WHAT IS POTASSIUM CITRATE
We have reached the point in the evolution of this site where the main stone risk factors are introduced and detailed, and the importance of citrate established. I have written about the price of potassium citrate because many patients and physicians have told me that it has risen steeply in recent months, and I would like to be of help.
A LOUD DISCLAIMER
In this post I will mention beverages and medications by name. Let me be clear: I have no financial relationships with the companies that produce or sell the products I write about here. Likewise neither I nor my colleagues at University of Chicago receive any support, financial or otherwise from these companies.
WHAT DOES CITRATE DO FOR PATIENTS?
It Can Reduce Formation of Uric Acid Stones
Some patients produce too acidic a urine which raises risk of uric acid stones, and they need supplemental alkali to make their urine less acidic. The use of potassium citrate to make urine less acidic will prevent uric acid stones in most patients who form them.
It Can Reduce Calcium Stone Formation in Patients with Low Urine Citrate
Some patients form calcium stones because they produce urine that is low in citrate, a valuable inhibitor of crystal formation. Most of the naturally occurring inhibitors in urine are complex molecules about which we can presently do nothing. But citrate is a small and easily measured molecule which we can prescribe and which will increase the urine citrate in at least some patients. Potassium citrate lowers urine calcium excretion. In so doing it reverses a key kidney stone risk factor. In trials potassium citrate reduced stone formation.
Potassium Citrate is Preferable to Sodium Citrate
I have a long list of sodium’s undesirable effects. It can raise blood pressure in large numbers of people, especially with age. It raises the amount of calcium lost in the urine, and that increase of calcium can raise supersaturation and promote calcium kidney stones. High sodium intake can reduce bone mineral retention. But, it may be that the sodium in sodium bicarbonate causes less of these problems than the sodium in sodium chloride – table salt. So I offer sodium bicarbonate as an alternative – with reservations.
Because sodium produces problems of its own, we tend to use potassium citrate as the preferred medication, and generations of stone patients have taken it. In several trials it has reduced new stone formation when given to patients whose urine is citrate deficient.
IS THERE A COST ISSUE?
I am not at all sure why the pricing of potassium citrate has become a topic I often hear about from patients, doctors, and just about everybody in the kidney stone world. Certainly the price must have increased, but I cannot find data on the web to prove the point. I also believe Medicare and perhaps other insurers have altered the status of this drug in their payment schedules. Perhaps some of you know more about the problem than I do and are willing to share what you know by way of a comment.
I did find on inspection of the Medicare lists of drug prices by insurance plan that some plans appear to include potassium citrate pills in their formularies at a preferred level and charge as little as $10 for what appears to be 90 pills. Others do not do this and publish higher prices, often as percentages of the retail cash price. Once again, I hope those of you with experiences in purchasing the drug will share what you know.
CAREFUL SHOPPING LOWERS PRICES
Listening to agitated, and worrisome stories about inflated prices for potassium citrate, I decided to try to be helpful. A Google search for prices of potassium citrate yielded a few promising shopping sites, and on study of the prices I found some much better than others. Note that in the following sections I present a lot of prices and arithmetic. Sometimes, when the message is very clear the results are rounded for simplicity. I give the basis for every calculation if you want absolute exact answers to the nearest penny. Likewise, because we are comparing prices, I have chosen 4 pills daily as my cost basis. The actual range can be from 2 to 6 pills or even more daily, so you will have to adjust costs to your own prescription.
SAM’S CLUB
GoodRx gives what I believe is the clearest list of prices. On their site, Sam’s Club was least expensive at $145 for 180 pills or $0.805 per pill. A typical 4 pills per day treatment option would therefore come to $290/quarter, which is still very pricey. The site gives a long list of other stores whose prices are even higher. Everyday health offers an approximate price for Cytra-K and Polycitra K of $50 – $99, but I could not be sure if this was for a month and likewise how much medication was in a dose.
CANADA
So far as I can tell, importing from Canada will not save you much money. I found Urocit K at $1.10 per tablet, which is higher than Sam’s Club. Another generic, K-Citra 10 was $0.79, which is about the same as Sam’s Club. Another less desirable canadian price was $0.52 per pill if you buy 90 pills, but it was for the 5 mEq size, 1/2 of the usual and therefore the corresponding price for 10 mEq would be $1.04/pill. Given that some costs must accrue for mailing, and there are issues with importing, I cannot see an advantage right now.
WHAT TO DO
Shop Well
Certainly web shopping is a good thing because in my modest and amateurish shopping efforts I found a tremendous range of prices. I am sure that many of you who read this post are far more skilled than I am at shopping for best prices. It is time for you to step forward and share your knowledge with all of us by posting a comment. Everyone will benefit and appreciate your contributions.
But even if you shop better than I did, retail pricing for this medication seems too high for most to afford. At even 4 pills a day, and at the best price I found ($290.00/quarter) we are over $1000.00 yearly for this one product. It seems to me that if your plan does not subsidize this medication, cost could be a serious issue.
Use Beverages
A useful publication reports the alkali content of commercial beverages. The ‘lemonade formula’ referred to on the graph is given as 1/2 cup ReaLemon© mixed with 7-1/2 cups of 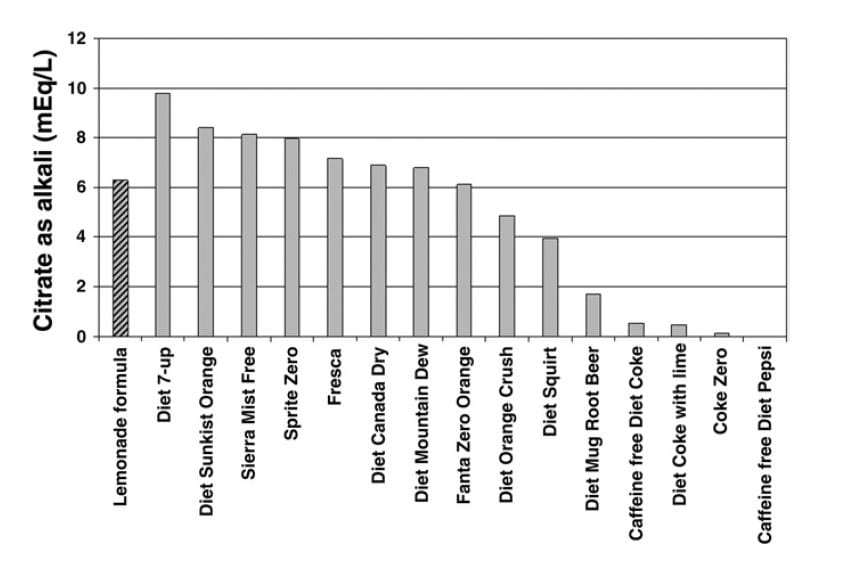 water and sweetened to taste with sugar or artificial sweetener. Diet 7-up was the winner with 10 mEq of citrate in a liter. A single Urocit K tablet contains 10 mEq of potassium citrate, as a comparison, so you would need 4 liters of the beverage daily to match 4 pills.
