
Uric acid stones, to me, means not just pure uric acid stones but any uric acid in stones. If this seems fey, let me explain. Uric acid is a peculiar kind of crystal. Low urine pH causes them and treatment that raise urine pH prevent them altogether. Whether they form combined with calcium stones or pure, treatment is the same.
Why then scruple over percentages? If I find uric acid in any stone, I look at urine pH with a yellow eye. Should it be low I treat it surely and on the moment so at least that crystal be banished forever.
The Profligate Punished by Neglect, Edward Penny 1774 catches the common motif of diet excess, obesity, diabetes, and gout – the joint manifestations of uric acid crystals. Note the abdominal fat denoted by his overly tight vest. All of these states can lower urine pH and lead to uric acid stones.
This article has a pragmatic leaning and eschews excessive scientific details. I have written a more mechanistic article that explores how the low urine pH might arise and cause uric acid stones. Read this one first unless you are already reasonably expert.
Who Are Uric Acid Stone Formers
Stone Analysis
Given my prior reasoning, I call patients who have any uric acid in their stones uric acid stone formers but reserve the right to use compound names when needed. If all stones are only uric acid, I call such patients pure uric acid stone formers. Those whose stones contain uric acid and other crystals I call mixed uric acid /x stone formers: mixed uric acid calcium oxalate, mixed uric acid calcium phosphate stone formers, as examples.
These niceties of naming have the practical value of calling to mind the perpetual need for dual or multiple treatments – for uric acid but also for whatever crystals might be present.
Radiographic Evaluation
Commonly uric acid stones show poorly on routine flat plate x rays having only carbon, nitrogen, oxygen and no heavier atoms such as calcium. On CT scans they do not look different from calcium stones but radiographic density can be measured and tends to be lower. As this article points out, machines differ in their results and evaluation may therefore be less than perfect. Dual energy scanners are more precise, but also prone to many potential artefacts. Multiple reports, by contrast, indicate that CT measurements of radiographic density can reliably distinguish uric acid stones from calcium stones.
A reasonable present view is that lower radiographic density is an excellent clue to uric acid in stones, but far from definitive as stone analysis is. I hesitate to classify a patient on scanning evidence alone.
Signs and Symptoms of Uric Acid Stones
Pigmented Stones and Crystals
Being stones, uric acid stones cause the usual problems of pain, obstruction, bleeding and infection. But they have some special features. The most obvious is stone color – red to orange because the crystals take up a variety of pigments mostly derived from hemoglobin breakdown. Recently scientists have determined the structure of one of these – urorosein. Sometimes, coarse or fine orange or red gravel passes, made up of uric acid crystals.
Rapid Crystallization, and Stone Growth
Because the crystals form not as a complex lattice like calcium with oxalate or with phosphate but simply as uric acid crystallizing with itself, the process can be swift to begin and require very little supersaturation. Said more technically the energy required to create the crystal is relatively low. This means the upper limit of metastability – the supersaturation needed to initiate crystal formation is not far from solubility, so values above 1 even if below 2, could suffice. Practically it means that bursts of supersaturation during the day can bring on showers of gravel and growth of stones.
Also, urine contains a lot of uric acid. Common daily losses of oxalate approximate perhaps 25 – 50 mg, compared to 600 – 1,000 mg of uric acid. The sheer amounts available when coupled to the rapid and facile crystal formation and growth allow stones to enlarge rapidly and achieve very large sizes, enough to fill the renal pelvis and calyces – so called staghorn stones.
Acute Uric Acid Nephropathy
Very uncommonly, sudden lowering of urine pH coupled with low urine volume can cause crystallizations in the terminal collecting ducts with acute kidney failure. This was once not uncommon during treatment of malignancies, but modern attention to uric acid surges from tumor killing has made it rare indeed. Today, one does not expect to see it apart from unusual situations.
Uric Acid Supersaturation
I made the figures for this section anew but from a lovely data file constructed some years ago by Joan Parks, who was my scientific colleague from 1976 until her retirement about 8 years ago. Her legacy of curated data files sustains a lot of my public writing, now, and she deserves a place in it. 
Effects of Urine pH
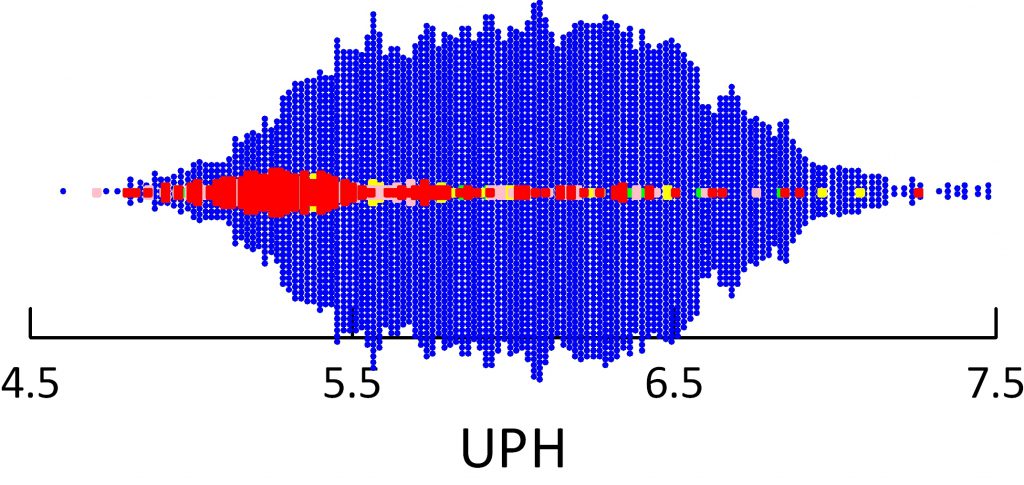
Uric acid crystals form like all crystals because of supersaturation. In this instance, that supersaturation varies remarkably with urine pH.
