 The title is my story’s conclusion.
The title is my story’s conclusion.
We know that calcium phosphate crystals in plaque, plugs, and the vast majority of stones are hydroxyapatite, yet when we measure supersaturation with respect to calcium phosphate we measure that for brushite, the pretty crystal in the big image that begins this work.
We do it because brushite crystals may initiate calcium oxalate stone disease, so it is wise to prevent their forming.
The beautiful image is a hand colored electron micrograph of brushite crystals by Kseniya Shuturminska. I have written about brushite on this site before, but this crystal is so important yet buried in that eerie world where atoms and molecules combine, it seemed to deserve a larger place – which I have now given it.
What Brushite Is
Put simply it is CaHPO4.2H2O, a calcium phosphate crystal. It forms more readily than hydroxyapatite or calcium oxalate.
In being a precursor form, brushite is similar to the ‘amorphous’ outer layers of bone mineral and to the first phase to deposit over plaque exposed to urine. Brushite can convert to hydroxyapatite. The work I focus on here shows it can nucleate calcium oxalate by losing surface calcium atoms to oxalate.
Brushite Comes First in Human Urine
As he added calcium to human urine, Charles Pak found the first crystals were brushite. Of 28 urine samples 25 contained only brushite crystals.
It was this observation that led him to work out the mathematics for calculating supersaturation with respect to brushite, and show that it was higher in stone formers than in normal subjects.
He predicted, correctly, that brushite could be a critical first step in formation of kidney stones.
Work many years later revealed exactly how brushite mediates between solution chemistry and stone crystal formation.
It is this work that I will now present to you.
Oxalate Dismantles Brushite Seeds
Brushite comes first, but oxalate can extract brushite calcium atoms to build calcium oxalate crystals in its stead.
The Constant Composition System
Solutions supersaturated with both brushite (1.2 fold) and calcium oxalate (1.8 fold) were kept at constant composition by a system of sensors and servo driven pumps that could replenish what was lost as crystals took up calcium, or oxalate, or phosphate. Because the pumps are regulated to keep solution concentrations constant, their rates of replenishment measure exactly uptake of calcium, phosphate, and oxalate by crystals as they form and grow. Put simply the pump rates measure crystal growth.
Brushite Pumps
One pair: Calcium and phosphate exactly calculated to make up for growth of brushite. As brushite grows it takes up equal amounts of calcium and phosphate from the solution. A calcium sensing electrode signals the calcium pump to add more. A pH electrode senses uptake of phosphate because, as brushite forms, HPO4= is selectively removed from solutions that contain it as well as H2PO4–. This drives the equilibrium: H2PO4– ↔ HPO4= + H+ to the right, so formation of brushite can be detected by a fall in solution pH.
We see dual signaling (calcium + pH) in the heavy curving line labeled “Brushite” on the figure just below. When that curve flattens, the electrodes are no longer signaling the calcium AND phosphate pumps: That means brushite is no longer growing.
Calcium Oxalate Monohydrate (COM) Pumps
One pair: Calcium and oxalate exactly calculated to make up for growth of calcium oxalate monohydrate (COM). As COM grows it takes up equal amounts of calcium and oxalate from the solution.
The sensors know to run this pair when the calcium electrode is signaling loss of calcium, and the pH electrode is signaling constant solution pH – calcium oxalate monohydrate formation does not alter pH of the solution.
We see single signaling (calcium + constant pH) in the thin curving line labeled “COM” on the figure just below.
I should note that the experimenters refer to calcium oxalate as the monohydrate. In fact they did not determine if it was the mono or the dihydrate but that does not matter to their results and I thought it best to use their nomenclature.
Likewise, I refer to pumps as in pairs, but that is only by way of explanation. There may be only one calcium pump to serve both the phosphate and oxalate pumps. It would not matter logically.
Dual Growth Conditions
When brushite and COM both grow, both sets of pumps run. The Brushite pumps replace calcium in exact proportion to phosphate (judged from pH change), the COM pumps replace calcium to keep solution calcium constant and add oxalate in exact proportion to it.
This ‘constant composition’ method was developed by George Nancollas, one of the authors.
Added Brushite Seeds Vanish into Calcium Oxalate
Evidence from Solution Chemistry
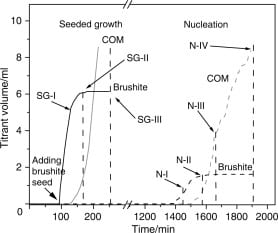
On the left side of the adjacent panel, brushite crystals were added at about 90 minutes (x axis), and as they grew titrant was added (the curve labeled “Brushite”) containing calcium and phosphate to keep the solution steady.
The volume of that solution is plotted along the vertical axis. Because it marks exactly how much calcium and phosphate must be added to keep the solution concentrations constant it is the exact rate at which the brushite seeds grew.
A sample of solids at SG-I contained ~40 mg, and – though not shown – would have had a molar ratio of calcium to phosphate of 1, that being the composition of brushite itself.
About 40 minutes later, the titrant solutions for calcium oxalate began (lighter curve labeled “COM”, calcium oxalate monohydrate) and rapidly rose as that for brushite fell to 0 – flat “Brushite” line.
The total solids at SG-II showed only 27 mg and the phosphate to calcium ratio was 0.72, consistent with the presence of calcium oxalate which has no phosphate. By the end of the experiment, SG-III, the solid material had a ratio of ~0, consistent with pure calcium oxalate.
Make no mistake. The sensors and pumps cannot be gotten around. Measurements shown in the paper prove the solution compositions were constant. So the “Brushite” and “COM” curves must mark growth of those crystals.
What stopped the brushite from growing?
Do not say the calcium oxalate took away so much calcium from the solution they could not grow. The pumps kept the solution calcium constant, phosphate, too.
The brushite crystals stopped taking up calcium and phosphate – the flattening of the brushite curve – because losses of calcium from their surfaces matched entry, so they could not grow and thereby donate protons to the solution and trigger the phosphate pump.
Evidence from Electron and Atomic Force Microscopy
Scanning electron microscopy (SEM) is intuitively obvious, like a light microscope but using electrons to get higher resolution – visualize smaller objects.
In panel a the solids harvested at SG-II in the picture above show brushite crystals (by SEM) roughening as they lose calcium and phosphate from their surfaces, and tiny calcium oxalate monohydrate crystals growing on them (Arrows mark COM crystals). Oxalate has taken the calcium off of brushite to make calcium oxalate. Deprived of their mates, phosphates drift into the water to live as roaming, solitary ions.
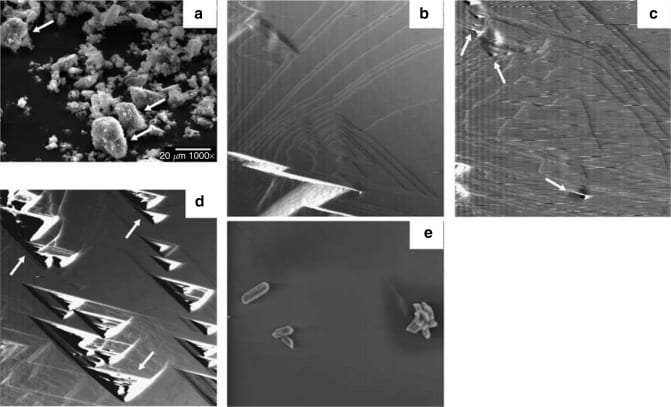 Atomic force microscopy is a lot wilder than EM. A very (very, very, very) tiny stylus sweeps over the crystal surface like a phonograph needle vibrating in the grooves of a vinyl LP record. In place of music encoded on vinyl, are the irregular planes calcium and phosphate make as they combine into brushite, or pits on those very same plates as calcium is pulled away by oxalate, and brushite dissolves.
Atomic force microscopy is a lot wilder than EM. A very (very, very, very) tiny stylus sweeps over the crystal surface like a phonograph needle vibrating in the grooves of a vinyl LP record. In place of music encoded on vinyl, are the irregular planes calcium and phosphate make as they combine into brushite, or pits on those very same plates as calcium is pulled away by oxalate, and brushite dissolves.
