when I take them to get them to start dissolving? What’s the time-release glue? I know it’s not recommended, but what will happen if I break it in half? thirds? quarters? before swallowing?
Hi Dave, What you see is the wax matrix. The active agent is almost always eluted. You can tell from your labs. Take a look. Regards, Fred Coe
Hi Fred,
Could you please take my last name off the previous comment. I made a mistake thinking it would be redacted automatically.
Hi Dave, Done. Best, Fred
Dear Dr. Coe, I have been suffering from Ca oxalate stones until age 50, where I was diagnosed with renal carcinoma (clear cell type). Subsequently I had partial nephrectomy surgery. After surgery I continued to grow kidney stones, no longer oxalate stones but predominantly uric acid stones. My doctor prescribed a combination of potassium citrate and Allopurinol in an effort to “dissolve” remaining kidney stones and to keep uric acid low (especially after I had my first gout attack). My question to you would be, is it really possible to completely dissolve existing urate stones inside the kidneys, based on a dose of 2x 10MEq and 100mg Allopurinol daily? I had passed horribly big yellow urate stones (around 12mm) and they are growing into this size in about one year. During passage they may have damaged some of the kidney function, and if so would that have to do with the sudden production of these kind of stones? I just wonder, if that K-Citra is going to work as intended. Keeping up my hopes…
Thanks
Lutz
Hi Lutz, Uric acid stones are caused by low urine pH in a mechanical – chemical actually – manner and prevented by raising the pH to about 6. You are taking a marginal dose of potassium citrate and perhaps new stones are forming because the pH is still too low. Allopurinol will stave off gout but has almost no benefit for uric acid stones. Here is what to do. Check out your 24 hour lab reports for uric acid stone risk. If your urine pH is indeed 6 on a 24 hour urine average, perhaps there are troughs – get some urine pH paper and check voidings during the day and overnight. After doing your own work, see your physician, show your results and get input about the best next step. I have written and stand behind this: Recurrent uric acid stones are virtually always preventable. As for dissolving stones, the odds are poor. They grow from small to large so the surface to volume ratio falls a lot. They shrink from outside in. You can try. I will wager you need more alkali but check first, as I have said. Regards, Fred Coe
I might have missed something in this article…
My question is..
I was put on 5000mg of Vitamin D and within 2 months developed 3 kidney stones, I never was bothered with this before, (I am 62)
My urologist told me D is the worst.
I still have one in the kidney but he feels it will not come up and through the utterers because of its location. He is waiting for it to grow in size so he can blast it.
He put me in touch with a kidney doctor who has me on 12.5 mg water pill..
He said this will be my life from now on.
I have read your article and I am hoping that this potassium citrate will keep stones at bay.
I have also read an article from Dr. Peterson, who claims magnesium and the citrate will dissolve or flatten the stones?
Can you please explain, in laymen’s terms?
Thank you,,,
Hi Mark, Although the vitamin D could have worsened things, it is likely that there was always something lurking and the vitamin simply exaggerated it. My guess is you have genetic hypercalciuria. The proper approach is 24 hour urine testing with appropriate fasting bloods which perhaps your physician has already obtained. If so, take a look. Be sure your evaluation has been orderly and complete. The diuretic at 12.5 mg daily is usually chlorthalidone or hydrochlorothiazide, and it is in treatment of hypercalciuria. Magnesium has never had a successful trial, I do not know why anyone touts it, and I do not know the article by Dr Patterson unless you mean this one:. It is a review of Canadian guidelines and I see no mention of magnesium as a prefered treatment. I do see the need for proper evaluation and I hope you have had such. Potassium citrate is a valuable treatment for prevention, as noted, if stones are calcium oxalate; for calcium phosphate trial data are limited. Citrate nor magnesium will flatten or dissolve calcium stones. Regards, Fred Coe
Thank you for your response .
The article was Dr. Dale Peterson, Wellness Clubs Of America.
Yes, I have had all the urine testing and blood work done.
I just don’t want to hear in a year or two that I have kidney failure from the diuretics, which has happened to a few people that I know. So I guess what you are saying is, the only thing for me, is the diuretics. Very sorry to hear this.. Again, I thank you for your time.