water and sweetened to taste with sugar or artificial sweetener. Diet 7-up was the winner with 10 mEq of citrate in a liter. A single Urocit K tablet contains 10 mEq of potassium citrate, as a comparison, so you would need 4 liters of the beverage daily to match 4 pills.
You Can Do Better
My colleague Dr. John Asplin has measured an additional group of products: Minute Maid Lemonade contains 10.3 mEq/liter of alkali, like Diet 7-up. Gatorade contains only 8.3 mEq/liter. But Crystal Light Lemonade contains 21.7 mEq of alkali, so it is the winner. Each liter substitutes for 2 potassium citrate pills, $1.60 a day, or $144 every 3 months.
We know About Classic Crystal Light
Crystal Light beverages include teas and other drinks. Our measurements refer to the classic or standard lemonade beverage. In what follows all of my remarks at bounded by that limitation. For example, I do not know if liters of the Crystal Light tea might contain excessive amounts of oxalate.
The Prices of Crystal Light
I did not research the price of Crystal Light Lemonade extensively, but Crystal Light Lemonade Pitcher Packs – 3-Pack – are $27.95 at Amazon. Each 3 pack provides 96 quarts of beverage. Each quart is about one liter (0.946 liters to be exact). The cost is therefore $27.95/96 or about $0.29 per 20 mEq (2 pills). This comes to $0.58 daily or $52 every three months. The Amazon site points out that prices might be lower at other stores. Please comment on the best prices you have found so everyone can benefit.
It is Not Just How Much Citrate is in the Beverage
You may have read, on a label or in a scientific paper, that some of the beverages I have listed contain quite a lot of citrate, yet we show them as inferior as an alkali. The reason has to do with the form of the citrate. If the drink is made up in a very acidic manner, much of the citrate is citric acid and will not produce alkali in the body when metabolized. It is only when the molecule is citrate itself, not the citric acid, that it can benefit you as an alkali. The graph and the additions by Dr. Asplin present the true alkali content.
Be Wary of Sugar
The beverages are mainly diet so they do not add to your caloric burden. If you sweeten them, or lace them with fruit juice, or add fruit juice or other flavorings to baking soda – see below, you will be adding calories to your diet and that may not be ideal.
But apart from weight gain, sugar has undesirable effects specific to kidney stone formers: It raises urine calcium losses. Even worse, as the article points out, urine flow rate falls as urine calcium increases, so supersaturation rises for two reasons.
What About Sodium Bicarbonate
It Has a Lot of Alkali for the Money
Baking Soda
According to Google, a teaspoon contains 4,500 mg of baking soda (sodium bicarbonate). Given the molecular weight of 84 mg/mEq (each molecule is one mEq of alkali) the teaspoon contains 53 mEq of sodium alkali. In principle, therefore, one can get alkali for nearly nothing by way of price. According to Dr. Asplin, who has – unbelievably – determined such matters, a teaspoon can contain up to 6,100 mg of baking soda depending on packing and whether the teaspoon is level or heaping.
To get 20 mEq of alkali from baking soda would require about 1/3 teaspoon. Given the variability of what a teaspoon holds, and the sheer problems of fractions of a teaspoon for every dose, I strongly recommend we abandon the remarkable cost savings from baking soda and use sodium bicarbonate tablets, which are very inexpensive and measure out the dose for you.
Sodium Bicarbonate Tablets
You can buy sodium bicarbonate tablets OTC and they are cheap. Concord, via Amazon, sells one hundred 650 mg tablets for $14.95 ($0.14 each). Rugby sells 1000 tablets of the same size for $25.77 ($0.026 each). Because each tablet contains only 7.7 mEq of alkali, it takes about 3 to match 2 K citrate pills (I realize 7.7 times 3 is 23.1 mEq but it approximates 20 mEq and the difference is not important). But that is only $0.075 for the three. So the price can come way down with this form of alkali.
It has a lot of Sodium, Too
But, alas, the 1/3 teaspoon, or the three 650 mg pills, deliver 20 mEq of sodium for each 20 mEq of alkali. The extra 20 mEq of sodium is 460 mg, about 20% of a full day’s sodium intake. For the 40 mEq (4 potassium citrate pills) we have used as a benchmark thus far, it is 40% of a full day’s sodium intake.
Whereas I am unconcerned to recommend beverages as replacements for potassium citrate pills, I have considerable reservation about sodium loads for reasons I have already mentioned and repeat here for emphasis. Excess sodium intake can raise blood pressure in those who are sensitive to salt. Although we have not as yet discussed urine calcium losses as a risk factor for stones, sodium loads will raise urine calcium, and are therefore not beneficial in that respect. If you are taking a diuretic to reduce urine calcium for stone prevention, sodium loads will reduce the efficacy of the treatment and promote losses of potassium. People with heart disease may develop worsening heart failure. Always ask your physician before using sodium bicarbonate as an alkali.
Even so, sodium bicarbonate is not sodium chloride – table salt. For physicians I have reviewed a few papers on the subject. If I sound ambivalent, I am. We may need a few more trials on this subject. In the mean time, all of my reservations hold sway. Use sodium bicarbonate sparingly.