In panel b are normal growth plates of brushite. In panels c and d pits (arrows) form, and in panel e calcium oxalate monohydrate crystals lie on the brushite surface.
Just What Have We Been Shown?
This brilliant science demonstrates what we usually see with underwater photography.
Brushite crystals, fully formed in their intricate lacework of calcium atoms and phosphate ion molecules locked together by powerful electrostatic forces, are submerged in water teeming with a rapacious predator. Oxalate ions rip calcium atoms out of the brushite structure, tear it apart, leaving behind actual holes where tissue has been eaten away to make a different crystal, the one that plagues most stone formers – calcium oxalate.
How do we know?
The Solutions Tell Us
Look back, at the vertical axis on the figure we just reviewed.
The graphs alone are enough to say the brushite grew and then stopped growing, and calcium oxalate crystals began to grow (oxalate is the only other ligand in the solution).They imply but cannot by themselves prove beyond doubt that calcium oxalate nourished itself by eating the calcium from the fabric of the brushite crystal.
Microscopy Tells Us Oxalate Destroys Brushite
The scanning EM is pretty graphic.
Brushite seeds look woebegone. Their surfaces are all roughed up. And all over it are tiny calcium oxalate crystals, making themselves out of brushite calcium.
The atomic force microscopy is even more graphic, isn’t is? There are the pits, the holes where growth plates are eaten into.
How much more do you need?
COM Replaces Brushite as a Solid
As a final proof, harvested solids turn from brushite (phosphate:calcium ratio = 1) to calcium oxalate (phosphate:calcium ratio = 0).
What Happens in Kidneys?
Oh, you will say, this is lovely but no one sprinkles brushite seed crystals into kidneys, or ureters. The science is not germane to the reality of my patient, my kidney, my husband’s kidneys.
We have more.
Same Solutions, No Seeds

I have brought the same figure back here, so you can see it along with this text. Take a look at the right side of it, where ‘N-I’ is.
Solution Behavior
The solution was as in the left side, SS of 1.2 for brushite, 1.8 for calcium oxalate. Pumps were ready, electrodes measuring. Nothing happened for nearly 1400 minutes – that is about one day.
Then, the pumps turned on, the ones for brushite – pH was falling along with calcium. Brushite grew merrily but by 100 minutes you can see the COM pumps were also running. By N-II brushite growth was over, and COM was taking off.
It is the same as before except instead of adding brushite seeds you just wait. Brushite forms all by itself. Then oxalate takes its calciums away to make calcium oxalate.
Analyses of the Solids Formed
The solid phase at N-I were pure brushite by x ray diffraction. Their ratio of phosphate to calcium was 1. No doubt pure brushite. Just as in urine, brushite is the first crystal to appear on the scene.
By N-II, x ray diffraction showed brushite + COM, and the ratio was 0.67. By N-III, COM predominated with some brushite. The ratio was 0.16. By N-IV, pure COM by X-ray and the ratio was 0.
Brushite formed, oxalate ate it all up to form calcium oxalate.
Pictures Proving Cannibalization
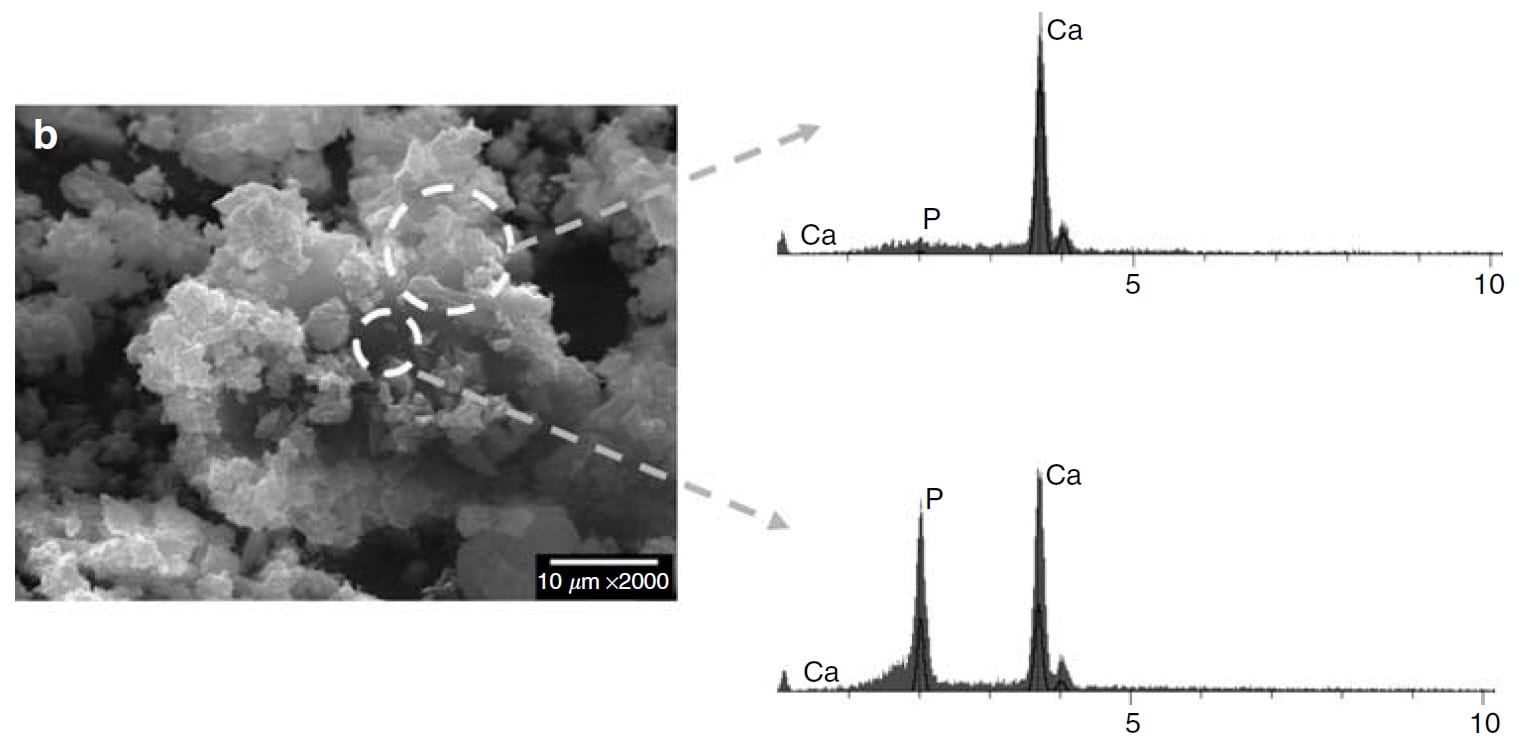
I have lavished much white space on this SEM picture.
Inside the larger ring is crystal without phosphate, just calcium. It is from the N-II solids that x ray diffraction proved was both brushite and COM. This portion is pure COM – no phosphate.
The inside core, within the smaller circle, is brushite. It has calcium and phosphate in nearly equal measure.
This is the perfect finish to the proof that oxalate attacks brushite from the outside, rips calcium off ifs surface to make itself, and gradually replaces the brushite, here reduced to a core.
You may wonder why there is no peak for oxalate. The reason is that its atoms (carbon, oxygen, and hydrogen) cannot be detected by this kind of analysis. So the partner to calcium is missing. Since there are no choices of partner except phosphate and oxalate, we can be sure the upper portion is indeed calcium oxalate.
What Does This Mean?
An Overview
It means what Charles Pak first envisioned.
As kidneys conserve water, their natural function, they concentrate calcium, phosphate, and oxalate, raising supersaturations for brushite and calcium oxalate. Brushite comes first, grows for a while. Then, oxalate begins to eat it up and make itself.
Brushite comes first because it is a more simple crystal, forms more readily. In fancy talk it has a lower energy of formation, needs less supersaturation, forms faster.