Hi Mark, If you have idiopathic hypercalciuria as a proposed cause of stones, low sodium diet with high diet calcium – for your bones, and high fluids may well be enough. Chlorthalidone in the dose mentioned has millions of patient uses as it is a first line antihypertensive. Allergic reactions are very rare and reverse on drug cessation. The drug has no known danger to produce kidney failure. Think about prevention in a larger context than just one drug – it is a low more flexible than that, and also do not be alarmed by a drug so old and so much used as this one. Regards, Fred Coe
Mark, …I’m a 68 year old male. After six surgeries in the ’90s, I had constant, continual, calcium kidney stones. I tried the Coke/asparagus technique – it worked, but…… Then, by chance I started drinking coconut water – WOW!….a year now – no stones. I am drinking ‘only’ 4 oz every-other-day. Do not “over-do” this… Good Luck
I forget to mention,, my stones are _”calcium”…..
I forget to mention, they are calcium….
my urine cit rate number is 360 and my ph 5.674 ss uric acid is 1.44 should i be taking potassium cit rate i have had 20 + stones in my life 3 surgery finally did a urine 24 hour test what will prevent the stones
Hi Charles, I cannot say if it is the right treatment – in principle – without knowing the stones you form. If they are uric acid, there is no question. If they are calcium oxalate it can be fine, but there are other complexities like the level of urine calcium, and sodium. If they are calcium phosphate, there is not enough trial data to support potassium citrate as a treatment: It may be good, or not. So you have had the tests, but need to know the stones, or guess what they were. Here is a way to think about the test results. Regards, Fred
I did 2 24 hr tests and the end result was my citrate levels were 220 and my production was well under at 980 and 1200. My doc suggested I take citrate medications and increase fluid intake but has not confirmed what I should do about the stones I have. I had 17 total last count after passing some and gaining others. I started out with around 14. Passing them is real painful and I was told the ones I have now is around 6 – 7 mm. The doc hasn’t said what I should do about the current stones since they will not dissolve. They just keep asking well do the bother you. YES!! Thus an emergency room visit, taking off from work, and coming to see you. Any thoughts?
Hi Ldowna, It is a dilemma. If you can stop new stones, perhaps the most conservative approach is to let those left pass as opposed to having a procedure. There is no point in surgery if new stones are actively forming. So, I would suggest you use the medication and be sure new ones are not forming, take some time to be sure. Then decide if surgery is worthwhile for you. In the absence of obstruction, infection, or important bleeding, surgery for pain is a personal not a medical choice. Regards, Fred Coe
My urologist wants me to build up to 4 Potassium Citrate pills qd. The only thing I (with help from pharmacist) can find are time-released formulations that are not to be crushed or split. I CANNOT TAKE THESE LARGE PILLS. If I am to take up to 4 qd, can I crush them afterall and take them throughout the day? What alternative can you suggest? I am a 70-year-old white female and have been forming stones for 47 years. Thank you.
Hi Monica, There are 1/2 size 5 mEq pills, and they are smaller. Try the – need two to do the work of one large one. Regards, Fred Coe
I’m trying hard to understand all of this but, just learning and much of this is still over my head. I’m trying to find a combination of therapies rather than megadoses of potassium citrate (45-60MEq/day). If sodium bicarb is effective in the same manner, I wonder whether magnesium bicarbonate (as in magnesium water) or potassium bicarbonate (or more likely a combination of all three) would be as effective as potassium citrate in raising citric acid levels in urine output.
Hi Katherine, It is best to think about prevention in an orderly way. The trick is to figure out what is wrong and aim at that. It is futile to choose a treatment without a diagnosis, and diagnosis is almost always possible. Regards, Fred Coe
I happened upon this website and I am both impressed and befuddled at the complexity of the topic. I have a bachelor’s degree in aerospace engineering, but this is way over my head.