How To Put It All Together
Compromise is the best policy, and I offer a general scheme which patients and physicians can use, if they wish, with their personal alterations. Be sure and check that your combinations provide the dosages your physician wants you to have.
Make a List of Equivalent Dosages
Each potassium citrate pill is 10 mEq; 2 are 20 mEq of alkali. Each liter of Crystal Light is just over 20 mEq of alkali. Each OTC 10 grain (650 mg) sodium bicarbonate tablet is 7.7 mEq of alkali so 3 make 23 mEq.
Make A Day’s Menu
Consider dividing the day’s alkali into 3 parts: Beverages; sodium bicarbonate; potassium citrate pills.
To Replace 2 Potassium Citrate Pills
If we only need 2 10 mEq potassium citrate pills (20 mEq), substitute 1 liter of Crystal Light (20 mEq). It is part of the day’s fluids, but also like a medication, so spread its use out over the day and, if possible, night.
To Replace 4 Potassium Citrate Pills
If we need 4 pills (40 mEq) consider 1 liter of Crystal Light and three sodium bicarbonate pills (20 mEq). The beverage and individual pills can be spread out through the day.
To Replace 6 Potassium Citrate Pills
If we need 6 pills (60 mEq), consider 2 liters of Crystal light (40 mEq) and three sodium bicarbonate pills (20 mEq) likewise spread out through the day. Reserve the potassium citrate pills for when you tire of the beverage or if the extra sodium is raising blood pressure or urine calcium.
Use Many Beverage Types But Keep the Dose of Alkali The Same
Crystal Light is convenient because of how much citrate it contains. But the chart shows many alternatives which can be used instead in larger volumes. Just remember to multiply so the total amount of alkali remains about the same. For example, you need 2 liters of Diet 7-Up to equal one liter of Crystal Light.
Be Inventive: Not All Days Need Be The Same
Mixing and matching is perfectly acceptable. Each day need not look like the one before so long as the correct amount of total alkali is used. The only drawback of a mix and match approach is confusion, so make lists and keep track. As a general rule, try to make the sodium component smaller than the beverage component. Keep the expensive potassium citrate pills as a convenience and source of variety. Obviously if sodium is contraindicated medically, and beverages are too tiresome as a source for all the alkali that is needed, potassium citrate pills can be used to replace sodium bicarbonate pills.
Not All Patients Need Potassium Citrate Or Any Other Alkali
This post is for those who have been told by their physicians to use alkali. Nothing I have written here should induce anyone to begin alkali unless their physician has prescribed or recommended it. Stone formation is complicated. Sometimes alkali can worsen stones, or even become a danger. Potassium can itself be dangerous if kidney function is below normal. Sodium loads are a problem for people with high blood pressure, heart disease, and other illnesses. Do not use sodium or potassium alkali or even high volumes of Crystal Light unless the physician who is treating your stones recommends you do so.
Stay Hopeful
Whatever caused the price rise, the changes in how insurers pay for this medication, or both, may be transitory. Millions of people have kidney stones in the US. Prices for 90 days of a standard treatment are so high that few can afford them without serious budgetary concerns. When so many people are affected, hopefully market or even political forces will countervail. In the meantime, between a few potassium citrate pills, a few liters of Crystal Light, and maybe some sodium bicarbonate, physicians can piece together an adequate regime of alkali for those patients who need it. Not every stone former does need alkali, of course.
ANOTHER AND FINAL DISCLAIMER
I have brought Crystal Light to your attention as an inexpensive substitute for some of the medicinal alkali your physicians may have prescribed. As in my initial ‘Loud Disclaimer’ I say here that I receive no financial or other benefits of any kind from the makers of this beverage, have not, in fact, ever tasted it, and do not currently plan to do so. My evidence for the value of Crystal Light comes from the work of Dr. John Asplin, and comparisons to the published work of Dr. Eisner and his colleagues.



Great information! My new kidney specialist increased my Potassium Citrate from 2 tablet per day to 4 and I am feeling the financial pinch. Wondering how effective the Potassium Citrate, Crystal Light, or bicarbonate is on brushite stones which seem to be fast growing and stubborn. And, unfortunately, Crystal Light really hurts my stomach (as does lemon juice), so it is not an option for me.
Hi Melissa, Brushite stones are a special and difficult problem and K citrate is often not very helpful. They are hard and grow rapidly so prevention is crucial. You need expert help. Perhaps your physicians might want to recommend a second opinion. If you tell me where you live I can try to identify expertise. With Telemedicine now commonplace, we can help from here concerning prevention, but people closer by would be ideal. Let me know. Regards, Fred Coe
My urogyn told me that Crystal Light is a bladder irritant. I have hypercalciuria and did not tolerate thiazides ( hospitalization for 2 days with hypokalemia) nor potassium citrate pills(diarrhea). So I was drinking two liters/ day of Crystal Light throughout the day besides other fluids. But I was urinating way to often and stretching out my bladder in an effort to reduce my risk to develop more kidney stones. Is Crystal Light a bonafide bladder irritant?
Hi Martha, I know of no evidence for which a claim. No patients taking it have ever had bladder irritation. Of course some people may experience it. But is it a proper treatment for your stones? Have you been fully evaluated? Regards, Fred Coe
i have been on diuretics for at least 2 years for hypercalcuria. It has not worked. My urine calcium is still very high. My Dr recently took me off them and put me on 4 potassium citrate pills/day. My urine calcium hasn’t changed and is still very high.
Any thoughts on why or next steps? My sodium levels are good.
Many thanks!