For patients and their physicians it means that urine brushite supersaturation is very important, perhaps most important of all. If it is below 1, the urine is deprived of a useful – perhaps essential pathway to calcium oxalate crystal formation.
What About Hydroxyapatite?
The phosphate in stones from the vast majority of calcium oxalate stone formers is not brushite, it is hydroxyapatite. So is the mineral in plaque, and tubule plugs. The story is not so different. Hydroxyapatite forms on brushite like calcium oxalate, and gradually eats it up, so all you have at the end in hydroxyapatite, no brushite. This is amply proven in experiments other than the one I made this article out of.
But to show you that work would make this article overly long.
What About Plaque as a Base for Calcium Oxalate Stones?
It is a different story. Plaque forms in kidneys. Kidneys make it.
When plaque is exposed to urine, urine calcium and phosphate ions create a very early phase of phosphate crystal over it, not exactly brushite but a crystal even less fully formed. Calcium oxalate grows over it, perhaps by oxalate pulling calcium out of the phosphate crystals. That has proven hard to study in the laboratory. Could calcium oxalate grow over plaque if brushite SS were well below 1? No one knows.
What About Plugs as a Base for Calcium Oxalate Stones?
Plugs form in the most terminal portions of collecting ducts, in a fluid virtually identical to the final urine. One can find calcium oxalate stones growing over them like with plaque. The details at the growth surface have not been studied as closely as for plaque.
How kidneys make plugs is not certain. I presume brushite forms and converts to hydroxyapatite, but that has not been shown directly. Surely brushite itself is scant, almost never found, but we have not looked for it in extremely microscopic amounts, either, especially in the smallest plugs.
Will tubules plug if brushite SS is kept well below 1? No one knows.
What About Stones Forming In Urine Like in the Reaction Vessels We Just Spent So Much Time On?
Plaque and plugs have taken up a lot of attention this past decade, and crystallization in urine a lot less.
Suppose crystals form in urine, why care? They can just migrate out, so tiny no one knows about them.
But maybe that is wrong. Even tiny crystals could lodge up in the crevices where papilla meet the medulla. Maybe in some people tiny crystals aggregate to make larger masses that will get stuck and grow. We need larger numbers of surgical observations, unselected, to get an idea of what fraction of stones are accounted for by plaque and plugging.
Perhaps quite a few are left unaccounted for. An open question.
Perhaps in those unaccounted for, stones crystals form in urine and grow into clinical menaces.
Effects of Uric Acid and Citrate
Solution Chemistry
Both parts of this graph are like the right hand portion of the figure I already have shown twice. In both experiments, the solution of 1.2 and 1.8 SS brushite and calcium oxalate was let stand until it chose to begin forming crystals.
The pumps and sensors were on throughout.
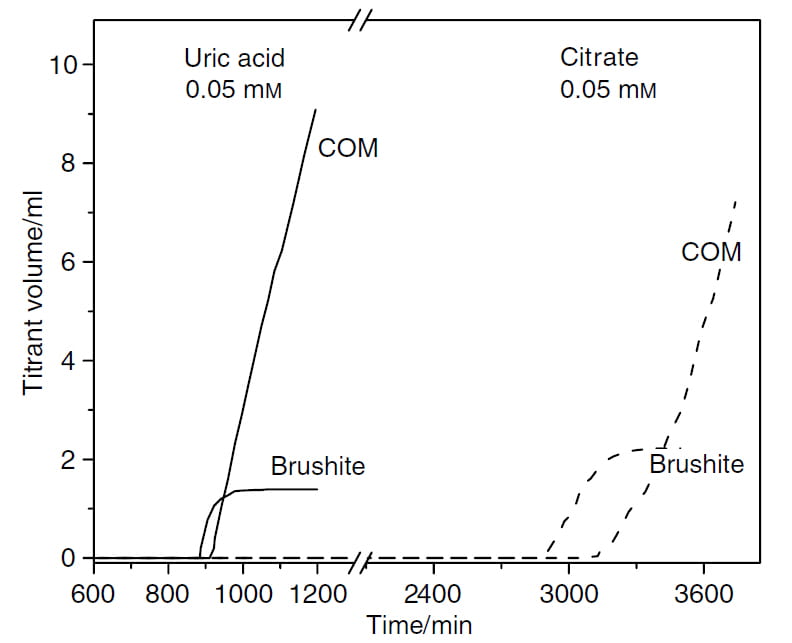
Uric Acid
If 0.05 mmol/l of uric acid was added to the solution – way too little to form crystals – brushite began forming by about 900 minutes instead of the nearly 1400 minutes without it.
Thereafter, calcium oxalate began to form only 30 minutes after brushite, much faster than without uric acid in the solution.
So uric acid in solution shortens the time needed for brushite to crystallize and also shortens the time needed for calcium oxalate be begin growing over brushite.
As a reasonable estimate human urine contains about 0.5 to 4 mmol/l of uric acid species. Typically there are as a mix of sodium, potassium and ammonium salts with only small amounts of the acid itself.
Citrate
At the same concentration, 0.05 mmol/l, citrate prolonged the onset of brushite crystallization to nearly 3000 minutes. Thereafter, brushite grew until halted by calcium oxalate crystallization. The interval between brushite nucleation and the start of calcium oxalate growth was lengthened to about 200-240 minutes. In general human urine contains about 0.5 to 4 mmol/l of citrate.
Appearance of Crystals
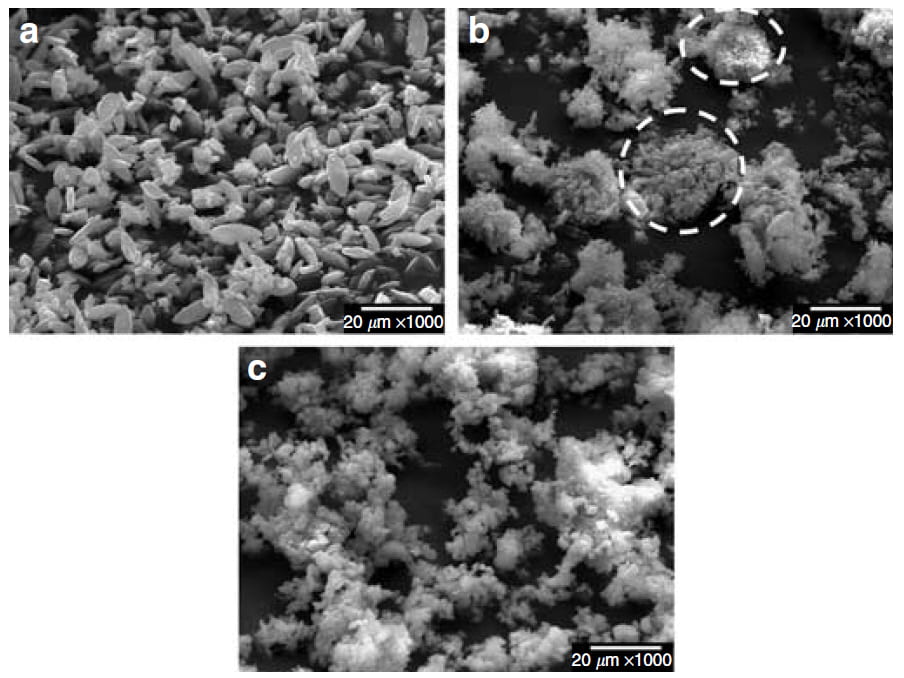
Calcium oxalate crystals that form on brushite are small and well separated when viewed by SEM (Panel a). Those grown with uric acid 0.05 mmol/l in the solution (Panel b) are a lot bigger because smaller crystals have aggregated together. This kind of aggregation is seen in human urine according to the authors of this paper.
By contrast, the crystals grown with 0.05 mmol/l of citrate in the solution (Panel c) are small and better dispersed.
Clinical Meaning
Citrate
Citrate is an established treatment for reduction of calcium oxalate stone recurrence. In general, it is thought to act by inhibiting formation of calcium oxalate nuclei and their subsequent growth.