On June 13, 2016 I submitted a 24 hour urine collection to Quest Labs in order to determine the StoneRisk Diag Profile:
STONERISK(r) DIAG PROFILE
Result Date: 06/17/16
Analyte Result Value Ref. Range Units Out of Range
TOTAL URINE VOLUME 1.43 >2 L/day L
PH URINE 6.2 5.5-7.0
CALCIUM URINE 300 <250 mg/day H
OXALATE URINE 49 <45 mg/day H
URIC ACID URINE 789 320 mg/day
SODIUM URINE 151 <200 mEq/day
SULFATE URINE 28 <30 mmol/day
PHOSPHORUS URINE 1115 60 mg/day
AMMONIUM URINE 23 14-62 mEq/day
POTASSIUM URlNE 74 19-135 mEq/day
CREATININE URINE 1799 800-2000 mg/day
CALCIUM OXALATE 3.75 < 2.00 H
BRUSHITE 3.94 < 2.00 H
SODIUM URATE 4.19 < 2.00 H
STRUVITE 1.70 < 75.00
URIC ACID 1.52 < 2.00
THE PATIENT HAS: See Below
Hypercalciuria
Hyperoxaluria
Hyperuricosuria
Low urine volume
SUPERSATURATION INDEX: See Below
Calcium oxalate
Brushite (Ca phosphate)
Monosodium urate
SUSPECTED PROBLEM IS: See Below
Hypercalciuric Nephroithiasis
Hyperoxaluric Nephrolithiasis
Hyperuricosuric Nephrolithiasis
It confuses me that I have both high Uric Acid (an acid) and high Oxylate (an alkaline base) at the same time. Does this mean I could have had both Uric Acid Stones and Calcium Oxylate stones at the same time???
On July 7, 2016 I had my bladder stones (infected with enterococcus faecalis) removed with a laser procedure. My surgeon and a different surgeon I saw six months before told me the same story. They promised that after the stones were pulverized, all debris will be removed and theta there would be no pain before or after surgery. THEY BOTH LIED BIGTIME. OUCH. Over the course of 48 hours from the time of the surgery, I passed small crystals with considerable pain. Now, I want to know how to prevent the formation of future stones.
What kind of stones were removed? The lab has not yet come back with the results. I will will return here to let you know. In the meanwhile, the surgeon has put me on Amoxicillin 2x/day, Potassium Citrate 1080mg 2x/day, Uroxatral 10mg 1x/day, Doc-Q-Lace 100mg 3x/day as needed. I'm also taking two 480mg capsules of Marshmallow Root 2x/day to soothe my urethra.
I've taken the Potassium Citrate from the evening of July 7 until today and my urine is still acidic Ph 5.0. I will now begin to try to give up meat, dairy and eggs and sugar as best I can. I think this will help.
I will keep you updated on my experience and the lab tests.
I will set up a new email account in order to answer anybody who has questions about the procedure and/or my experience. I will post the email address as soon as I get it set up.
Hi Joe, Your results suggest you have idiopathic hypercalciuri causing the high urine calcium and also a high urine oxalate excretion which could be from high oxalate diet or low calcium diet or both. That the stones were bladder stones is a bit unusual and suggests perhaps bladder outflow obstruction (prostate??); you did not say if there are kidney stones. The approach to treatment is to reduce your urine supersaturation with respect to the crystals in your stones and we do not know those crystals as yet, so let us know. The infection is an additional issue and I presume your urologist is treating it. It points to bladder outflow obstruction, as do the bladder stones. Regards, Fred coe
It is now 1:30 pm. About 20 minutes ago I was pissing razor blades with bloody discharge at the end– the pain was horrible– but I could not pass the stone (if there is a stone in there). My surgeon called me back right away after I left a message. He told me to increase my Potassium Citrate to 6 (1080mg) pills a day divided into 3 doses. He also told me to take AZO to soothe the irritation in the urethra. He said he was very sure he got everything out. He suggested he can do a cystoscopy in the office to check if there is a particle there but he said most of his patients think there is a particle there but it is just irritation from the procedure.
I just sent my son to the drugstore to get the AZO.
I’ll add to this If I don’t pass out from the pain the next time I pee.
I am 81 years old & have passes kidney stones & have had urinary tract infections about every 5 months for the past 2 years. I have had no pain through all of this. I have a large prostate. My urologist in trying to figure this out has blasted my kidney stones a year ago &
just last Wednesday prescribed Potassium Chloride 20 meq (2 times per day. Since last Thursday I have been urinating on the hour each day and during my sleep at night and thus, not getting any sleep.