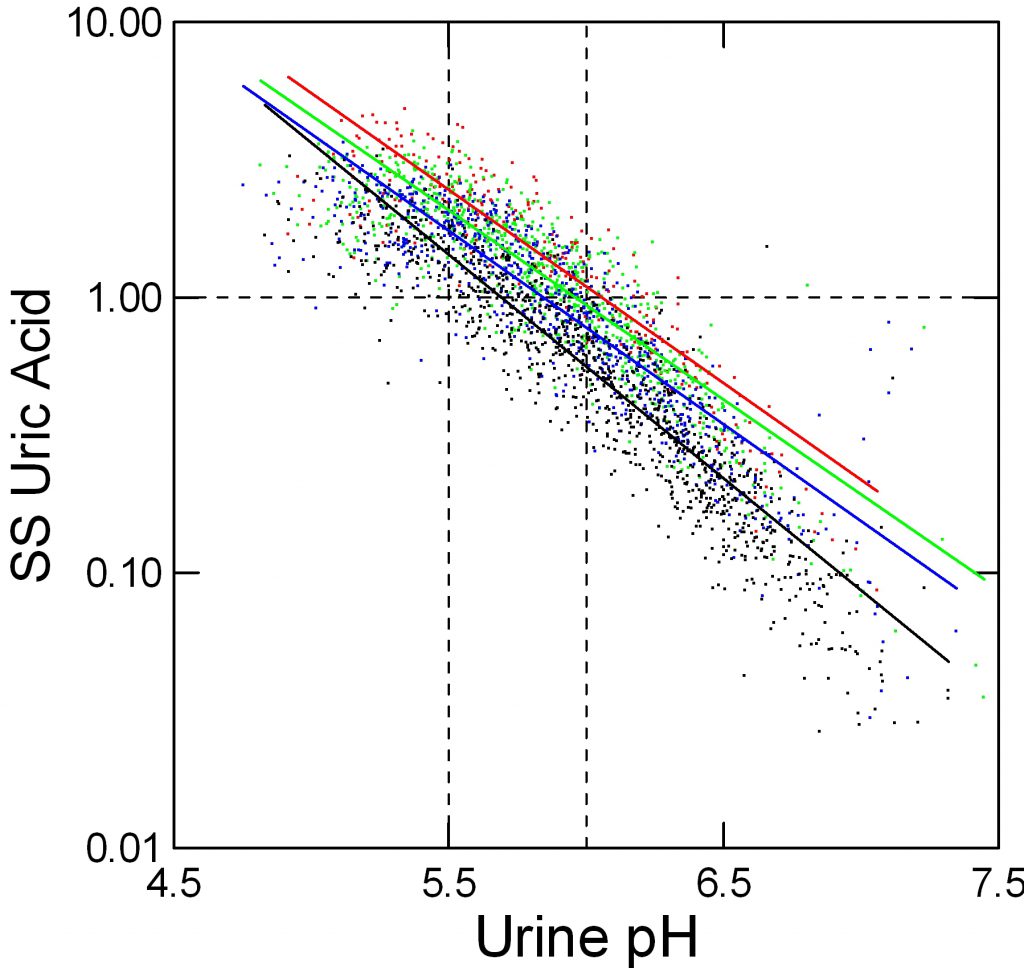
In the figure, supersaturation ranges from 0.01 to 10 fold. The dashed line at 1 represents equilibrium, or saturation, the level where crystals neither form nor dissolve. The horizontal axis shows urine pH. The dashed lines at 5.5 – acid urine and 6 neither acid nor alkaline urine are for visual reference.
The tiny points each are one 24 hour urine from patients and normal people. Like an ancient Persian scimitar, points curve downward from 8 to 0.03 as pH rises from 4.5 to 7.5.
Effects of Urine Volume
Urine volume matters. Low volumes (red) 0.5 to 1 liter/day give higher supersaturation than 1 to 1.5 liters/day (green), and 1.5 to 2 and above 2 liters/day (blue and black) lower supersaturation progressively.
But pH trumps volume. At pH 5.5, the whose distance from red to black varies supersaturation between about 2 and 5 fold (use the lines for averages) whereas raising pH from 5.5 to 6 brings almost all the points down below 1. Below 5.5 virtually no points are below 1 at even above 2 liters of urine volume daily.
Effects of Uric Acid Excretion
In speaking about excretion of uric acid we need to insert a note about the molecular species involved.
Form of Uric Acid in Urine
Uric acid is a weak acid, which means it can take up or donate a proton to water. When it has its proton, that proton neutralizes much of its charge, so water molecules cannot themselves form charge bonds with it to keep it in solution. This means that the molecule becomes very poorly soluble and tends to crystallize.
When it loses its proton into solution, it has a charged site for water to relate to and also requires a counterion, which in urine will be sodium, potassium, and ammonium ion. These ‘salts’ of urate – the name for uric acid when it has given up its proton and is a charged ion – can themselves form crystals just like calcium and oxalate form a salt – calcium oxalate – that can crystallize. But all three salts have much higher solubility than uric acid itself.
Effect of Uric Acid Excretion on Supersaturation
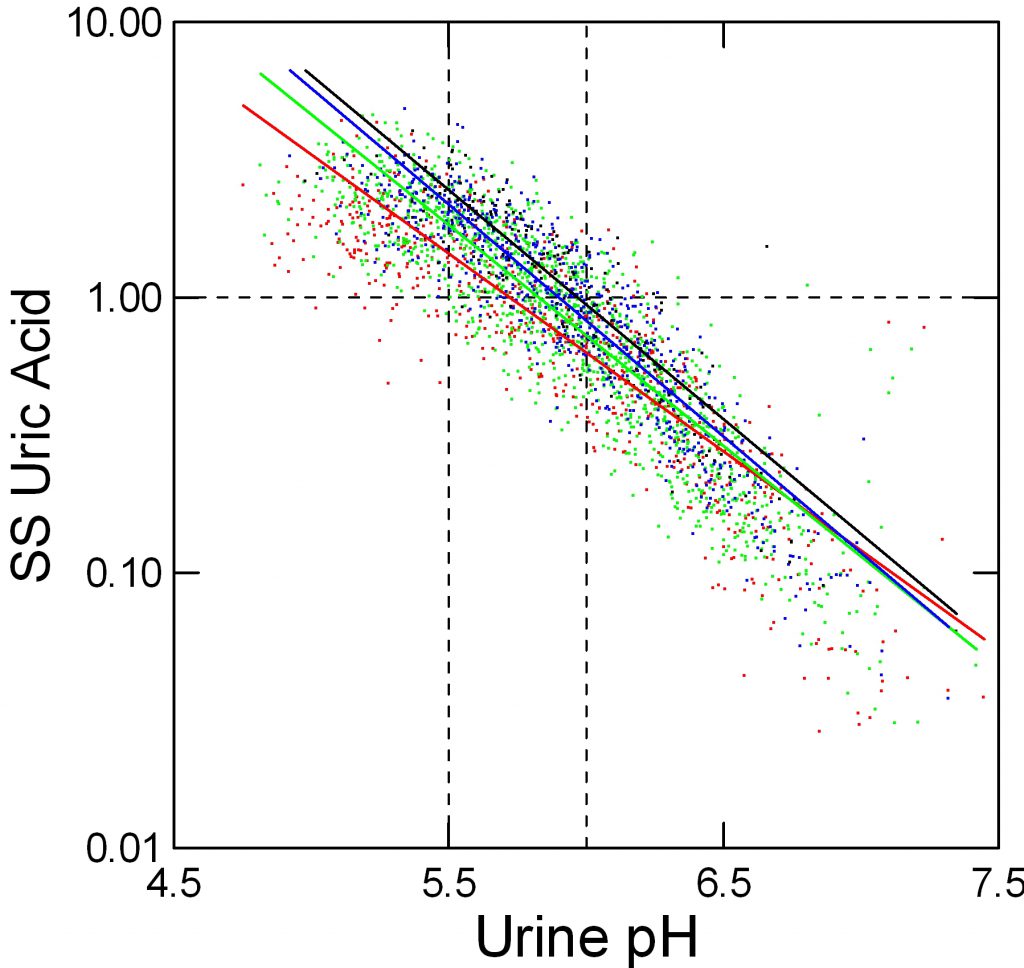
When we measure and report urine uric acid excretion we show the sum of all salts and the acid in one number. Obviously this total should affect supersaturation, but the effect is relatively small because so much depends on pH that sets the percentage of uric acid per se – the fraction that has its proton and is therefore poorly soluble.