This work says about the same but shows that citrate acts at both of the steps: It slows nucleation of brushite, and slows formation of calcium oxalate out of the calcium in brushite.
The concentration of citrate needed is far below those in urine. Perhaps that is because this system lacks the many complexities of urine so citrate effects are more visible.
Uric Acid
Long ago, I proposed that abnormally high urine uric acid excretion raised risk of calcium oxalate stones, and that lowering it with the drug allopurinol seemed to reduce new stone formation. Subsequently, Bruce Ettinger published a double blind random allocation prospective trial that showed allopurinol did indeed reduce new calcium oxalate stone production in patients with elevated urine uric acid excretion rates.
But how uric acid might promote calcium oxalate stones was not clear. Our group and others like us thought perhaps crystals of uric acid might act for calcium oxalate like brushite does, but we could not prove it. Likewise for other ideas including adsorption and removal of crystal inhibitors from urine, and “salting out” of calcium oxalate salts by urate salts.
Lacking a clear mechanism, allopurinol has languished as a stone treatment despite having a superb trial to support it. It shows an odd role science plays in medical practice. Physicians, myself included, seem to need an explanation for how a treatment works before we are willing to use it with enthusiasm, even when trial evidence is excellent.
These experiments may have disclosed yet another way uric acid could indeed foster calcium oxalate stones. They suggest a way to explore the matter further, and also suggest we may have under-used allopurinol and done less than full justice to the wonderful trial Bruce Ettinger did for us decades ago.
The Strangeness of Brushite Stones
Here and there we find patients whose stones contain brushite – in part or altogether, and frankly they seem to me a mystery worth solving. How does so vulnerable a crystal survive among such predators as oxalate and hydroxyapatite? What saves its calcium atoms from the oxalate molecules that roam about in the urine? Why does it not lapse into hydroxyapatite?
Something must stabilize brushite, protect it from dissolution. Brushite is not safe in urine or any other solution that has a significant calcium oxalate supersaturation and a pH high enough to permit hydroxyapatite to form.
I will not take this up here, but plan to, one day. It baffles me, astounds me, really. Yet I not rarely care for brushite stone formers, knowing nothing of why they exist.
Risk of Forming Stones Begins at a Brushite SS Above One
Prediction from Basic Science
The present work I have summarized, and the many other demonstrations of brushite as a crystal precursor predict that brushite supersaturation should predict stone forming. In fact, given brushite is like tinder and supersaturation its match, one would expect risk to increase at a SS just above 1.
That appears to be the case.
Empirical Evidence the Prediction is Not False
I have shown this figure elsewhere, and only bring it back here for convenience.
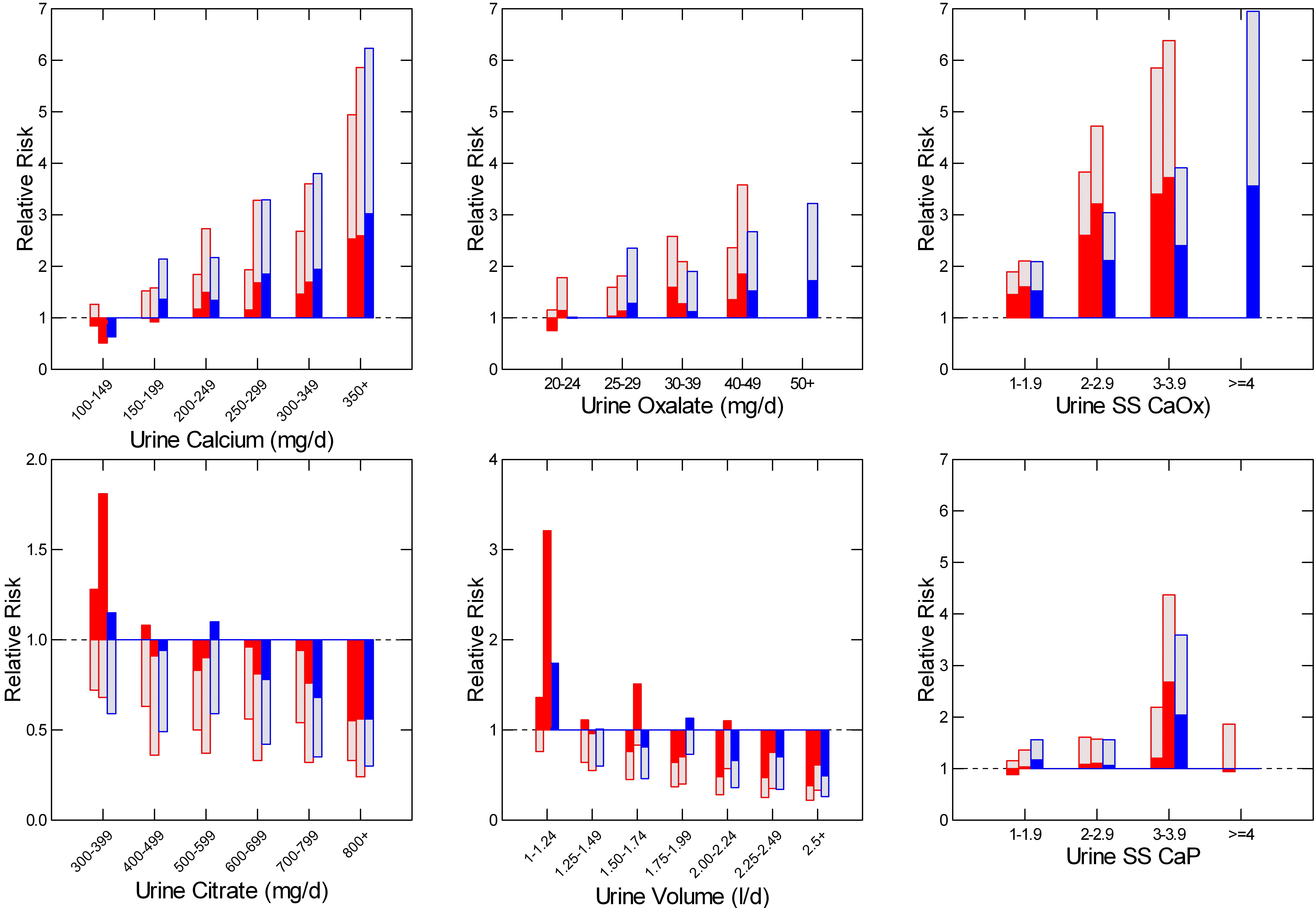
The vertical axes show the relative risk of becoming a stone former among women (red) and men (blue) in three large cohorts followed over many years as part of a long term effort to establish risk factors for a multiplicity of diseases.
Over time, some people began forming stones, and 24 your urines were obtained. As controls, 24 hour urine samples were also obtained from a properly chosen set of well matched people who did not form stones.
Daily excretion of calcium, oxalate, citrate, and water – urine volume, were predictive of stone risk. In all four cases, risk rose significantly as the excretion rate rose (calcium and oxalate) or fell (Citrate and water – urine volume).
Risk rose with SS for calcium oxalate as well, as one might expect. But the units here are odd. They represent the actual SS divided by the mean SS among a group of about 50 normal people (SS mean = ~3). So a value of 1 is about a SS of 3 fold and the first risk group is therefore SS from 3 to 6.
For SS with brushite (CaP) SS values are not so corrected, and risk begins as SS equals or exceeds 1. This is shown by the height of the bars that register the mean relative risk, and by the fact that in 2 of the 3 cohorts the lower 95th percentile for relative risk lies about 1.
Reservations and Science
I have shown you basic science pointing to a role of brushite to initiate the common stone, and to empirical science in the form of epidemiology supporting brushite as a risk factor for onset of new stones.
Urine Chemistry is Far More Complex
The complex environment of urine that contains about1800 peptides, many of them affecting crystals, may mute the effects of brushite or even obliterate them. It is not possible to measure in urine what I have shown you here. For example in whole urine, crystals induced by adding excess oxalate are not affected by seeds of brushite. One might note that initiating crystallization by adding oxalate is almost certain to complicate effects of phosphate crystals, as it creates by definition sufficient supersaturation to initiate calcium oxalate crystals de novo.