I took the pills for the past 4 days and at noon today, Aug., 8, 2016,
I have dizzy spells when I get up to walk; urinating once every 20 minutes (sometimes just a dribble); vomited once; I feel weak; drink water; heavy breathing. Tomorrow I go in for a CT scan of my bladder. Could the potassium pills have given me these complications? I’d appreciate your answers to this. Thank you.
Hi Anthony, It sounds like the prostate is a major issue in that retained bladder urine can lead to infections and bladder stones. But the kidney stones would be from some other cause; it is important to know what they are made of, and presumably that was determined by stone analysis on the fragments a year ago. I would have thought the pills were potassium citrate, not potassium chloride, but in any case vomiting weakness, and heavy breathing sound like serious symptoms which your physician should evaluate right away. I would not delay this. Regards, Fred Coe
My dr recently prescribed potassium citrate er 15 meq tb taken twice daily. After a couple years of frequency, urgency, burning, bladder spasms, visible pus in urine with elevated white cell count but no bladder infection found via culturing, I was finally diagnosed with cystitis cystica. I am 64 yoa. I had been two months on Doxycyclne, phenazopyridine, gabapentin, AZO, with no help to frequency or urgency until the potassium citrate was added. I came here trying to understand why after all this time of hourly urinating day and night, I am beginning to go less often. Slept 5 hours last night before needing to go. Although not a vegetarian, I have eaten mostly salads with some chicken at times for years because it is easier on my colon. I notice that most of your posters have stones and worse problems. I don’t pretend to understand much of your article but I read the whole thing and did pick out that vegetarians get their PH out of balance. Sounds like maybe I fall into that category?
Hi Crystal, Potassium citrate inhibits crystal formation, and crystals are often part of the bladder condition you refer to. I suspect that is why it is of some help. If it continues to work I see no reason to not use it. Be sure your physicians keep track of your blood potassium – I am sure they would. Regards, Fred Coe
Hi Dr. Coe,
Hope all is well with you. I started the traditional chlorthalidone and potassium citrate last year for Ca stones. I developed symptoms that looked like heartburn, apparently because of stomach irritation related to the potassium. So my doctor prescribed omeprazole.
To make a long story short, we found that I’m one of a handful of people for whom PPIs, especially rabeprazole in my case, cause hypokalemia – much more than the chlorthalidone alone. Weaning off the PPI is taking months. Taking more K citrate tablets only feeds the vicious cycle. Amiloride didn’t help significantly.
Enteric coated K citrate tablets would seem to make sense, but apparently are considered dangerous to the intestine.
So I have a very practical question…Do you have any thoughts on a way to get, say, 10 mEq of potassium citrate, or an equivalent, with the least risk of stomach irritation?
For example any idea whether dissolving the contents of ten or eleven OTC 99 mg (0.92 mEq) K citrate capsules in, say, 8 oz of water taken with a meal might be more or less irritating to the stomach than the usual 10 mEq extended release tablet?
Grateful for any help you might offer! Take care,
Al
Hi, Marked potassium loss with chlorthalidone usually means urine sodium is too high. Try to lower it to below 65 mEq (1500 mg) daily before using the CTD and add amiloride at the same time as the drug. That may lessen the need for potassium. You could use plain potassium chloride to replace potassium losses and these are slow release and also in liquid form. Regards, Fred Coe
Hi Dr. Coe,
Thank you for several options. Yes, I’m on a low salt diet.
I’ve found potassium chloride much more irritating. But potassium citrate remains an interesting option because it also provides beneficial citrate. You’ve mentioned Cytra-K, which is available as a liquid. They claim it is well tolerated.
Do you have any experience or ideas about whether drinking that in a glass of water or juice would tend to be more or less irritating than the same dose as an extended release tablet? Best regards,
Al
Hi Al, The best way to negotiate these details of medication use is to work with your pharmacist and physician to find what works for you. I am too far from the action to be a safe or useful guide. Regards, Fred Coe
Hi Dr. Coe,
Thank you for your kind help! Just to close the loop for anyone interested:
We have not been able to find any research. However, they all agreed that, in their opinion, the ER tablet seemed to be as good as it is going to get. That would slowly release the potassium over long as 8 hours. One could try sipping liquid all day, but it would be hard to spread it out more than the ER tablet would. Factors beyond that would involve pure speculation. Best regards,
Al
Thanks, Al. Fred
Here in Europe one can get potassium citrate capsules (no extended/slow release).