Hi Barbara, I have two suggestions for your physicians. Is your serum 1,25D very high and PTH low? Alternatively is your serum phosphate low? Both could explain the stubborn high urine calcium. Regards, Fred Coe
Wondering about your thoughts on using Alka-Seltzer Gold in rotation with other interventions if sodium is not an issue – yes it’s expensive in branded form, but more interested in the bicarbonate idea…here is the formula (note it contains no aspirin):
https://www.livewell.bayer.com/sites/g/files/vrxlpx31351/files/2022-06/alka-seltzer-gold.pdf
Hi Jacalyn, Not so good if you want alkali. It contains potassium and sodium bicarbonate but also citric acid; The latter is really an acid and will decompose about a third of the alkali into carbon dioxide gas – bubbles but nothing for you. So, no. Fred
I was just put on potassium citrate by the nephrolojist,I get a 16 oz bottle of liquid for $32.00 thru good rx.. at meijers pharmacy. my problem is kidney stones from too much uric acid in my urine. I’ve had to take laxatives eveynight for 20 years due to colon surgery. could these laxatives be causing the uric acid build up??
Hi Janice, Uric acid stones occur because urine pH is low and K citrate can cure them. Laxatives can lower urine pH by increasing GI alkali loss, and k citrate is also an effective prevention. It is not the amount of uric acid in the urine, it is always the acidity being too great (low pH). One issue with laxative use is uric acid vs. ammonium urate stones. Be sure it is uric acid stones that you form. The urate stones should also respond to potassium citrate but may have special issues. Regards, Fred Coe
In your book, which I just downloaded, it mentions veggies as a source of citrate. I’ve been on a Mediterranean diet for 5 years. I ate 10 servings fruit and veggies for my 24 hour urine. My citrate was 47. That’s not a typo. The 5mm stone was found by accident during an Xray for another condition. I was not informed at that time. Still 5mm, It made itself well known 18 months later when it decided to move. Removed a month later, with no symptoms in between. Since it obviously started forming well before then, I wonder what role citrate played, if any? Also, what role is my mostly plant based diet having on citrate or stones? Urine pH runs between 6.3 and 6.8. I am now on 1/2 lemon juice spread through day and 10mg K citrate bid. Next 24 hour is next month.
Hi Kim, with such a diet and so low a citrate and stones, possibly you have an underlying disease beyond simple stone forming. Since your urine pH is not low, citrate should be much higher than you report. Was the stone calcium oxalate or calcium phosphate, incidentally? Given your odd results, perhaps your physicians might want to consider referral to a convenient university program. I do telehealth from my university, but I imagine any fine stone center will do as well. But whatever, you have some odd condition that can be identified and treated. Regards, Fred Coe
Hello Dr Coe, I’ve been taking OTC potassium citrate from amazon in the UK and have tried 3 different brand to increase my citrate but it’s still dangerously low. Do you think it’s wise I seek prescription Potassium citrate and see any difference. The trouble is the UK NHS does not provide this so I need to seek worldwide sources. thanks again
Hi Jonathan, Do the OTC products contain less citrate than the prescription pills? The latter contain 10 mEq of citrate alkali each and the usual treatment is about 20 to 40 mEq/d. The US has many OTC supplements with sufficient alkali – perhaps some are also in the UK. Food grade potassium citrate is available from routine stores, and inexpensive balances have allowed many to weight out 1080 mg doses – 10 mEq each. But more to the point, is your urine citrate so low as to pose a risk of stones (below 400 mg/d)? If so, is there perhaps an underlying reason like renal tubular acidosis? Regards, Fred Coe
I’ve been making kidney stones, Calcium Oxalate, for 40 some years. Most were small and passable. Twice in that time I need surgical intervention to help with passing the stones. There were also large gaps without stones. About 20 years ago a urologist suggested Oxalate could be an issue and recommended that I stop eating spinach, I was eating a lot of it, but I wasn’t given much more information than that and there wasn’t any follow up. After stopping with spinach the stones reduced in size and quality.
Most recently I had a couple of stones that were not passable. I reviewed my diet and what other causes could be causing them. I learned that almonds and other nuts where other foods that contained a lot oxalate and I had been consuming a lot of those as well. I cut those out of my diet as well. I’ve also use an app to prompt me for better hydration of water. The latest urine collection reflects these changes. I don’t have a baseline of values before the diet/hydration changes.
Urine Volume(liters/day) 2.66
SS CaOx 2.56
Urine Calcium (mg/day) 110
Urine Oxalate (mg/day) 32
Urine Citrate (mg/day) 216
SS CaP 0.07
24 Hour Urine pH 5.313
SS Uric Acid 1.64
Urine Uric Acid (g/day) 0.746
As a ballpark figure how much Potassium Citrate would it take to change my levels to the suggested levels? Other suggestions? I am seeing a urologist and he is giving me a change and increasing my citrate by diet instead of taking pills with a follow up 24 hour collection. I just have no idea how much lemon water, crystal light… to try between now and the test. Also how quickly is the urine collection effected?
Hi Mike, it looks as though you are most likely to form uric acid stones from the low urine pH. Your urine oxalate is modest and calcium normal and SS CaOx below the level at which stones form. By contrast you have UA SS above 1 because of the low pH. Do you know the composition of your recent stones? Usual starting doses to raise urine pH and stop uric acid crystallization are 10 mEq tabs, 2 twice a day, with adjustments based on follow up 24 hour testing. Regards, Fred Coe
All tested stones were Calcium Oxalate. That’s not to say that others, and there have been many, were not other types of stones.
The above testing was after changing my diet from having been NO spinach for years to removing all almonds and almond products and some other higher oxalate foods starting a few weeks before the test.
Thank you, Mike
Hi Dr. Coe,
I am recurrent stone former and my stone composition is calcium oxalate. I made changes to my diet by removing high oxalate food but I’m still forming stones.
My recent 24 hr urine test confirmed I have low citric acid. Here are the numbers along with other details:
TOTAL URINE VOLUME: 1.33 L
PH URINE: 5.9
CALCIUM, 24 HOUR URINE: 135
OXALATE, 24 HOUR URINE: 42
URIC ACID, 24 HOUR URINE: 654
CITRIC ACID, 24 HOUR URINE: 2.86 L
SODIUM, 24 HOUR URINE: 82
SULFATE, 24 HOUR URINE: 18
PHOSPHORUS, 24 HOUR URINE: 713
MAGNESIUM, 24 HOUR URINE: 33 L
POTASSIUM, 24 HOUR URINE: 39
CREATININE, 24 HOUR URINE: 1148
BRUSHITE: 1.03
CALCIUM OXALATE: 3.21 H
SODIUM URATE: 2.08 H
URIC ACID: 2.47 H
My urologist prescribed potassium citrate 10mEq twice daily. The first one I took didn’t sit well with my stomach, and kept my heart beat in high 90s through out the day.