Here, red, green, blue and black stand for below 500, 500 to 750, 750 to 1,000, and over 1,000 mg/d of urine uric acid excretion respectively. As for urine volume, the total amount of uric acid matters; a fivefold increase from below 500 mg to over 1,000 mg/day raises supersaturation at pH 5.5 from about 1.2 to about 3 fold.
Urine pH of Stone Formers
One presumes that urine pH of uric acid stone formers must lie below that of other kinds of stone formers, and numerous reviews and case descriptions have proven this true.
My own collected data make the point as well as any.
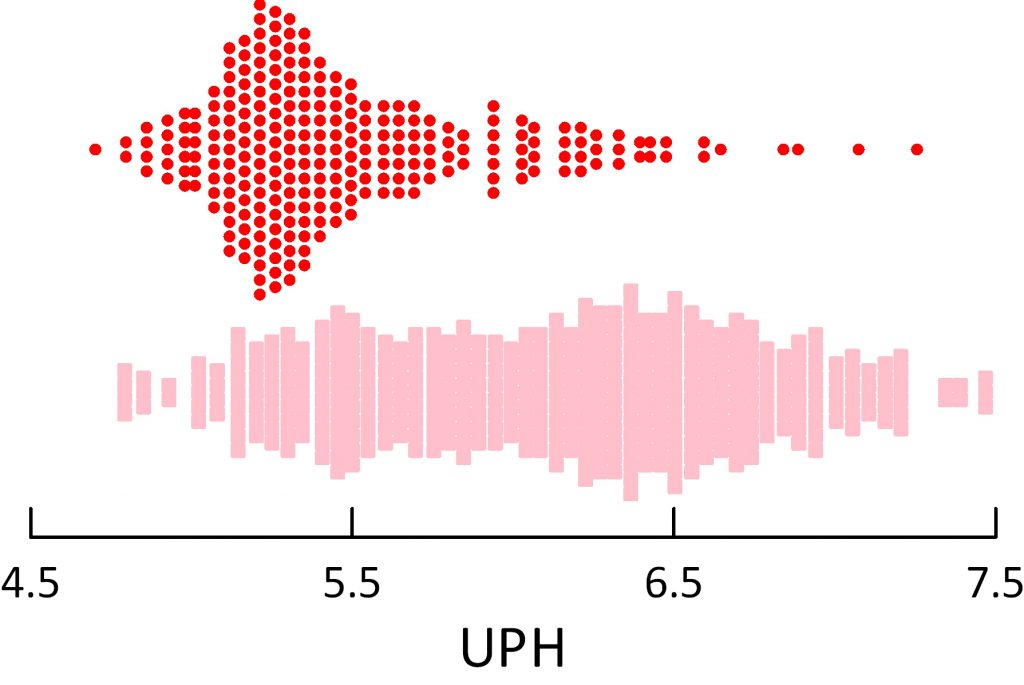
The dot distribution just below shows individual 24 hour urine pH measurements for calcium oxalate (blue), calcium phosphate (green) and uric acid (red) stone formers. Here I include among uric acid stone formers those with both pure and mixed stones.
Calcium oxalate stone former pH ranges widely with an average at about 5.8 pH units. Calcium phosphate stone formers average a lot higher – around 6.4.
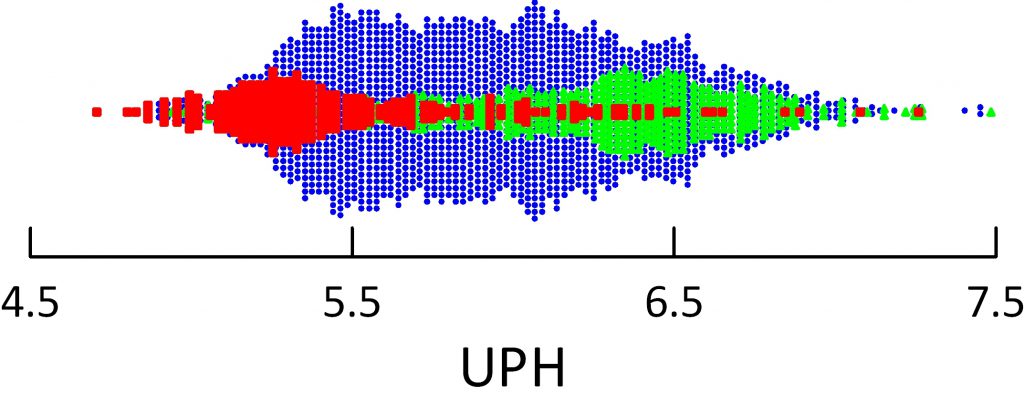 Uric acid stone formers lie in an acid range. Their average is about 5.3 – 5.4 and only a tiny scattering of points range above 6. So uric acid stone formers produce a very acid urine compared to other stone formers, and the pH is exactly in the range to produce supersaturation that can drive formation of uric acid stones and hold them steady or cause them to grow.
Uric acid stone formers lie in an acid range. Their average is about 5.3 – 5.4 and only a tiny scattering of points range above 6. So uric acid stone formers produce a very acid urine compared to other stone formers, and the pH is exactly in the range to produce supersaturation that can drive formation of uric acid stones and hold them steady or cause them to grow.
To see this, just look back on the graphs showing supersaturation vs. urine pH. Below 5.5 values almost all lie above 1 – solubility – meaning that crystals can form and grow.
UA Fraction in Stones
The General Pattern
I have said that any uric acid in stones means pH should be raised because at least that portion of the stone burden might dissolve or at leasts not grow.
 The figure below shows urine pH associated not with the kind of patient – calcium oxalate, calcium phosphate or uric acid stone former, but by the fraction of a given stone made up of uric acid.
The figure below shows urine pH associated not with the kind of patient – calcium oxalate, calcium phosphate or uric acid stone former, but by the fraction of a given stone made up of uric acid.
Blue means no uric acid at all. Red means 100% of the stone is uric acid, and pink and green lesser amounts. With a scattered few points as exceptions, stones made of mainly uric acid go with urine pH values mostly below 5.5.
The graph makes a point we often speak of but rarely show. Oxalic acid has a very low pKa – is a strong weak acid. So it has charges available for binding to calcium that very hardly at all with urine pH all the way down to 4.5, about the lowest value human kidneys attain. So these stone crystals are indifferent to pH.
Paucity of Mixed Stones
I makes another point, too, one that a patient emphasized in a comment to this article and that I failed to mention in the original version. Of all stones that contain any uric acid, at least in my collection of data, most are mainly composed of uric acid. See where the red – pure uric acid – stones make up the largest mass in the figure just above?
This is not to say that patients are uncommon who make both calcium and uric acid stones – mixed stone formers. The graph shows the stones themselves. People who make both kinds of stones need treatment with alkali so they will stop making uric acid crystals. They also may need treatment against their calcium stones. Stones that contain both uric acid and calcium – usually calcium oxalate – mean the patients may need treatment against both their uric acid stone formation – that would be alkali, and for their calcium stone forming.
So in the final analysis, whether the stones are mixed, or patients form both uric acid and calcium stones the answer is the same: Treat against both crystals.
Who Produce Uric Acid Stones?
Low pH Without Intestinal Disease
Genetic Factors
In identical twin studies, urine pH had only a 60% concordance compared to over 90% for calcium excretion. In a larger study urine pH seems as heritable as urine calcium excretion. Of interest, such dietary traits as sugar, calcium, and protein consumption that could influence stone formation also had significant heritability.