I myself take them for potassium loss by hydrochlorothiazid (diuretic). I take 200 mg capsules, 5 times a day, after food and with a full glass of water – no stomach problems. And I use ranitidine, which is much better than PPI’s in general (eg safer for the heart).
For uric acid stones prevention (together with diet): potassium citrate 500 mg, 3 times a day (combined with sodium bicarbonate 500 mg 4 times a day) is given here in the Netherlands.
Needed to put you the little note to finally give many thanks yet again for the precious things you’ve featured on this site. It was really particularly generous with people like you to grant freely what many individuals could have distributed for an ebook to earn some dough on their own, primarily seeing that you might well have done it if you ever wanted. The advice in addition worked to be a good way to fully grasp that most people have the same fervor just as my personal own to figure out a good deal more in regard to this matter. I am certain there are lots of more enjoyable situations in the future for those who look over your site.
testing the speed of the site
Dear Dr. Coe,
Potassium citrate is given to prevent or even dissolve some type sof stones that grow in an acid environment.
Now, I would like to ask you: When you make the urine neutral or alkali by taking a potassiam citrate supplement – could that cause other types of stone to form (those that grow in an alkali environment)? And if so, could that already happen when one takes 20-30 mEq long term?
Hi Leon, Calcium phosphate stones are favored by high pH but if urine citrate rises it inhibits such crystals. The question of potassium citrate and calcium phosphate stones is an open research issue and needs a trial. Regards, Fred Coe
Thank you very much for your reply, Dr. Coe, that is reassuring.
I have one more question Dr. Coe:
I myself am taking potassium citrate not to prevent kidney stones but for potassium loss by hydrochlorothiazid and because I (like most people) get too little dietary potassium (at least not the recommended 4700 mg).
I would like to ask: Did you ever examine possible negative consequences of getting so much citrate? Is anything known? For example, could it raise the risk for some types of cancer?
Hi Leon, citrate is metabolized as citric acid which is why it is an alkali, and urine citrate rises because of the alkali so the production of alkali from the oral citrate is balanced by a loss of urine citrate – more or less null outcome after a few days. Citrate itself being metabilized does not rise in blood. It is a central metabolite in the Krebs cycle = citric acid cycle. SO, no. Regards, Fred Coe
Again, thank you very much for your reassuring reply!
I was considering supplements for hypertension and came across citrulline and arginine but then found out that they might stimulate some types of cancer, so I was wondering about the citrate – I didn’t know it doesn’t rise in blood – contrary to amino acids of course.
Hi, Let me be clear; blood citrate may rise, slightly, with citrate dosing, but there are no links to cancer. As for the other two molecules you mention, I would be sceptical of real links as well – but they are not my focus. Regards, Fred Coe
Hi, I think there is still a lot of research to be done, but there is some reason to suppose citrulline and arginine in (excessive) dosis might stimulate (not cause) the growth of some cancers:
Citrulline and arginine increase the growth of the Ward colon tumor in parenterally fed rats.
https://www.ncbi.nlm.nih.gov/pubmed/8844725
Arginine dependence of tumor cells: targeting a chink in cancer’s armor
https://www.ncbi.nlm.nih.gov/pubmed/27109103
I worry much less about citrate and I think the benefits of additional amounts of potassium by far outweigh the minute (if any) and unproven/undiscovered risk of citrate. Judging from the research I saw, additional potassium for most people might prevent many CVD, strokes and MI and might be far superior to, for example, statins. But of course, potassium cannot be patented and there is nothing to gain for the pharmaceuticval industry…
Thank you again for your help – I’m sure your patients must be very happy with you.
Kind regards, Leon
My husband had stage three kidney disease (40). Dr prescribed potassium citrate 1080, 2 pills twice a day. Two months, now had blood test now stage four (25) My pharmacist says it’s the pot cit. Dr says no, we’re afraid?