The Miscreant Crystal May be Another Phosphate Phase
The Nancollas group has reported that amorphous calcium phosphate phases may be critical as opposed to brushite, citing that urine may not be supersaturated with brushite as a general rule. This latter is contradicted by the epidemiology findings just above. The relative risk of stone forming with higher CaP supersaturation arises from higher values in those who became stone formers.
However, the way we calculate brushite supersaturation may not include all possible kinds of calcium phosphate phases, so our measurements could be imperfect. They could over estimate “true” brushite supersaturation.
Medicine Requires Action Despite Uncertainty
I cannot resolve these matters, but that does not affect my main point. Time and more experiments will eventually sort out the details.
Right now clinicians do not have measurements for supersaturation of amorphous calcium phosphates, so whatever they do cannot matter in clinical practice. What we do measure is a risk factor for stones, and what basic science we have points to brushite as a good source of trouble.
As I have said, no risk, some gain, suffices.
Acknowledgment
I want to thank Dr John Asplin for his careful reading of this article. The material is very complex and it is reassuring to have someone as expert as he as a reviewer. I have incorporated his additions.
Professor George Nancollas
George Nancollas invented the constant composition technique that drove this research. I knew him well, and heard portions of this research long before the final publication.
George was a distinguished chemist utterly devoted to the problems of biological crystallization.

At the ROCK society, our small group of like minded scientists interested in stone disease, George was a favored guest, brilliant speaker and discussant, and lovely person as well. Howsoever lacking a sophistication to match his, we were nevertheless politely welcomed to chat with him about what he did and knew, and felt – so long as he was around – somehow smarter about the whole matter.
He was aware of the brushite/calcium oxalate issue and also of the uric acid connection to calcium oxalate stones. It was because of clinical investigators like myself and others that he did the uric acid portion of the present work.
He died Jan 11 2016. His 420 publications remain for any to use that have wit and desire enough, and a taste for their ascetic beauty.
Ashes denote that Fire was —
Revere the Grayest Pile
For the Departed Creature’s sake
That hovered there awhile —
Fire exists the first in light
And then consolidates
Only the Chemist can disclose
Into what Carbonates.
Emily Dickinson


Excellent analogy for the patient who is dedicated to do their very best to eradicate the stone forming processes. Thank you Dr. Coe for your constant vigilance!
Thank you, Jolie. Fred
Thank you for this information. My medically complex 38 yr old daughter was found to have multiple kidney stones bilaterally at an ER visit. The stones were removed through ureterscopy. Bilateral stents were placed and removed 1 week later, completely encrusted and pathology reported it to be 100% brushite.
3 months later CT scan is again showing multiple kidney stones.
stone that was analyzed : 70% calcium phosphate apatite 30 % calcium oxalate dihydrate.
Any thoughts as to why her body is suddenly making all these kidney stones and do you think the brushite encrusted stents are important in directing treatment ?
Thank you for the important work you are doing.
Hi Kathy, Indeed I do. Brushite forms rapidly and that it forms on stents makes me worry it forms in her sans stents. I guess your daughter has an elevated 24 hour urine calcium excretion and a high urine pH and I know she requires very skilled treatment to stop this condition. If she is not already being treated in a university stone center, she might ask her physicians if they might want to obtain consultation from one that is convenient to where she lives. I find phosphate stone prevention very difficult, and worry about its consequences. Regards, Fred Coe
So to a layman such as me, what can I do in simple terms to reduce brushite levels? And will this reduce my calcium/oxalate stone formation if I have MSK?
Hi Kelly, Here is the good news, Kelly, and I should make it clearer. Everything we do to reduce stone risk should lower CaP supersaturation, but in a given case one would need to tweak it aiming at that SS below 1 – for example, maybe just a bit more fluids, less sodium, less sugar, possibly thiazide, maybe swapping out something else for potassium citrate, maybe adjusting diet for less alkali loads. Fred
Dr Coe,
In Regards to your answer to Kelly H. on Jan 6th you mention “maybe adjusting diet for less alkali loads”, can you give me some fruits and vegetables that would be the worst for raising the urine PH? I eat a lot of fruits/vegs including clementines, grapes, apples, pears, bananas, dried apricots/prunes, tomatoes, cucs, broccoli, and red bell peppers and these are daily. I have Calcium Phosphate stones. Thank you.
Hi Lisa, In general plants provide a lot of alkali in the form of potassium – anions, like potassium citrate, or malate. Honestly I do not have a catalog of plant alkali by species as you ask about. I am not sure where to get one from. Perhaps the best answer for the moment is to get adequate protein 0.8-1.2 gm/kg/d , fats as usually prescribed in diet plans, and fill in with fruits and veggies to get 80 – 100 or so mEq/d of potassium (around 3200 – 4000 mg of potassium). This article details what is known for stones in general, the special problem of high pH calcium phosphate stone formers does not as yet have a proper set of menu lists for those plants, as I said.
Dr Coe, appreciate your response and I have a couple more questions.
I have been experimenting with PH strips at home and found it very interesting being a Ca Phosphate stone former that my PH is the highest in the morning after night fasting and it’s at its best in the evening after I’ve had all my meals for the day which includes lots of fruits and veggies. Why is that, it seems it would be higher after all the fruits and veggies for the day and not after night fasting or is it that it takes that long for the PH to raise after eating plant alkali?
Another question, you mention 0.8-1.2 gm/kg/d of protein, do you mean for the weight you are currently at or your ideal weight? I significantly changed my diet with less sodium between 1000-1500 a day and extremely lowered my added sugars to 25-30 grams of added per day which I think is my biggest achievement and of course when you do all this you will drop in weight and I believe I’m a bit underweight now so need to know which weight to use for the protein amount. Thank you for all your time.
Hi Lisa, Ideal weight is best. Consider what you would weigh at an ideal BMI. Here is the CDC BMI calculator and the ideal range. You know your height, assume 20 is an ideal midpoint, estimate your weight at that BMI and use the 0.8-1.2 protein per KG at that ideal weight. Regards, Fred
Dr.Coe:
I have dealt with kidney stones for over 20 years. First experience managed by urologist for 12 years, insurance changed and left to primary care. First, large one in left kidney required lithotripsy 3times to break up. Type: calcium based (told) and MSK. Nothing in the right s kidney until a couple years ago. Smaller and fewer. Last surgery stone analysis was 80 % brushite and 20%appetite. Going to a nephrologist last year (his dx as IH) and a half ago to find treatment because I have again another large stone in left kidney and two small ones on the right. 24 he urine only showed low citrate. Treatment of potassium citrate 10 mqs twice daily, hydrochlorothiazide 12.5 was at 25 1 x a day (potassium dropped), potassium chloride ER 1 x day . Had yearly X-ray yesterday, showed same stones in both kidneys with little change. Left one was recently termed “staghorn” 29 mm. Had for a couple years. Unfortunately my urologist retired in December and recommended I just monitor…but said I would need PNCL procedure. Going to see nephrologist this week? Is my medicine still appropriate as this article mentions changing out the potassium citrate???? What do I do to safely protect my kidney – wait out PNCL or get it? Anything us I should be aware of?
Thank you for your time!