Systemic Disorders
Historically, uric acid stones have been linked to gout. A recent but brief review repeats that fact. Likewise, another review.
Given low urine pH drives uric acid crystallization, one has to ask whether some kinds of patients might be expected to produce acid urines. In answer, those most common are obese, older, diabetic, hypertensive, and prone to modest reduction of kidney function. Obesity itself, without necessarily overt diabetes correlates with lower urine pH in a progressive manner – as obesity increases urine pH falls.
Resistance to the actions of insulin – so called insulin resistance – is often invoked as a general paradigm to encompass the general class of abnormalities that lower urine pH. Metabolic syndrome, a mix of insulin resistance with lipid and vascular abnormalities is linked to kidney stones. But not to uric acid stones per se. Attempts to link uric acid stones to gut bacteria – use of the genetically defined biome -failed in a tiny study to disclose any species unique to uric acid stones.
Kidney Physiology
At least one specific abnormality that produces the low pH is an inadequate production of ammonia with which kidneys can remove acid. I plan another article on uric acid stone formation that will review the underlying disease mechanisms, and do not wish to burden this text with more detail. The linked articles from the group at UT Southwestern Medical School give access to the best current work on the subject. Essentially uric acid stone formers respond to acid load with less ammonia than normal people. Insulin resistance probably produces the renal tubule abnormality.
The issue is complex, as illustrated by a recent publication that found no evidence for low urine ammonia in uric acid stone formers. But the conditions of that study – mere measurements made in uric acid stone formers with comparisons to normal ranges hardly have any power to test the ammonia hypothesis.
In an elegant analysis of a single patient, Kamel and his colleagues point out two matters I use in my own work. Urine ammonia needs to be viewed in relation to urine sulfate – the net acid load. Likewise, the low urine ammonia of their case was accompanied by a high urine citrate – this occurs when proximal tubule cells consider themselves in an alkaline state that would cause a fall in ammonia production.
I have allowed myself a bit more about the urine pH than perhaps is ideal, and will end here. Either I will write another article on this subject or expand this one with my own data on ammonia and citrate.
Intestinal Causes
Intestinal Diseases
Any organic cause of diarrhea can lower urine pH because the fluids contain appreciable bicarbonate, the main blood buffer. In turn kidneys increase acid excretion in compensation. This requires both an increase of ammonia excretion and lowering of urine pH. Common situations include small bowel resection for such conditions as Crohn disease and partial or complete loss of colon. The latter, ileostomy, can cause marked alkali loss with acid urine and uric acid stones.
Chronic intestinal fluid losses also deplete body sodium and potassium. The 24 hour urine is very valuable for assessing both as excretion rates fall with such losses. Repletion with a mixture of sodium and potassium alkali is often valuable.
Bariatric Surgery
Howsoever valuable, these can result in both enteric hyperoxaluria and chronic alkali loss so calcium oxalate and uric acid stones do occur. The former are more common. This recent and excellent review details new stone frequencies but stone analyses are not widely reported so I cannot state the balance between calcium oxalate and uric acid crystals. Treatment with potassium alkali is recommended to increase citrate and pH.
Overuse of Laxatives
By increasing Gi fluid and alkali losses one might think these drugs would cause uric acid stones. In fact, a recent review of reported cases – not many! – suggests that mainly low urine volumes from fluid loss causes calcium stones. Not uric acid, in fact, but ammonium urate stones have been documented. I presume they represent induction of ammonia production by the potassium depletion from the diarrhea. As ammonia increases urine pH can rise despite loss of alkali and the higher pH would favor the ammonium acid urate salt.
What Happens With Treatment
Changes in Urine pH
In principle, potassium alkali in the proper dose will raise urine pH and abolish uric acid formation. The reality of practice has a bit less perfection.
These are data from my own work.
The original pretreatment urine pH values are at the top of the figure, for reference, in red. Below them, in pink squares the treatment data show a large shift toward high urine pH so that a majority of values lie above the pretreatment ones. But some patients did not take their medications, and in some I miscalculated the dose needed.
Even with this natural variation in physician intent and patient willingness, the shift of pH with treatment was drastic in my own practice.
Given the powerful dominance of pH over supersaturation, I decided to not add a figure showing that supersaturation fell – it would be redundant.
Treatment Complexities
Potassium
Although potassium alkali – potassium citrate or potassium bicarbonate preparations are an obvious and widely used treatment, the kind of patients involved – often older, diabetic – may not tolerate large amounts of extra potassium without increasing serum potassium. Especially, common and effective blood pressure medications such as angiotensin converting enzyme inhibitors or receptor blockers can worsen the risk. Typically most clinicians are aware of the problem and proceed based on serum potassium level and whether kidney function is normal or not. Sometimes I use a low dose of thiazide diuretic along with potassium citrate – the diuretic to foster renal potassium loss. This can enable patients to get more alkali without risk of raising serum potassium.
Sodium
In the intestinal diseases, sodium depletion may be great enough one wants to use sodium alkali. I prefer inexpensive sodium bicarbonate tablets bought over the counter, being cheap and easy to use. Two provide about 13 mEq of base.
Dosing
I almost always begin with 40 mEq daily and repeat the 24 hour urine measurements. Spot urine pH testing with pH paper never impresses me as very useful because results scatter and, after all, what most matters is average supersaturation over the day. These crystals can form and dissolve rather rapidly, and one hopes to achieve 24 hour average SS below 1. Overnight is clearly a high risk because of lower urine volumes so a nighttime dose of alkali before bed seems reasonable. If I need to I increase dosing in 20 mEq/day increments.
Effect on Uric Acid Stones
Uric acid in stones has a different meaning than we attach to calcium oxalate or calcium phosphate, or even cystine. This crystal can be prevented by raising urine pH within the common physiological range between 4.5 and 6. This means that simple alkali treatment should and will prevent such crystals in stones. Likewise, lowering supersaturation below 1 must eventually reduce kidney stone mass. Put another way, not guile or special knowledge but simply persistence with alkali use must inevitably stop uric acid crystallization.
Even so, data are hard to come by. This small report says that 91% of 24 uric acid stone formers treated with potassium citrate had no recurrence after a mean of 31 months.
No Formal Trials
A look on PubMed found no prospective uric acid stone prevention trials.
(For the purists, this was my search: ((“prevention and control”[Subheading] OR (“prevention”[All Fields] AND “control”[All Fields]) OR “prevention and control”[All Fields] OR “prevention”[All Fields]) AND (“uric acid”[MeSH Terms] OR (“uric”[All Fields] AND “acid”[All Fields]) OR “uric acid”[All Fields]) AND (“calculi”[MeSH Terms] OR “calculi”[All Fields] OR “stones”[All Fields])) AND Clinical Trial[ptyp])
I am not surprised. Given all we know can we assign such patients to a control group that does not receive alkali? Given the ease of use should one even try to do so?
I say not.