I am wondering if I can replace this with just mixing lemon juice in water three times a day after/with meal, few drops of lemon in one glass of water.
Will that be enough or should I start taking Crystal Light – if yes what should be the dosage and timings?
Also I heard lemon mixed with water can cause damage to your teeth. Is that true and is there a way to avoid it?
Thank you very much for your insight and articles, very helpful and life changing.
Hi Adam, Your urine volume is SO low it is itself a good cause of stones. I believe you meant your urine citrate was 286 mg/d, not 2.86. I notice your urine magnesium is also low and wonder why: proton pump drug for reflux, diuretics,, bowel disease, perhaps under collected urine?? I would not trust this urine because of these issues and would get another with care to follow the collection rules closely. If like this one, then fluids are your most important beginning. Any alkali will increase urine citrate unless there exists a primary citrate problem. Crystal Light is fine, there are many OTC alkali products.Regards, Fred Coe
One word, GoodRx (www.goodrx.com).
After my deductible’s exhausted, I’d be paying $722 a year for 4 pills a day of GENERIC potassium citrate at CVS ($0.50 each).
GoodRx provides a coupon that gets the price per pill down to $0.30 at Costco (which allows non-members to use their pharmacy).
That’s $432 per year for a simple potassium salt (albeit a time release one).
I’d go vegetarian but my innards don’t handle fruit and vegetables that well.
Not that they appreciate the horse pills all that much.
I actually have to eat more food to keep the pills from bothering me.
I’m writing the stones off to old age.
I got the first stone that I’m aware of last year.
3 score and 10.
After that, good luck!
Hello Dr. Coe, food grade potassium citrate is very cheap, can we use that to make a drink? will it be effective as pills? Thanks. – Jingkun
Hi Jingkun, Many have thought about this and done it. Just be sure you have a balance capable of weighing out a proper dose – 1000 mg is about 10 mEq of the KCitrate crystals. And be sure your physicians know what you are doing and approve. Regards, Fred Coe
Hi Dr Coe,
I have a history of high uric acid and uric acid stones, but, based on your suggestion which was confirmed by my primary care doctor, since around 2019-2020, in addition to Allupurinol (300mg/day), I am also consuming Pottasium Citrate 10ml/twice a day.
I have been monitoring my urine pH and it’s always between 6.0 to 6.5 and no stones have been formed so far.
In the past, whenever I had the stones, my creatinine level was below normal this is why, in 2021, when my creatinine level was below normal, my primary doctor sent me to do an ultrasound to check if there was any stone.
Ultrasound came back reporting there was no stone, however, the ultrasound incidentally found a lesion on my left kidney.
Since then, I went to another ultrasound series and most recently, doing CT every 6 months and then met with a urologic oncologist.
The lesion is growing marginally from 11mm from the initial CT in Oct 2021, 18mm in Jan 2023, 20mm in July 2023, and 23mm in Feb 2024.
During my initial meeting with the urologic oncologist in March 2023, he said no biopsy was needed at that time for 2 reasons:
Even if a biopsy said that the lesion is cancerous, the right course of action at that time remains the same, active monitoring that is.
A biopsy can say that it was benign, however, it may change to cancerous later on.
I met a urologic oncologist a few days ago and he suggested surgery to take out the lesion.
I wonder if, in your opinion, my history of high uric acid and specifically uric acid stone has to do with my kidney lesion and if so, what do you suggest me doing? I, of course, will always confirm everything with the specialist first. Many thanks in advance!
Hi Niko, There are no links between lesions as you describe and uric acid stones. I am not an expert on management of kidney lesions, and would suggest you follow the advice of your physicians. As for the oncologist vs your other physician I can have no opinion. But lesions that are enlarging and might be malignant are best done away with as a general rule. Best, Fred Coe
Hi,
I am not sure if someone has already asked this question, but if I am having a hard time finding Crystal Light lemonade, do Crystal Light lemon-lime and/or Crystal Light pink lemonade have comparable levels of potassium citrate? Both flavours do have potassium citrate listed in the ingredients.
Thanks
Hi Paul, I do not know. We measured what is in the article. But there are now a lot of OTC alkali. The article compares them. Regards, Fred Coe
Hi Dr Coe
My sister recently sent me a link of online pharmacy owned by Mark Cuban- called cost plus. I checked out their prices and seem quite unbelievably cheap. Is this real ? Also just wondering what the ER stands for after the name Potassium Citrate ER. Says it’s a generic brand.
~ Thanks for your wonderful work and write up about this.
https://costplusdrugs.com/medications/potassium-citrate-er-10meq-1080mg-tablet-extended-release-urocit-k/
Hi Shelly, Good. ER mean extended release. I presume this is a valuable addition for patients. Thanks for pointing it out. Fred
Hello Dr. Coe. I saw my nephrologist last week after doing my 24 hr urine, ultrasound, and blood work. He told me my citrate level was at 0. It had been 1.5 on a previous appt. I take 3 k Citra every day. I was taking four a day but I kept getting chancres in my mouth. He suggested effervescent potassium citrate but the pharmacist said it is $180.00 for 3 months. I wondered if you would know any reason how a citrate level could go this low. I follow the kidney stone diet with Jill Harris.
Thank you.
Cathy A.
PS. I really don’t want to start crystal light if it contains aspartame.