Dear Cathi, You do not have MSK, you have brushite stones which involves numerous calcium deposits in the kidney tissue. Brushite may have formed in the left kidney in part because of your hypercalciuria and also because of 3 SWL treatments to that kidney. Potassium citrate is not necessarily ideal in general as it raises urine pH; the important index is SS with respect to calcium phosphate which should be below 1. If potassium citrate raises urine citrate more than urine pH that SS may fall but often the drug raises CaP SS. The OHCTZ is a bit short acting, and perhaps your physicians might prefer chlorthalidone or indapamide which are long acting, and potassium chloride as a replacement. As for PCNL that is a surgical decision. I would consider an opinion at a university based stone center as to whether PCNL is absolutely required as it inevitably damages kidney tissue. Regards, Fred Coe
I have Calcium phosphate stones with a very high brushite in my stone risk analysis. Is there ANYTHING (besides increasing my water intake and lowering my salt) that can help to prevent these? They also run in my family. I am so tired of being in pain and getting lithotripsy procedures. Please help ;(
Hi Tracey, Brushite stones are often very major problems. My attitude is to lower urine calcium with long acting thiazide type diuretics like chlorthalidone at a low dose (12.5 mg/d) along with low diet sodium to help the drug work better and of course high fluids. The goal is to lower brushite supersaturation, as in the article. You would be wise to ask your physicians for a possible second opinion from an expert of their choice to be sure everything is being done. The same for your family. I know this disease all too well, and consider it difficult to treat. Best, Fred Coe
Hello, I am a 17m calcium phosphate former with what my urologist says is a case of IDRTA. I only have crystal attacks(seen before in an ultrasound as hundreds of tiny stones 1<mm) which have gone from almost daily to every few months. Without medication I have a very high urine ph(6.9), uric acid(800) and low citrate(217). My calcium was at 184 with 60meq sodium. Ultimately those lead to a calcium phosphate ss of 5, uric acid ss was low at 0.27.
In a second 24hr urine I responded to 20 meq of k citrate with my cap ss falling to a 4 even alongside very high sodium intake at the time(lead to 288 hypercalciuria). K citrate increased my ph to 7.4 and citrate to 290.
After multiple more 24hr urine tests my uro has decided to keep me on 30meq k citrate and 300mg allopurinol daily. I also aim for 3L of liquid a day, aiming for 1L after waking up. I do not have any of the 24hr urine tests after the latest dose of medication but my urologist is satisfied with the results. My urine is still bouncing between cloudy and clear seemingly on sodium intake. A CT showed that my kidneys are now completely stone free. I am planning on requesting the results of these 24hr urines at my next appointment and adhering to more strict dietary restrictions. Due to a high bp I am determined to decrease sodium intake further although i am not adhering to this "determination" well.
My biggest question is could allopurinol simply be only positive to Cap SS in its effect on uric acid? Also would someone of my age be a potential candidate for thiazide if my further sodium restrictions aren't enough? There has been massive progress so far on my condition but I'd love to do as much as I can.
Thank you!
Hi Michael, You presumably have hydroxyapatite stones because of your very high urine pH. The cloudy/clear bouncing shows how on the edge your urine really is. Allopurinol has no role here I can imagine unless the stones are some odd urate salt. I would add a thiazide to lower urine calcium as low as possible, and of course as low a diet sodium as possible. A sodium goal would be below 1500 mg (65 mEq)/day. Thiazide, incidentally, tends to lower urine pH a slight bit. Regards, Fred Coe
Dr. Coe,
What do you think of stone analysis like these?
Stone Analysis DNR
Stone Analysis – 1st Constituent
RESULT: 70% Calcium phosphate (apatite)
Stone Analysis – 2nd Constituent
RESULT: 20% Calcium oxalate monohydrate
Stone Analysis – 3rd Constituent
RESULT: 10% Calcium oxalate dihydrate
The above are the result of my second stone. (2019) Which required Ureteroscopy for removal.
History: The first stone(s) occurred when I was 29 (2011-2012) which did not receive analysis. Was passed on its own.
Hi Amanda, You form calcium phosphate stones, and belong in that class of patient. Here is a nice review of them. Urine pH is usually above 6.3, and in women this is because of increased absorption of alkali from food. Treatment is like that for common calcium oxalate stones except that urine volume and calcium excretion predominate and diet and urine oxalate are usually minor players. Regards, Fred Coe
Hello Doctor- I have continuously acquired kidney stones my whole life. The only solution I have ever been given by doctors is; 1. drink more water, 2. limit animal protein, and 3. reduce sodium. My recent kidney stone analysis is comprised of these components dated 7/21/2021;
1. 20% calcium oxalate (monohydrate and dihydrate)
2. 60% calcium monohydrogen phosphate dihydrate (brushite)
3. 20% calcium phosphate (hydroxy-and carbonate-apatite)
one of my stones was 9mm
Any advise, help, or analysis would be greatly appreciated. Thank you for your time.
Hi James, Brushite stones are very hard to prevent, and often the kidneys have considerable brushite deposits new stones grow on. I presume your 24 hour urine calcium is high as is urine pH, and the only workable approach is to add medication. I usually want 3 liters/d of urine volume, 1500 mg diet sodium, and a thiazide diuretic such as chlorthalidone 12.5 to 25 mg/d as needed to lower urine brushite SS below 1. Regards, Fred Coe
21 year old man came to see me with recurrent stone formation ( 2 in 2 years – imaging study showed tiny stones in kidneys) her sister also has similar problem since her younger years.
TOTAL URINE VOLUME >2.00 L/day 1.12Low
Comment: See Note 1
pH Urine 5.5 – 7.0 6.5
Calcium Urine 24 Hour <250.0 mg/day 237
Comment: See Note 1
Oxalate, Urine – per 24h <45 mg/day 35
Comment: See Note 1
Uric Acid, Urine – per 24h 320 mg/day 469
Comment: See Note 1
Sodium, Urine – per 24h <200 mEq/day 112
Comment: See Note 1
Sulfate, Urine – per 24h <30 mmol/day 19
Comment: See Note 1
Phorphorus, 24 Hour Urine 60.0 mg/day 117
Comment: See Note 1
Ammonium Urine 14 – 62 mEq/day 29
Comment: See Note 1
Potassium, Urine – per 24h 19 – 135 mEq/day 77
Comment: See Note 1
Creatinine Urine 800 – 2,000 mg/day 1,696
Comment: See Note 1
Calcium Oxalate <2.00 3.31High
Brushite <2.00 5.93High
Sodium Acid Urate <2.00 5.05High
Struvite <75.00 8.36
Uric Acid <2.00 0.98
The Patient Has:
Comment: Hyperuricosuria
Low urine volume
SUPERSATURATION INDEX WITH RESPECT TO
Comment: Calcium oxalate
Brushite (Ca phosphate)
Monosodium urate
SUSPECTED PROBLEM IS:
Comment: Hyperuricosuric Nephrolithiasis
Hoping to get your thoughts.
Thank you
Hello Dr, The low volume, hefty urine calcium, and pH of 6.5 have raised brushite SS very high. I think the person is hypercalciuria but urine Na is low enough at 112 to moderate the urine calcium. His urine NH4 exceeds his urine acid load – SO4 – as we have seen in CaP stone formers. I would start with a lot higher urine volume and work from there concerning the BR SS. Regards, Fred
Hi Dr Coe, I work for a large group of Radiologists and am writing about my husband who has had issues with calcium oxalate stones for many years. He was diagnosed with CLL 2 years ago and started on Imbruvica the end of April this year. In August he started passing bladder stones which were analyzed and had a mixed ratio 10% calcium oxalate monohydrate, 30% calcium oxalate dihydrate and 60% CAHPO4. He continues to pass stones on nearly a daily basis. Last week he went in for L Renal stent removal/replacement. The stent was encrusted with stones some of which were analyzed and found to be brushite 100% CAHPO4. My husband’s BMI is 22.38. Thank you for your thoughts.