Greetings, Dr. Coe, from Deep South Texas. Is it ever appropriate for a urologist to advise consuming 1 tablespoon of sodium bicarbonate three times daily–yes, 1 tablespoon, which I verified with him the next day–to dissolve an 8 mm uric acid stone? He also prescribed Potassium Citrate ER 15 MEQ twice daily, which might be fine, but the Arm & Hammer stuff is kicking my butt. I’m a 61-year-old male on Ozempic 0.25 mg once weekly, Atorvastatin 40 mg Q.D., Lisinopril-HCTZ 20-12.5 mg Q.D., and Tamulosin HCL 04. mg Q.D. This is my second stone in four months, with the earlier stone being the first of my life. It was removed during a ureteroscopy (I believe) requiring no overnight hospital stay. Thanks for any guidance you can provide.
Hi Dr Berger, Whatever will raise urine pH to a 24 hour average above 6 will prevent uric acid stones and help dissolve present stones. Frankly I prefer potassium citrate, and in general need to use 60 mEq in 3 divided doses to get the effect I need. Sodium bicarbonate is cheap and will work but lots of patients hate it. Best, Fred
Hello Dr. Coe – Great article. I recently passed a 6mm stone that was 80% uric acid and 20% calcium oxilate. Also a CAT scan revealed that I have two stones in my other kidney that are 16mm in size. I have Crohns disease and have had surgery for it so I’ve been told this is a major contributing factor to the many stones I get. Assuming that the two large stones are mixed uric acid stones like the one that passed can they be dissolved by raising the pH in my urine or will they most likely need surgery to be removed? The pH in my urine is 5.0 which is pretty low. I see a urologist about them in a month. Thank you!
Hi Richard, It is very important to raise urine pH so uric acid stops crystallizing. This can be from sodium bicarbonate, sodium citrate, or potassium citrate and your physician can help you choose the best for you. Uric acid will dissolve, calcium oxalate will not. The Hounsfield units of radiographic density of the stones on CT can help tell, and UA stones have low values in the 100’s of units. Regards, Fred Coe
Hi again. I am the 80/20 uric acid calcihm Oxalate song former. I have Another question this time about magnesium glycinate. I started talking 500mg qd of this magnesium supplement along for another reason along with my K citrate. I have noticed however that my urinary ph is raised to seven which it has never been and for a much longer period of time. As I mention on 30meq k citrate extended released twice a day, the most I can get my urine ph to is 6.4-6.8 and that’s only for about two hours after taking the k citrate. While my ph is higher, there are conflicting thoughts on the effect of magnesium on stone formation. What are your thoughts. Thanks and happy Thanksgiving
Hi Joe, The magnesium itself has no effect on urine pH, but the glycinate will be metabolized as glycine taking up a proton and behaving as an alkali. Perhaps the addition of this extra alkali is causing the higher urine pH. Magnesium has no proven benefit for any stone type. Regards, Fred Coe
Greetings, Dr. Coe.
I have been prescribed Potassium Citrate ER, 10 MEQ, 4 pills, 3 x day for uric acid kidney stones and am concerned about taking too much. My pharmacist states that this can be a dangerous medication in that it affects all of the muscles in the body, and that this is the reason for frequent testing. The muscles in my arms ache, even when I sleep. When I wake in the morning, my legs feel “heavy.” Although I reside in the Chicago area, my doctors are at Mayo Clinic. The pH testing kit that I was instructed to get is extremely difficult to decipher. My internist retired and I’m about to meet with a new internist. Any thoughts on how I should approach my concern?
Hi Becky, Uric acid stones are treated only by raising urine pH, but 12 pills a day seems high. I might be concerned, although I do not know the details of your particular condition. Since you are in Chicago, and if you are concerned, we can see you at University of Chicago 773 702 1475. If that is not possible, perhaps I can suggest alternatives, or you can journey back to Mayo Clinic which is certainly an excellent facility. Regards, Fred Coe
Dr. Coe,
I am so impressed with this website and information disbursement need it supplies. The questions and answers section is almost as important as the articles themselves – discussion/clarification is always good. There is not enough reliable sites and sometimes information is conflicting.
I am currently wanting to do diet coaching, and I have a friend that wants to lose weight, but I don’t want to misinform him. He is obese class I with kidney stone issues (mixed uric acid and oxalate stones but mostly uric acid) He currently takes allopurinol for an elevated uric acid blood level. He does not have a problem with gout, only kidney stones. At first he had strange side effects with this medication (such as involuntary muscle movements that creeped him out, for lack of a better expression!). Is this an effective treatment for uric acid stones? I am not sure he takes a urine alkalizing agent or not, but he mentioned eating alkaline foods. Can diet be enough to correct urine pH. He stopped exercising last spring when he passed copious amounts of stones (in the hundreds according to him), in fear of “knocking stones loose” or creating more of them. I have read as far as weight loss not to lose to fast as this could precipitate an attack. What are your thoughts about diet/exercise regarding these types of kidney stones? There is so many ” don’t eat this and don’t eat that , when you look at low-purine and low oxalate diets that there would be nothing left to eat. My friend sure likes his meats and limiting that to 6 oz/day as some places recommend, would almost devastate him. He does have hypertension, and has had cardiac problems such as pericarditis. No sign yet of diabetes yet, but did have a slightly elevated fasting bloodsugar level (under 110 mg/dl). As I understand in your comments, diet restrictions are only minimally helpful in preventing these type of stones, is this right? I am not sure if he takes any urine alkalizing medication, but as I understand, diet alone is not effective enough!? If you could recommend a reliable source for diet recommendations and your thoughts on diet, exercise and weight-loss for people with these problems would be highly appreciated. Thanks!
Hi Charlotta, I did not realize in the earlier question below that you were a nurse. Allopurinol is useless for uric acid stones, because they arise from protonation of the urate N-9 site that is the only polar region on the uric acid molecule. The pKa for this site is 5.3, so when urine pH is below 5.7 or so the concentration of uric acid begins to exceed that of urate, and uric acid per se is very insoluble – 90 mg/l or so. No amount of allopurinol will lower uric acid so low, and the drug has risks. I never find diet sufficient to raise urine pH in uric acid stone formers. When renal function is adequate to tolerate potassium loads, I use K citrate 40 – 60 mEq/day seeking a pH over 24 hours of 6. Low protein diet, low purine diet, neither makes any difference. As for the diabetes, it is a notorious cause of low urine pH and uric acid stones, as is obesity – that lowers urine pH all by itself. Given his hypertension, lower diet sodium – to 1500 mg/d is ideal, but hard to attain. I hope this is useful to you. Best, Fred
Hi. Dr. I am a uric acid stone former 80/20 the 20 being calcium Oxalate. I take 30 meq pot citrate twice a day but my urinary ph rises to 6.8 only for about two to three hours after I take the potassium and then back to a low 5 ph. . Is this enough time to dissolve the crystals? I’ve been asymptomatic for 15 months but
Hi Joe, the dosage is not right given what you are saying. If your pH dips to 5 after only l3l hours ask your physicians if you might not be better off with taking 20 mEq three or even four times a day. Uric acid does dissolve, but if it is forming and dissolving that is not as good as just not forming at all. Regards, Fred Coe
Thanks!
Hello, Dr. Coe. I am a little confused by what I am reading here and in other places, and wondered if you might help me understand better.
I have, within just the last couple of years probably, developed uric stones. These have caused me a bit of trouble, including several laser liths and sepsis from obstruction. While I had some calcium-based stones years ago, these stones have come back tested as 100% uric.