Hi Cathy, Urine citrate is almost never 0. Possibly your urine volume is so high the citrate concentration is too low to measure. Possibly you have a reason for very low citrate (although not 0) such as bowel disease, potassium depletion, renal tubular acidosis. I imagine your physicians are puzzled as well and search for the answer. Rarely a 0 citrate is lab error. Regards, Fred Coe
Dr Coe,
You mentioned here that a patient rarely would have 0 urine citrate and that possibly the urine volume was high. Does this mean that if we keep our urine volume high say 3.75-4L/day that shows in the 24 hour urine that could actually lower our urine citrate level?
Hi Diane, No. The lab has a concentration below which it will not measure urine citrate for patients – purely a convention of how they calibrate machines. When urine volumes are very high – >4 liters/d – and urine citrate is lowish the concentration can fall below detection. But usually this is not a problem in normals. Consider 500 mg/d of citrate and 5 liters of urine – 100 mg/liter, 10 mg%. That is easily measured. But for that volume, a lower citrate of perhaps 250 mg would be close to the lab lower limit of detection on the machines. So higher volumes have nothing to do with how much citrate you lose but whether or not the lab can measure it. Regards, Fred Coe
Dr Coe, I submitted a question to this page a month ago. Did you get it? If not, I’ll try to write it up again. Thanks!
Hi, I must have answered because your question does not show up in the que. Perhaps it somehow was lost. Please consider sending it again. Fred
Hi Dr. Coe. The stones I get are always calcium oxalate. For 3 years I’ve followed a 4g / day. I’ve done many 24-hour urine tests, for the most recent (listed below) my intake of sodium was 809mg, oxalate 220mg, calcium 1234, and potassium 4632. Unfortunately, my Urine Citrate ranges from 256-414. My questions:
1. What dossage of NOW Potassium Citrate Powder should I try?
2. My Urine pH is always very high. Is that an issue for me?
3. Do you think I can cut back to 3L of h2o / day, since I have spend a lot of time peeing?
FYI: I take 25mg of HCTZ (12.5 twice / day, 12 hours apart) to decrease my urinary calcium level (without the HCTZ my urinary calcium was ~300). I’m also on 40mEq of Potassium Chloride (1 pill twice / day, 12 hours apart) to counteract the side effects of HCTZ.
Volume 4.21
SS CaOx 2.29
Urine Calcium 158
Urine Oxalate 33
Urine Citrate 414
SS CaP 0.97
Urine pH 6.828
SS Urice Acid 0.04
Urine Uric Acid 392
Sodium 44
Potassium 118
Magnesium 159
Phosphorus 965
Ammonium 40
Chloride 91
Sulfate 24
Urea Nitrogen 7.18
Protein Catabloic Rate 0.9
Creatinine NA
Creatinine/Kg 19
Calcium/Kg 2.5
Calcium/Creatinine 132
Hi Cameron, I am surprised the stones are calcium oxalate not calcium phosphate. I would stop the K citrate and use K chloride so the urine pH can be lower. Your urine citrate is is lowish and your urine ammonia is above your sulfate acid load. Perhaps your urine pH will fall with KCl, as this pattern can reflect potassium depletion from your cells- serum potassium is not a good gauge of this. People prone to calcium phosphate stones have high urine ammonia excretion lowish citrate and high pH from what appears to be a genetic cause – not as yet defined – and you may be like that. See if your physician might agree with this. Of course I am far away and do not know details. Regards, Fred Coe
I’ve had numerous stones tested through the years and they always are Calcium Oxalate Dihydrate (Weddellite) 15%, Calcium Oxalate Monohydrate (Whewellite) 70%, Carbonate Apatite (Dahllite) 15%.
The results I posted above were with me taking 40mEq Klor-Con tablets (2×20), 50mg HCTZ (2×25), and drinking 1/2 cup of lemon juice with 4 liters water. This test’s Ammonium vs Sulfate result is an anomaly. All my previous results had the two basically even, or with Sulfate being higher. Not sure why this one was so different, since I didn’t have any diet change.
My first 24-hour urine test back in 2021 had my Urine Calcium at 290 & Urine Citrate at 579. I had already changed my diet to a be < 1000mg sodium, so we added the HCTZ which worked wonderfully in lowering my Urine Calcium to the 150s, but also lowered my Citrate to 259. We then added the 40mEq of K chloride, but in the 3 tests since then, my Citrate has been 414, 339, and 414. My Urine pH has always been ~7, but I'm a vegetarian, rarely even eating eggs, but have multiple servings of dairy every day.
Since I always get calcium oxalate stones and my results seem good (other than my Citrate & pH), should I just not worry about it? Or should I ask my urologist to increase my K Chloride from 40 to 80mEq? Or should I try the K citrate powder? Or…? 🙂
Hi Cameron, This question is perhaps beyond what I should say given I do not really know your entire medical situation. But 15% calcium phosphate does raise my eyelids a bit. Perhaps more KCl might be a benefit. Ask your physician. Best, Fred Coe
Hmmm. Something really weird is happening. Sorry for all the posts. Here’s my final try….
“For 3 years I’ve followed a 4,000mg / day.”
I have used the correct information in my response. Fred
Are these recommendations the same or similar for cystine stone prevention?
Best,
John
Cystine stones are so special a case each instance needs its own evaluation using a special kind of urine testing. Fred
I’m on vacation and had a first gout attack. I’ve been drinking as much water and Crystal Light as I can and it seems to have improved. My toe is now back to normal size and the “nodule” seems smaller. As I can’t visit my doctor (who is in a different country) is there anything else I should be doing or not doing at this time?
Thank you.
Hi Sally, OTC NSAIDS help as does colchicine. But if it is getting better wait until you get back to your doctor. Fred
Dear Dr. Coe,
Thank you for your invaluable website. A few years ago, prior to my lithotripsy, I learned a lot from your great notes.
Inspired by your explanation of how helpful potassium citrate can be, I’ve developed a cost-effective homemade solution. By mixing ½ teaspoon of potassium bicarbonate with ¼ teaspoon of citric acid in a glass of water, I create a 21 mEq potassium citrate drink. The mixture effervesces for about 30 seconds, indicating the reaction’s completion. The slight excess of citric acid ensures full conversion of the bicarbonate, resulting in a mild citrus flavor.