Hi Bernadette, I presume your physician prescribed potassium citrate for stone prevention and that your husband has kidney disease whose cause is known. The potassium citrate should not of itself altered kidney function. I would speak with your physician about whether a nephrologist might be useful here as a consultant. Use of potassium supplements in someone with reduced kidney function is best reviewed by such a specialist. Regards, Fred Coe
I have a very large existing stone that has firmly lodged in my kidney. it is KUB monitored every 6 months and hasn’t moved for years. If it should move I would have limited options, as I have had surgical removal of a stone before and lithotripsy stone-breaking would simply create an unmanageable number of smaller chunks.
I have been prescribed, and taking, 1080 mg tabs 2/x day Potassium citrate the past two years as a way to help prevent new stone formation.
I am now experiencing consistent lower back pack pain and regular spasms for the past two months. Chiropractic attempts seem to rule out standard muscle strain.
My question: Do P-C pills “eat away” at existing stones enough to dislodge them?
Hi Bill, the only stones I know for sure will dissolve are uric acid. You have not said what your stones are made of. As for shock wave lithotripsy I would generally prefer ureteroscopic procedures unless the stone is very large. If very large one might need percutaneous removal. I would find out the composition of past stones, and ask if the present stone might be uric acid based on its radiographic density – measurable on your CT scans if any have been done. If it is not uric acid, you might want to be evaluated for factors in urine that have promoted stones, because more can always form. Regards, Fred Coe
Hello Dr. Coe,
What a fascinating, informative read. Truly one of the most insightful things I’ve read so far in my never ending search for relief. I’m a 34 year old male of Asian decent. For the past 13 months now, I’ve had to be rushed to the ER 3 times (give or take 4 months apart) for a kidney stone. Every single time it’s been anywhere from 4-6 mm calcium oxalate stones that are so jagged and sharp. The pain has been extreme each time, and it’s always the same routine. Morphine in ER, IV drips, cat scans, then I’m sent home with a prescription of flomax, Percocet and anti-nausea pills. I drink up to one gallon of water daily. I avoid salty and fatty foods. I only take a multivitamin. I don’t know what to do. Last week was the third time I was rushed to the ER. Please help. I feel like I’m out of options.
Hi Karlo, It sounds like recurrent calcium oxalate stones and no prevention. You need a program approach, and here is my choice. This article pulls together the other articles about how to be evaluated and how to use your physicians in the most efficient manner. It is truly time to do this. Stones are crystals, at heart, crystals obey physical laws, we know the laws, so it is just getting things done. Regards, Fred Coe
Thank you so much for taking the time to respond doctor. I just have to say that I’m very grateful for your continued work in trying to help people out there. You really are a hero and you’re making the world a better place just by being the compassionate you. I’ll read everything I can from your site and share what I can with fellow sufferers. My deepest gratitude kind sir.
Hello Dr. Coe – First, I want to thank you for such an informative blog. I really appreciate your time and effort in providing such authoritative information from a true expert in the field of kidney stones. I was researching kidney stones on the internet and there is so much junk and pseudoscience; it is re-assuring to have an authoritative medical source. I suffered my first kidney stone this past weekend(Male, age 41) and my left lower back hurt like the dickens for about 3 hours! I was in a lot of pain and I commiserated, albeit briefly, with my wife on child birth. That crazy woman, how could she do that twice! The ER doctor said it was probably a small stone passing from the kidney through the ureter into the bladder. He recommend lots of water, gave me filters so I could possibly catch the stone, and a followup with my primary car physician.
In researching kidney stones on the internet, citrate intake is recommend to reduce the formation of stones; but some websites actually seem to suggest that this will somehow “dissolve” the stones. Is this true? Does anything “dissolve” a stone? My lay understating is that citrate inhibits formation of new stones by binding with oxalates. Does citrate help with all types of kidney stones and does it matter if I’m using potassium citrate or if I’m ingesting citrate via fresh lemon juice. Thanks Doc! – Joe
Hi Jose, You are a wise reader. No, calcium stones do not dissolve, or if they do it is a rarity. Prevention begins with knowing what is the cause, and here is a good plan to find out. Here is an overview which includes the five steps and broader perspectives. The analysis of the stone is crucial – the problem is crystals and you need to know what they are in the stone. As for citrate, it is used when it should be used, and that is just sometimes, depending on what is wrong. It is often not the right thing, and as for lemon juice any and all vegetables and fruits are good potassium and citrate sources. Take a look at the real diet story. Regards, Fred Coe