I didn’t appear to form them – or at least notice them – until I went on a low carb, somewhat keto diet a couple of years ago when my husband became diabetic. Your article seems to indicate that this type of diet doesn’t have much affect on uric stone formation, but much of what else I read says it really has an effect.
Am I misunderstanding something on either end? My very recent 24-hr urine came back with a bit high uric (around 1200), and I had a slightly elevated serum calcium level. I have been working to reduce my animal protein intake.
I have upcoming appointments with both my endocrinologist (for my thyroid issues, but she works with stone formers) and the urologist. I’m just trying to understand how a higher protein diet may or may not affect uric stone formation before I discuss things with my doctors, since that diet seems to have affected me in this way.
Thanks so much for your guidance to everyone.
Hi Kerry, You are reading the right thing. At a urine pH above 6 the solubility of urate species is so high that the total urine ‘uric acid’ excretion has no significance at all. This is because the solubility of a acid form is 95 mg/liter, that of the sodium or potassium alkaline form is over 1000 mg/l. Allopurinol, low protein diet, all as basically silly as compared with alkali enough to raise the urine pH above 6 on a steady basis throughout the day and night. The only failure possible is not enough alkali, a bad choice of alkali, or poor timing, and your physicians can certainly tend to that. You also speak about an elevated serum calcium level. That is something else altogether and separate as a problem. If serum calcium is high, fasting, on repeated measurements, you certainly have something that needs treatment, like primary hyperparathyroidism. That is not a cause of uric acid stones but of calcium stones, and nothing prevents you having two problems. Low diet protein can have no effect on serum calcium. Regards, Fred Coe
Thank you so much for your reply. I suspect both physicians will be looking at allopurinol because it’s been brought up before, but I didn’t have the opportunity to do any research before I had the sepsis issue. I’m glad to know this information before my appts because I’m certain the uric level/diet will be brought up. I continue to see much reference to the keto diet “keeping urologists in business”. I will read more to learn how to work with these stones.
My fasting calcium came back at 10 this time – first was not fasting. Usually I’m in the mid 9’s fasting. So hopefully that non-fast higher one was just a one-off. 🙂
Thank you again for your generous guidance. Much appreciated.
Hi Kerry, The keto diet, by being deficient in sugars, stimulated ketone acid production. Urine pH falls but does not stay at its minimum long term as urine ammonia production rises. But given 24 hour testing we never need to guess. The 24 hour urine pH is low enough <5.5 or so, to permit uric acid crystallization or it is not. Potassium alkali – see article you wrote on – is enough to bring the pH to 6 or more, or it is not. Urine uric acid excretion is what it is, but the pH matters most. I did not mention the matter, but of course you always want 2 liters of urine or more. Regards, Fred Coe
Hello Dr. Coe,
I saw an earlier comment that you responded to and I had a few follow-up questions. Here is the thread:
Thanks so much, Dr. Coe. I’m trying to get a sense of what you mean by “rapidly.” Could uric acid crystals form in only one missed day of citrate treatment? During my first stone episode 9 months ago, I did see small red crystals in my urine but have not seen anything since.
Reply
Fredric Coe, MD
July 16, 2017
Hi Kim, Yes they can. Uric acid crystallized when urine becomes acid, and in a day red crystals mean uric acid. Take your med. Regards, Fred Coe
I have seemingly had rapid formation, all seemingly on days where my salt intake was higher than normal. I eat very low carb (<20g/day) mostly beef and eggs. I drink only water and black coffee (once in the morning). I've been eating this way for over 18 months and the little red/orange gravel has only started in the last 3 months here and there. I can go weeks without any and then 1 will appear. Sometimes a few. If I increase my water intake they go away – though the last one I was surprised as I had consumed a good bit of water that day.
How does salt intake effect formation of crystals?
Does the Ph of the water I drink have any influence?
Thank you. This site is amazing.
Hi James, Thanks for providing the thread. What I said to Kim is as best I know. The pH of drinking water matters not at all as water has no buffering capacity. Sodium has no role either. It is all urine pH and treatment is all potassium alkali, either citrate or bicarbonate. Regards, Fred Coe
Hello Dr Coe,
I am a 66 year old male with a history of occasional kidney stones in the past. Last January 2019 I suffered heart AFIB and have been ok since. I take lipitor for cholesterol, losartan and carvediolol for blood pressure.
In the last several weeks I noticed that I am passing on almost a daily basis kidney stone gravel. Had this analyzed and was told by the lab that they are 80% uric acid Dihydrate and 20% calcium oaxalate Monohydrate.
Before the lab test I had started taking B6 – 400 mg per day thinking that these stones were mainly oaxalate, but the lab test corrected my assumption, although it probably would not hurt to continue this to prevent oaxalate issues, I imagine.
The ultrasound shows only one 5mm calcification on the right kidney that may represent a peripheral calculus or vascular in nature in the kidney. No hydronephrosis and no masses. Otherwise both kidneys are normal.
As I stated I pass gravel daily; for example today, I passed about a dozen little stones already and it is only 4pm here in Florida. Very concerned about this daily gravel passing situation.
I see that in your article it mentions potassium citrate as a treatment to raise urine ph, and will start on that right away. How much should be taken daily?
I would really appreciate any other suggestions you may have to increase my ph and other ways to resolve this issue.
Thank you for your consideration of my email. Have a great day.
John
Hi John, You are reading the right article. The uric acid gravel will vanish when you take enough potassium citrate to raise urine pH to about 6. You need 24 hour urine testing before treatment, as the dose depends on the urine pH – how low it is, and the urine ammonia excretion. That is for your physician to consider. Generally 20 to 40 mEq/d of potassium citrate works, but I have used as much as 80 or more, depending. Your physician needs to supervise everything as your tolerance for potassium is also a consideration. But uric acid vanishes when pH reaches 6, and you are done. Regards, Fred Coe
Thank you! Your reply is greatly appreciated.
Hello Doctor Coe,
I have followed your advice and settled on a 30 mEq daily of potassium citrate. The results after the first day were very successful and have not passed any more gravel or stones since starting almost a month ago. Before this treatment it was a daily event, two or three times per day.
Wanted to mention for others’ benefit, that potassium citrate in pill form at these dosages is very expensive, so instead I purchased it in bulk powder form on line and saved a large amount of money. Particularly when looking at having to continue this potassium treatment on an ongoing basis.
You have changed my depressed state of mind of having to live with these stones and I thank you very much for your recommendations. Your help is greatly appreciated.
John
Hi John, The food material is fine, but be sure about weighing it out right. Potassium can be dangerous. Be sure your physician knows you are doing your own pharmacy work and she/he agrees. Likewise, get your 24 hour urine checked on treatment, because if pH goes to high calcium phosphate stones can be promoted with very unfortunate outcomes. Regards, Fred Coe
Hi Doctor,
Just saw your reply to my last email.
I use the measuring tool that comes in the potassium phosphate bag in what it amounts to 30mEq daily. I continue to measure my urine ph and it stays around 6.75 to 7ish in the urine test kit measurement sticks.
No more stones!
Appreciate your advice greatly.
Thank you.
John
Hi Doctor,
Just saw your reply to my last email.
I continue to measure my urine ph and it stays around 6.75 to 7ish in the urine test kit measurement sticks.
No more stones!
Appreciate your advice greatly.
Thank you.