Dr. Coe,
I have passed over 600 stones since first occurence in 2005 (that I was told was kidney stone—lots of flank pain/sickness in childhood now has me wondering if I had stones further back).I am 45 now. My last stone analysis, when hospitalized as recent as Dec 2021, was 20% calcium oxalate monohydrate, 40% calcium monohydrogen phosphate dihydrate (brushite), and 40% calcium phosphate (hydroxy- and carbonate- apatite). DX with MSK and Bone/Mineral Disease in 2016. My question is this….after reading much of your articles on here I started reviewing lab results. Specifically very low AGT (usually 7-8) and AST (usually 8-10). In Dec while in hospital, my TP was L (5.9) as was Alb (3.3). I read in one of your articles about possible liver issues that effect kidney. I am slowly heading to renal failure pre every doctor I have seen, including Mayo. All say no cure but something underlying. I have even been to Pak Center in Texas. I keep seeing a correlation with PH1 that everything seems to point to. Mayo currently shows I am in an active study with patients who have PH1 so I have requested my gene study results. Since car accident in 2007 where bone would not heal and almost caused it to be amputated, I have had over 32 surgeries to address my bones (12 on left arm) and 20 on my kidneys and 2 heart ablations. In 2019, I was rushed into emergency surgery 10 times in 10 consecutive months due to sepsis. I am desperate for answers. Could you possibly offer guidance on where to look or who to see to try to explore this further? Thank you in advance
Hi Stephanie, I doubt oxalate is causing brushite stones and such severe bone disease, but there is little I can say given you have reams of information at Dallas and Mayo that no doubt tell a story. If your physicians at either center believe I can be helpful I can review things via telehealth, but there is so much expertise at both places I cannot imagine they will think so. Perhaps you might mention this to them, and let me know. Regards, Fred Coe
Thank you for replying, I’m just catching up. I have been very ill with both kidneys. Stented since Jan 30. Currently recovering from PCNL on rt on 4/5/22 with left scheduled 4/18 (if I can mentally work up doing it again). I have RSD so pain factors in so much to the whole process. Stone composition didn’t chg but % was greatly different. 10% calcium oxalate monohydrate,
20% calcium monohydrogen
phosphate dihydrate (brushite), and
70% calcium phosphate (hydroxy-
and carbonate- apatite). The latter is getting higher it seems. Dr. Moe @ Pak Center is doing a analysis of my 24 hr urine completed on 4/3-4/4. Next appt with him is in mid-May. I’ve been told I’m an enigma and stump all doctors seen so far. I have read so much of your articles trying to learn but admit it’s very Greek to me.i would love to see if you would be interested in becoming involved. I want to save my kidneys. If interested, could you email me on how to contact? I will inform them of your previous comment for telehealth contact. Thank you in advance .
Hi Stephanie, I already wrote to you personally so I can say again here what I said to you. The Pak center is outstanding and Orson Moe a brilliant physician who is world renowned for his research. If he is your physician, you can have none better. You are not an enigma to him. Best, Fred Coe
Dr Coe, My stone drama is nothing compared to patients writing you for help. Nevertheless after having experience pain from passing small stones 3 different times, I have decided to be more pro active with my health management. I am 58 years old.
First encounter with passing stone was back on April of 2020. It was 3 mm. The next ones were 2mm and a 3mm. Latest CT on March 2023 showed a 2mm on the right kidney and 3 small ones on the left ranging in 2-3 mm in size. I was never able to collect a specimen for stone determination.
Had a 24H urine collection (results are inside parentheses) back on Sept 2022, three mos after I have been on potassium citrate 15 meq twice a day. Based on results, dose was increased to 30 meq twice a day. Recent 24H collection done on March 2023 showed : ph 7.4 (from 7.1 on Sept 2022), calcium 204 (204), oxalate 30 (50), citric acid 726 (853), uric acid 802 (895), sodium 324 (223), brushite 2.43 (2.1), sodium urate 5.2 (2.74). My serum uric acid is normal.
I have been trying to drastically cut my salt intake. I have been conscientious with increasing fluid intake and decreasing purine intake.
Should I lower potassium citrate dose? Would I benefit from a low dose allopurinol? Would highly appreciate any comments/recommendations with drug management.
Thank you so much for listening. God bless.
Hi Marlon, I cannot quite see the rationale for potassium citrate given your initial urine citrate was not low (853?) and the urine pH is so high that CaP SS is very high indeed. Your 24 hour urine sodium is remarkably high and warrants lowering for health’s sake alone. But in the scanty measures you put up I see little initial stone risk apart from the high urine pH. Perhaps your urine pH is indeed high sans treatment and the stones are indeed calcium phosphate – that might be a clue to the causes. With so little to go on I cannot help more, but perhaps your physicians might want to consider what I have written. Regards, Fred Coe
Dr Cole, I have been reading all your articles for about a year now, learned of you from kidney stone diet FB group. My husband has passed hundreds of stones since his 20s. He’s 46 now. He has had 4 lithrotripsys. He stopped going to doctors years ago in his 20s due to frustration of being young and in pain feeling like no one was taking him seriously so he just passed them on his own. Recently in the past few years, they have gotten worse. He went to ER in 2022 and had a 3.5 CM Staghorn (40%oxalate 60%phosphate, hydroxy and carbon-apatite) removed by PCNL.. He had stent for a month and it was so badly encrusted the urologist said it was the second worse stent he had ever seen. It was a horrible experience for him and there was no followup scheduled after that or any offer to find out what was causing them, just told to drink more water and stay away from oxalates..I made appointment with primary and asked for referral to nephrologist . His pain from the stone being removed only eased up temporarily and had already started back up by the time we saw nephrologist about 2 months after surgery. We got a 24 hour urine this is the results..Urine Volume 1.47 SSCaOx 6.05 Calcium 295 Citrate 279 SSCao 2.37 Dietary Sodium 188 PH was 6.036. He was put on 12.5mg of hydrochlorothiazide and ordered another 24 hour urine. We have been watching sodium more closely, drinking 3 liters water minimum daily. I’m not sure of the results of second 24 hour urine. He went to the appointment alone and didn’t get a copy but at that appointment they put him on 1600mg potassium citrate 3 times daily and referred him back to urologist for a 2.5 CM Stone in the same kidney that his 3.5 CM was in at this time last year. He had a Uteroscopy done a few weeks ago and this stone was 10%oxalate(monohydrate) 90%Brushite. I’m planning to get a copy of his last 24 hour urine. I know we can’t have a full picture without that but I’m just concerned with his Urine PH and all the Potassium Citrate he is taking and how quickly they are growing especially now that they are Brushite. Any advice?
Hi David, The stones are brushite, a calcium phosphate crystal. I presume a main cause is high urinie calcium (295 mg/d) + low urine citrate (279 mg/d) and low urine volume (1.47 L/d). Given the massive surgery he certainly should have as aggressive a prevention regimen as possible. Hydrochlorothiazide was proven rather ineffective in a recent trial so his physicians might want to consider longer acting agents. Higher urine volume is certainly easy enough – about 2.5 l/d of urine. Reduced diet sodium (1500 mg vs the measured 188 mEq x 23 mg/mEq or 4324 mg) would be prudent. Potassium citrate is questionable – raises urine pH which could increase risk of calcium phosphate stones and may not raise urine citrate very well – repeat 24 hour urine can distinguish matters. In all brushite stones are very concerning.It is a triggering crystal and the stones can grow rapidly. If possible perhaps David might want care at a university based stone center as his condition is far outside the usual common stone. Regards, Fred Coe
Thank you so much for taking the time to respond. We are so very grateful for all of the knowledge you continue to share!
Thank you, David. Fred
Hi Dr. Coen, I have crystals in my urine in my last blood tes. I am taking vitamin B2 for migraines and capsicum for osteoporosis, also Lovothyroxine .75mg
Cal hydroxypatile 40%and citrate aspirate 1000 mg. Phodforus as (calcium hydroxyapatite 500mg 40%.and Mg aspirate and taurine 500 mg 119%
By Country Life Cal / Mg
Will this has anything to do with the Crystals?
Thank you so much, God bless you.
Hi Maria, Indeed the calcium hydroxyapatite can cause them and I would stop it and see if the crystals stop. Regards, Fred
I had my first kidney stone when I was 25 and it was calcium oxalate. I could mostly pass those on my own and they occurred throughout my late 20s. I went approximately 10 to 12 years without having any stones passing or obstructing and then I had another big issue in 2021. Those were so large. I had to have surgical intervention and they were also calcium oxalate. At that time I did a urine study and it said that I had high levels of calcium and low citric acid. I’ve since continued to have problems, but they are forming more rapidly and the last analysis after surgery showed that it was calcium, phosphate, and brushite. I have elevated uric acid levels as well and my calcium is elevated in the blood as well. I was prescribed potassium citrate, but it was not tolerated so I discontinued. I was also prescribed allopurinol which I stayed on for a time but I’m no longer taking it. I just discovered that my uric acid levels are still elevated so I suppose I need to go back on that. My parathyroid number was normal. I’ve been trying to follow a kidney stone diet but with all of the conflicting information I’ve been given I’m a little confused as to what else I should be doing to try to help slow the production of these. It seems I form about one a year in my left kidney. I don’t seem to have issues with the kidney stones that are in the right side. I will eventually have to have them all surgically removed I was told.