On Amazon.com, a 2 pound bag of citric acid is priced at $10 USD, and potassium bicarbonate is available for $29 USD per kilogram. Consuming two 21 mEq drinks daily results in an approximate daily cost of 12 cents, totaling about $45 annually. (I have also switched to using potassium bicarb as a healthy substitute for baking soda in recipes.)
To enhance the taste, I add ice cubes, a packet of sweetener, and a splash of lime juice. I recommend verifying these calculations using tools like ChatGPT which do a good job of detailing all the molecular math behind the recipe.
Best regards,
John A in Toronto
I checked the recipe against 4 AI’s and discovered an error in the initial chatgpt math related to the density of K bicarb. The recipe results in approximately 27 meq of K citrate. If you decide to post the recipe, I suggest the edit: replace 21 with 27. Here is the question I posted to chatgpt, llama, copilot and claude: “My recipe of 1/2 tsp of potassium bicarbonate plus 1/4 tsp of citric acid in a glass of water results in a 21 meq potassium citrate drink. Correct?”. The difference in results all related to the assumed density of K bicarb. I weighed 1 level cup of the K bicarb I have and the weight was 258.5 mg. Given this followup information, the AI’s all came to the same 27 meq calculation.
I weighed a cup of the two ingredients, so the AI’s now all agree that 1/2 tsp of K bicarb weighs 2.69 gm and 1/4 tsp of citric acid weighs 1.2 gm. This results in the 27 meq calculation. The daily cost is corrected to 18 cents and the annual cost is $66.
I asked Gemini pro model to calculate the mEq of K in 1/2 tsp of K bicarbonate.
Here’s how to calculate the mEq of potassium in 1/2 teaspoon of potassium bicarbonate:
1. Find the Molar Mass of Potassium Bicarbonate (KHCO3):
K (Potassium): 39.10 g/mol
H (Hydrogen): 1.01 g/mol
C (Carbon): 12.01 g/mol
O (Oxygen): 16.00 g/mol (x3 = 48.00 g/mol)
Total Molar Mass: 39.10 + 1.01 + 12.01 + 48.00 = 100.12 g/mol
2. Determine the Density of Potassium Bicarbonate:
The density of potassium bicarbonate is approximately 2.17 g/mL.
3. Convert Teaspoons to Milliliters:
1 teaspoon = 4.93 mL
1/2 teaspoon = 4.93 mL / 2 = 2.465 mL
4. Calculate the Mass of Potassium Bicarbonate in 1/2 Teaspoon:
Mass = Volume x Density
Mass = 2.465 mL * 2.17 g/mL = 5.35g
5. Calculate the Moles of Potassium Bicarbonate:
Moles = Mass / Molar Mass
Moles = 5.35 g / 100.12 g/mol = 0.0534 mol
6. Determine Moles of Potassium Ions:
The chemical formula KHCO3 shows that there’s one potassium ion (K+) for every molecule of potassium bicarbonate.
Therefore, moles of K+ = 0.0534 mol
7. Convert Moles to Milliequivalents (mEq):
For monovalent ions like K+ (charge of +1), the number of milliequivalents is equal to the number of millimoles.
Millimoles of K+ = 0.0534 mol * 1000 mmol/mol = 53.4 mmol
mEq of K+ = 53.4 mEq
Therefore, there are approximately 53.4 mEq of potassium in 1/2 teaspoon of potassium bicarbonate.
They got what appears to be 2x your value. This is an important issue.
I did a rough calculation: if the density is 2 then the 1/2 tsp is really 1 tsp full of the k bicarbonate powder or 5000 mg. The MW is about 100 which makes 50 mEq more or less, just like Gemini got.
So people would get about 50 mEq of potassium from your recipe and I am warning people in general to be careful and check with their physicians if they can tolerate such a dose. Frankly, 1/4 tsp would be safer, but I am concerned about such a small measure to work with a potassium based medication – errors that look small might be too large. Regards, Fred
Hi Dr. Coe,
Thanks very much for recalculating that using Gemini Pro!
I see the source of our discrepancy—the Gemini Pro calculation used the solid crystalline density of potassium bicarbonate, which is significantly higher than the density of the granulated powder form typically used in recipes.
This is similar to sodium chloride: a solid chunk of NaCl has a density of 2.16 g/mL, while granulated table salt is around 1.2 g/mL (USDA), and Morton Kosher salt even lower at 0.86 g/ml.
In my case, I’ve measured potassium bicarbonate powder directly and consistently find it weighs about 6 grams per teaspoon, which works out to approximately 1.18 g/mL—significantly lower than the crystalline density. But, as with salt, different vendors may have K bicarb powder of different densities. Because of this, I’ve moved to measuring by weight rather than volume to avoid estimating density altogether.
I now make weekly batches based on my prescribed dose of 20 mEq potassium citrate twice daily. For one week (14 doses), I require 280 mEq total (20 mEq × 2 doses/day × 7 days). This corresponds to 0.28 moles of potassium bicarbonate, and thus about 28 grams (0.28 moles * 100.115 gm/mole).
To prepare the solution, I combine 28 grams of potassium bicarbonate with 28 grams citric acid (an amount sufficient for full conversion into potassium citrate plus a bit extra for a pleasant tartness) and dissolve it into 3.5 cups of water, making a 1/4 cup serving equal to 20 mEq of potassium citrate.
With each ¼ cup serving, I add ice cubes, a packet of sweetener, and about 8 ounces of water. I’ve stopped adding lime juice, as the drink is pleasantly tart as-is.
Thanks again for taking the time to double-check—I appreciate your insights!
Best regards,
John
Hi John, Sounds good for you but I would advise other readers to steer clear unless you have the desire, skill, and patience to be sure about the density of the potassium bicarbonate you are using. This is a work around for a very skilled patient, and I admire it, but not for everyone. Fred
And finally (I hope) the higher weight we are now using for what 1/2 teaspoon of k bicarb weighs means we need more citric acid to ensure all bicarb is converted. Bicarb is bitter, citric acid is tart (so tastier). So we need 1/2 teaspoon of citric acid. i’ve been using 1/4 teaspoon and it tasted fine, but I am quite generous with the lime juice I add. Once I got all four AI models to agree on the 1/2 tsp of K bi for 2.69 gm and 1/2 tsp of CA for 2.41 gm, they quickly agreed on annual costs of a bit less than $77. And daily costs of a bit less than 21 cents.