John
Do I need a prescription to get Potassium Alkali. If so, can I do enough OTC Potassium Citrate on my own to get comparable results or any benefit? Thank you for the courtesy of your reply.
Hi Steve, Many people buy food grade potassium citrate and a home scale that can weight out in grams. A single 10 mEq potassium citrate tablet contains 1.080 gm of potassium citrate and that is the dose form physicians use. But this requires you work with your physician who says how much and agrees to home weights. Potassium can be dangerous, so she/he is crucial in all this. Regards, Fred Coe
Thank you Fredric!
Hi Dr. Coe,
What would you suggest for someone that has in 24 hour urine test, pH 5, uric acid, 595 mg, calcium 109, citrates 886, fosforus 1.0, oxalates 31. Urine volume 3200 ml. I did not catch the stone, it was seen in a ultrasound and had one of 3.2 mm.
thanks a lot
Maria
Hi Maria, I would suspect uric acid stones, and would raise the urine pH to a bit above 6. Potassium citrate will do that but you need to check a urine while on it because you do not want the pH to rise much about 6.5. If there are other stones in your kidneys an ultra low dose CT will find them, and you can tell from the CT if they are uric acid or not. I would do that. Regards, Fred Coe
Hi Dr. thanks a lot. So far I am trying to take care of diet, as in that particular case I had had a E. coli after the stone came out. I have been trying to follow the diet Jill recommends because my pH in september was 7.00 and I wasnt paying attention to the risk of oxalates (potatoes, lots of almonds, etc). Now I just trying to organize my diet in terms of acid and alkaline foods. What would you say would be the amount of protein I should take? I have a mediteranean diet (olive oil, veggies, fruit, but also fish, mostly poultry, some beef, yoghurt, dark bread, oats). Sometimes we just have too much protein. My blood uric acid is ok. Thanks
Hi Maria, At pH 7 uric acid cannot form, but it is so high that calcium phosphate stones could. Protein intake needs to be about 1 gram/kg body weight/d, and I am sure Jill is helping with that. Likewise she is excellent at your oxalate. Just be sure you use your 24 hour testing and avoid high supersaturations for calcium phosphate as well as calcium oxalate. Regards, Fred Coe
Hello Dr, Coe…. greatly appreciated the article. I’ve undergone 31 surgeries over the past two years. Last pathology report showed my 16mm stone as 10% oxalate and 90% uric acid. I’ve tried the potassium citrate in the past; however the urologist quickly took me off and tried something different. I’ve only had one 24-hr study. Maybe because I’m in the hospital more than not? I’ve recently moved (Bakersfield, CA) and soon will start with a new urologist. Any suggestions on what I should be asking for? Diet? Looks like it’s time for me to take control! I’m exhausted and frankly unable to bear the pain with one more overly large stone trying to pass.
Hi Lisa, uric acid stones are cured and can be dissolved with potassium citrate. The dose needed is that which raises urine pH above 6, to about 6.5. Usually this is 2 – 3 standard 10 mEq tablets. I have to say it is not good practice to fail of using potassium citrate for uric acid stones, and I imagine your physician stopped it because of a sudden event and then somehow it was not restarted. I suggest reminding him/her about the absolute need for this treatment, which prevents uric acid from crystallizing. It looks like LA is nearest to you. At UCLA kidney stone center, 4 of the five faculty list stones as a major interest. I would trust any of these physicians. Regards, Fred Coe
Good day,
Was wondering if potassium citrate is something that can be taken for extended periods of time. I have done 1 month cycles. But was wondering if I can do 6 month cycles etc etc
Hi John, You need it every day forever. Uric acid crystallizes when urine becomes acidic, the potassium citrate prevents that. It is already in your blood – potassium and citrate – so what you take is a supplement. Use it daily. Lots of veggies and fruits provide potassium with citrate – like molecules so that can reduce the needed dose. Do not cycle it, use it. Regards, Fred Coe
Hi Dr. Coe, I am a mixed stone former (80% Uric Acid Dihydrate & 20% Calcium Oxalate Monohydrate). Based on everything I read on your site I have had the correct testing done by my doctors. I see both a urologist & a nephrologist. Just as you say on your site; through supersaturation I form stones quickly. Yes, I know I don’t drink enough! I’m also obese, have high blood pressure and uncontrolled diabetes. I have had four lithotripsies within the past 4 years. My doctor has prescribed 40 mEq of Potassium Citrate daily. I really, really want to take it as I know it would help; however, I have terrible side effects ; severe stomach pain, ulcers and GERD. I have also had many UTIs; 5 last year and 2 already this year. My urine ph is 5.5. Is there anything else I can take that would work the same way as Potassium Citrate? Is there some other form of it besides tablets? Thank you for any guidance you can provide.
Hi Sharon, Indeed you are the very archetype of uric acid stone former. You need the alkali. Lots of alternatives exist. There are liquid sodium/potassium citrate liquids, Crystal light lemonade has 20 mEq of potassium citrate per liter. You can take sodium bicarbonate pills, if needed, and your physician can prescribe a tiny dose of a diuretic if the sodium load raises your blood pressure – it usually will not being bicarbonate not sodium chloride. Your pharmacist can recommend alternatives forms of alkali with different flavors. But you need the alkali and a urine pH above 6 – cures the stones. Regards, Fred Coe
hello,
I have a kidney stone and gout(pain in joints), am from country with no adequate resources to check for the type of crystal. I have seen different readings connecting uric acid with joint pain as well. can I be sure it is a uric acid stone from this? how could the potassium citrate affect some one with bad gastric problem?
please give a good suggestion on how to eat, drink, and live my life and good home remedies to get potassium citrate from food.
thank you.
Hi Daniel, If you have stones and gout the stones may well be uric acid. A CT scan can help as the radiographic density is a clue – in the article you are posting on. Potassium citrate can be unpleasant for people with gastric problems, but sodium bicarbonate is usually not a problem and it will dissolve uric acid stones. But you need at least the urine pH, which can be measured with inexpensive pH paper. It needs to be above 6. Of course you may not have gout or uric acid stones, and if your stones were calcium and you took sodium bicarbonate things might worsen. As for food, fruits and veggies are high in potassium and molecules like citrate that are metabolized to produce alkali. Put another way, there is a lot of uncertainty here, and therefore some concerns about how to proceed. My main issue is your stone. Can you get a CT and then a measure of its density. That one measure would make a great difference. Regards, Fred Coe
thank you very much for your response. I will get CT scan get the density of the stones. On sodium bicarbonate, can I only take it when urine pH is above 6? on urine pH when is the perfect time to measure it, as amount of water intake clearly affect urine solution? on stone density which one have a higher density between the crystals, is their a standard to followed for radiographer? and finally which medical pills are best from your huge experience to be considered? thank you very much. I am glad i found wise person to take advice from.