Hi Stephanie, Very complex. You have converted from CaOx to brushite stones which grow very rapidly indeed. One presumes high urine calcium and low citrate are a main cause. Often brushite can occur in one kidney and CaOx in the other. The elevated uric acid is presumably in the blood. Increased blood calcium which you mention is something special and suggests primary hyperparathyroidism which is curable via a rather commonplace surgery.There are two articles on it on the site. The hormone level can be normal for a while so high serum calcium is a sole reflection. Read the articles and see if they apply to you. I would suggest your physicians seriously consider this diagnosis. Regards, Fred Coe
Doctor Coe,
Below is a summary of my kidney stone and surgical history, At the current rate I can grow a brushite stone at the rate of 1cm every month and a half in my right kidney. I have taken 1620mg Potassium Citrate 2X a day and 12.5mg Hydrochlorothiazide 2X a day for about a year and does not seem to help. Now my urologist has suggested taking L-Methionine supplement. My urologist has only did a urine study once because he says if I have stones it is not acurite. I have a copy of the report if you would like to see it. I need help and ask if you could look at my records in detail or see me? Do you know of any studies I could be a part of? My urologist has never had a patient with this frequent and amount of stone formation and seems only interested in surgery. I have suffered both physically, emotionally and financially. Any help is appreciated.
7/23/07 Left kidney 14mm had ESWL
1/8/2010 ESWL Right Kidney
? Right kidney Ureteroscopy
4/29/20 ESWL
12/2/20 ESWL Right Kidney
4/27/22 PNCL 4CM Right Kidney Balloon broke had Stone Street
4/29/22 PNCL and Ureteroscopy
11/28/22 ESWL Right Kidney 3CM Staghorn
10/10/23 PNCL Right Kidney 3.5CM 7/5/23 Remove tumor on neck
10/12/23 PNCL addition hole in back
10/19/23 ESWL Right Kidney
10/27/23 Ureterscopy on Right Kidney
11/30/23 PNCL and Ureterscopy Left kidney 2.5CM
Doctor says I have a unique Physiology and the Kidney stone was in a sack so after he broke it up could not get the pieces out. Need to have another surgery.
12/21/23 Uretercopy on Left Kidney
4/23/24 Uretercopy on Right Kidney [2] 1CM stones at funnel of Kidney to the tube
6/4/20 CT Scan 12mm + 5.5mm Left Kidney, 4.5mm + 1MM +1MM Right Kidney
11/6/20 CT Scan 1.4CM Left Kidney, 5MM Right Kidney
2/14/22 CT Scan Right 3.4cm, Left 1.6cm
4/28/22 CT Scan Right 3.5cm, Left 1.8cm
5/23/22 CT Scan Right 4mm, Left 1.5cm
11/10/22 CT Scan Right 1.4cm, 1.1cm,1cm, Left 1.7cm
10/3/23 CT Scan Right 2.9cm X 1.5cm, Left no report
11/13/23 CT Scan Right 6mm and Numerous stones clustered, Left 11mm X 11mm cyst?
3/18/24 CT Scan Right [2] 1CM Stones, Left clustered stones at bottom of Kidney
Hi John, Brushite stones are indeed worrisome, fast growing, space filling, and difficult to control. I would obtain care via a university kidney stone program. No doubt your state contains several such. Telehealth is useful for consulting on prevention matters, but needs to connect with personal physicians who are interested in receiving such collaboration. Depending on your state laws I or people like me can consult for physicians there. But a program in your own state may be best given the amount of trouble you are having. If you tell me where you live I could try to find help for you. Regards, Fred Coe
Thank you for taking time to answer me. I live in Gulf Shores Alabama and currently see Dr Younger at University Urology in Mobile. He is a part of the University of South Alabama, but as I mentioned he seems only interested in stone management by surgery. Any help would be greatly appreciated.
Thank you, John
Hi John, Perhaps you might find a nephrologist at the university more interested in prevention. I can help only via telehealth and given state control of medical practice I would have to consult our compliance officers concerning the rules. Regards, Fred Coe
Dr Coe,
I would be interested in a telehealth visit. I have not been able to locate any doctors near me that have brushite stone formation as their main area of study.
Thanks,
John
Hi John, I guess you read the draft of the article – it is really not ready yet. Indeed I can do this depending on your state and its laws. If not Illinois or Indiana we will need to figure out what barriers to jump over. Just email me directly – coe@uchicago.edu. Regards, Fred Coe
I have a 25+ year history of kidney stones, passing at least 25. Two ureteroscope surgeries for stuck stones. Stones determined to be Calcium Oxalate. Have been taking Potassium Citrate for about 20 years and Allopurinol before that. To be honest, I wasn’t that consistent with the Potassium Citrate until recently when a stone started forming again last year leading to flank pain and urgency. Taking 1080 Potassium Citrate 2x day and Chanka Piedra 2x day, plus vitamins etc. Stone has not passed, but it may have dissolved? I have felt pieces of stone have been expelled, but could not find them. Recent 24 hour urinalysis showed Calcium at 471mg and Brushite at 7.36. I believe the calcium was high because I was told that calcium binds with the oxalate to prevent stone formation, so I added calcium supplements to my diet. Everything else fell into the normal range, including Oxalates, Uric Acid, and PH. Citric Acid was high at 1340mg. Diet is basically NSNG/Keto, but not too strict. Good fluid intake and regular exerciser. 58 y/o male. Had CT, but awaiting results. Blood work also normal except for Calcium just slightly elevated at 10.6 (10.3 normal). Any advice or changes for better prevention?
Hi Paul, I am not so sure. That urine calcium may be high because of genetic hypercalciuria or primary hyperparathyroidism. Your physicians need to delve in more. Best, Fred Coe
I so enjoy reading your articles, not only for the science, but for the wit and erudition. I have a 35 yo w 100% Brushite stones and am at my wit’s end. Based on the above, I will try Allopurinol and double down on the K citrate.
And a passing question: do you have any insight as to why the 24 hr urines from Mayo are finding so much more Ca Phos than Litholink? When we switched labs, I noted a significant change. Most of these folks are not Ca Phos stone formers, yet their SS Ca Phos (sometimes both types) are elevated.
Thank you in advance. I wish there were more thinkers like you in medicine.
Hi Dr McNally, Brushite stones are really an issue! Be sure and get the urine calcium down as much as possible – I use chlorthalidone liberally and low diet sodium. As for Mayo, they show free energy changes and the uniting differs from LL which shows SS ratios. They also may be using a different calculating engine – I have not asked. I use LL. Glad you like reading the site. I love writing it. Best, Fred
Are you aware of any relationship between remdesivir and the development of kidney stones? I have a patient family that insists this stone started after patient was treated with this for COVID. Thank so much for all you do.
Hi Dr McNally, Here is the one article I could find on PubMed: https://www.sciencedirect.com/science/article/pii/S0731708524002887?via%3Dihub. First analytical confirmation of drug-induced crystal nephropathy in felines caused by GS-441524, the active metabolite of Remdesivir. The article demonstrates stones in cats given this metabolite of remdesivir and proves they are made of the drug metabolite GS=441524. Authors refer to tissue deposits and tubule plugging but the article does not show these. A recent CJASN review of drug induced kidney disease https://journals.lww.com/cjasn/fulltext/2022/08000/drug_induced_acute_kidney_injury.19.aspx does not mention this metabolite or drug. So, yes, it is possible the drug caused stones by self crystallization. Stone analysis would have shown the organic crystal. Best, Fred