Hi John, I answered with a Gemini calculation that looks about right to me. The density of K bicarb is about 2 and there is about 54 mEq of K in 1/2 tsp of the k bicarbonate. Your value is in error by 2.
As for citric acid, it is complex. If you add too much you will begin to lower the pH so the citric acid species will be a higher and higher fraction of the total – less and less alkali for the patient. Here is how one calculates the amount of citric acid:
1. The Balanced Chemical Reaction:
Potassium bicarbonate (KHCO3) reacts with citric acid (H3C6H5O7) to produce potassium citrate (K3C6H5O7), water (H2O), and carbon dioxide (CO2). The balanced equation is:
3 KHCO3 + H3C6H5O7 -> K3C6H5O7 + 3 H2O + 3 CO2
2. Understanding Milliequivalents (mEq):
mEq represents the amount of a substance that will react with or replace 1 millimole of hydrogen ions (H+).
For bases like KHCO3, the mEq is equal to the millimoles multiplied by the number of replaceable hydroxide ions (OH-) or equivalent basic groups. KHCO3 has one bicarbonate ion (HCO3-), which can accept one H+. So, 1 mmol of KHCO3 = 1 mEq of KHCO3.
For acids like citric acid (H3C6H5O7), the mEq is equal to the millimoles multiplied by the number of replaceable hydrogen ions (H+). Citric acid is tribasic – it has three replaceable hydrogen ions. So, 1 mmol of citric acid = 3 mEq of citric acid.
3. The Key Relationship:
For complete neutralization, the mEq of the acid must equal the mEq of the base. We know we have 53.4 mEq of KHCO3. Therefore, we need 53.4 mEq of citric acid.
4. Calculate Millimoles of Citric Acid:
Since 1 mmol of citric acid provides 3 mEq, we can calculate the millimoles needed:
Millimoles of citric acid = 53.4 mEq / 3 mEq/mmol = 17.8 mmol
5. Convert Millimoles to Grams:
The molar mass of anhydrous citric acid (H3C6H5O7) is 192.12 g/mol.
Grams of citric acid = 17.8 mmol * (1 g / 1000 mmol) * 192.12 g/mol = 3.42 g
6. Convert Grams to Teaspoons:
This is the trickiest part because the density of citric acid can vary depending on its granular form (fine powder, coarse crystals, etc.). We need a density to convert mass to volume. A commonly used density for granular citric acid is approximately 0.85 g/mL. However, this can vary, and it’s crucial to use the density of the specific citric acid you have. If you have a food-grade citric acid, the packaging might list a density or a serving size in grams and teaspoons, which you could use to calculate the density.
Using 0.85 g/mL as an example:
Volume (mL) = Mass (g) / Density (g/mL) = 3.42 g / 0.85 g/mL = 4.02 mL
1 teaspoon = 4.93 mL
Teaspoons of citric acid = 4.02 mL / 4.93 mL/teaspoon = 0.82 teaspoons
Important Considerations and Caveats:
Density is Crucial: The final answer in teaspoons is highly dependent on the density of the citric acid you are using. The 0.85 g/mL value is an estimate. If you have a different density, you must use that value in the calculation.
Anhydrous vs. Monohydrate: The calculation above assumes anhydrous citric acid (no water molecules attached). Citric acid is also commonly found as a monohydrate (H3C6H5O7 · H2O), which has a higher molar mass (210.14 g/mol). If you have the monohydrate, you’ll need a slightly larger mass (and therefore volume) to achieve the same number of moles of citric acid. You would need 3.74g of the monohydrate. This will change the final volume.
Complete Neutralization: This calculation assumes complete neutralization, meaning all the bicarbonate is reacted. In practice, you might use a pH indicator or meter to ensure you’ve reached the desired endpoint.
Precision: For precise work, use a scale to measure the citric acid by mass (grams), as this is much more accurate than measuring by volume (teaspoons).
In summary, assuming a density of 0.85 g/mL for granular anhydrous citric acid, you would need approximately 0.82 teaspoons to neutralize 53.4 mEq of potassium bicarbonate. However, you should verify the density of your specific citric acid for an accurate result, and preferably measure by mass (grams) rather than volume. If using the monohydrate form, you would need closer to 0.89 teaspoons, again assuming the same density. FROM GEMINI
So with 1.2 tsp of K bicarbonate you get 53.4 mEq of bicarbonate and potassium. To neutralize it you need 0.82 tsp of citric acid. But the amount depends on the density of the product as noted and that needs to be checked.
All in all, this approach is probably fine for people who can and will go to the trouble to get things right for their particular citric acid product, but it may not be a goo thing for people in general to try. So I am against endorsing this and suggest people do not use this approach unless they can understand the chemistry and perform the needed additions accurately. INcidently I checked the GEMINI calculation for the amount of K bicarbonate and it is correct. Regards, Fred Coe
Should I be worried about the aspartame that is in Cristal light lemonade? I would use it to increase my Citrate levels but am worried about daily aspartame use.
Hi Theresa, A brief search using Perplexity indicates a lack of support for aspartame as posing any human health hazards. “Conclusion
In summary, while some epidemiological studies in humans have reported weak associations between high aspartame intake and certain health risks (cancer, cardiovascular events, migraines in sensitive individuals), these findings are inconsistent and often confounded by other factors. Regulatory agencies, including the FDA, WHO, and JECFA, conclude that aspartame is safe for the general population at current intake levels, except for individuals with phenylketonuria. The overall human evidence for harm remains limited and inconclusive, and further high-quality research is needed” Big Sugar has long falsified science to maintain its hold on us, this has included efforts to discredit safety of substitutes. Best, Fred Coe