Hi Daniel, Find out about the stone density before you do anything. If they are dense, like calcium stones, the advice about alkali would be wrong. The density is measured from a CT and the procedure is standard for all physicians to perform. Regards, Fred Coe
Some good news, Dr. Coe. I started a trial of baking soda Friday evening, .5 tsp twice a day, morning and night. After 13 PH readings over the course of approximately 36 hours, my PH average is now 6.53. The PH average over the course of the past 7 readings is 6.72. Before taking the baking soda, the average was 5.4. No heart arrythmias as I was experiencing on the pottasium citrate, and so far, no effect on my blood pressure. I’m hoping the higher PH will desolve my current stones (one 8 mm) and prevent new stones from forming. The only thing I don’t like is the taste of the baking soda water solution. Yuck. I ordered some sodium bicarb pills online, 325 mg. Im planning to take 2 a day. I hope that’s the correct dose and in addition, that I can take the pills without having to disolve them in water first. I asked my Urologist about the sodium bicarb beofre taking it. He told me he doesn’t treat his patients with it and referred me to a nephrologist.
Hi Dave, I am glad you are doing this. Two pills contain about 325 mg/pill x 2 = 650 mg/84 mg per mmeq sodium bicarbonate = 7.7 meq of base. So you need 2 pills 4 times a day to get any effect. Just swallow them. Fred
I am facing a PCNL treatment for a uric acid stone in my left kidney. My urologist does not believe it can pass and is recommending the PCNL treatment. Obviously, I don’t want it. Can sodium bicarbonate dissolve the stone? If so, how much is considered a safe, conservative amount to take. My urologist is dubious that either potassium citrate or bicarbonate would reduce the size of the stone. I have roughly a month and half before my next appointment. Thank you for any information!
Hi Steve, It is a pity that uric acid stone got big enough to require a PERC. Your surgeon is probably right but I would push to try dissolving it. The stones are often built up of many smaller stones, so with a high urine pH – 6.5 – they sometimes come apart into smaller ones. There is no harm in trying, and the one month is not a deadline. Of course, stay on K citrate life long, so no more form. Regards, Fred Coe
Thank you for the response, Dr. Coe. If my urologists does not want to go to attempt to try either citrate or bicarbonate, is it safe for me to attempt to do it on my own? What is the usual amount of time it takes to break up a uric acid stone with either of these substances and how will I know if it is working (short of passing the stone)?
Hi Steve, Not really. Perhaps the stone compromises drainage within the kidney, she/he would know and you would not. pieces may detach and cause obstruction – this needs an active physician. If you want to try alkali and your physician will not support that desire, I would suggest you ask her/him to help you get a second opinion. Alternatively, you are free to choose a second opinion. Do not venture off apart from physician recommendation – it is not safe. Regards, Fred Coe
Thank you for the valuable info, Dr. Coe. I will follow your recommendations to the letter…take care!
Hi Dr. Coe,
I’m a 56 year old man who has stage 3 kidney disease, controlled diabetes, and normal blood pressure. After going to the emergency room last year in June with an incredible amount of pain. I had a ureteroscopy to remove a 7mm kidney stone. It was my first
Kidney stone surgery. For whatever reason, no stone analysis was done at the time. About 3 weeks later, I peed out a bunch of small stones which were caught in a strainer. After lab analysis, the stones were determined to be made up of 100% uric acid. Blood work and a 24 hour urine analysis soon after revealed that I had a high parathyroid level, normal calcium, a urine PH of 5.4, a high urine super saturation of both uric acid and CaOx, and 1 liter of collected urine. I refused to follow my doctors recommendation at the time to take Potassium Citrate because I thought things might improve on their own and wanted to avoid experiencing the unpleasant medication side effects. About 3 weeks ago, I had those same tests again with very close to the same results above. The urine PH this time was 5.36. I also had a ultrasound which revealed about 5 kidney stones, 4 under 3 mm and 1 that was 8mm. The doctor again wanted me to start taking the potassium citrate, Urocit-k. I decided to do that, but first wanted to try a change in diet to see if it would make a difference, and it did in a very short period of time. I stopped eating fast food, I had been eating it 2 to 3 times a week. I cut down on my meat consumption and stopped drinking so much seltzer water. After 4 days my urine PH, which I’ve been testing at least 3 times a day with PH meter, jumped from an average of 5.4 to 6.2. My questions are why do you think the diet may have made a difference and if the PH remains over 6, is it necessary that I take the potassium citrate? Thanks.
Hi Dave, as in the article, pH is all there is to uric acid stones, so if you got to 6 on 24 hour testing you are done. But I am not sure if you are doing spot testing or 24 hour urine. Spot testing is not ideal as stones form in response to the integrated pH and one does have overnight etc. My advice – very confident – is to use 24 hour urine testing to be sure your diet has raised 24 hour urine pH to 6. I suspect it did not and you will need alkali. Regards, Fred Coe
Thanks, Dr. Coe..
Just one follow up.. I was prescribed and started taking potassium citrate, which unfortunately, I wasn’t able to tolerate, even at the lowest dose, 5 mEQ. When I take it, I get heart arrhythmias. Anything else I can do or try at his point or should I just resign myself to the possibility that I’ll have many more stone surgeries in the future? I bought a PH meter awhile ago and have been testing myself multiple times a day. My PH is still low, averaging about 5.4.
With regards to my 8 mm non obstructive stone, do you think it should be removed as soon as possible? My urologist didn’t think it needed to be.
Thanks..
Hi Dave, Raise the urine pH another way, then. There are many options including sodium bicarbonate and sodium citrate alkali. The non obstructive stone may be uric acid and might shrink as you raise urine pH. Regards, Fred Coe
Hello Doctor,
So, I am a 23 year old woman who just had a Lithotripsy of a 6mm stone. My urologist however found I had 8 smaller ones still between my two kidneys. I had been experiencing/presumably passing stones since I was about 18 and experience what I now can identify as kidney pain nearly every day. I have a primarily very healthy plant based diet and don’t do fast food or tea or soda or dairy. I am also really active, despite having discomfort. Now, I did a 24 hour urine sample and my results shocked my urologist. My oxalate levels, which was thought to be my problem, were normal, however my uric acid level was at 1,099, my calcium at 324, and my magnesium at 175. So, he referred me to a kidney specialist in my area. This all being said, it’s been a month and I haven’t heard from the specialist yet and am just baffled as to how my uric acid is so high. I strained my stone I ended up having to pass in the hospital and it was a darker color, not the red/orange you described. This is all just so puzzling to me. My dad and his dad had chronic kidney stones, however my dad’s are potassium based. Any advice here?
Hi Elena, I suspect the high calcium is a cause of your stones and the high uric acid a reflection of a high purine diet and I do not feel confused at all. Here is a good article on stones in general. Uric acid stones occur because of a low urine pH – see if yours is low. High urine calcium is genetic, and here is an article about that topic. All very treatable. Regards, Fred Coe
Hello Dr. Coe,
Thank you for sharing your knowledge about this painful affliction. I truly appreciate your work. I am a uric acid stone former.
Would taking apple cider vinegar tablets help in any way to prevent stone formation?
Thank you again,
Tony
Hi Tony, No. As the article points out proper dosing with medicinal alkali is curative so why play around? Low urine pH causes uric acid stones, alkali to raise urine pH prevents them and can even cause some to dissolve. Just get real alkali, raise urine pH above 6 on a 24 hour average, and you are done with them. Vinegar is nice in salads, I guess, but hardly a match for a sure cure. Regards, fred
Hi Dr Coe,
When you say 40 mEq of potassium citrate is it the same for the extended release tablet and is it 40 mEq in one dose or throughout the day?
Hi John, the usual form is 10 mEq tablets, and I favor 2 twice a day to spread out the effect. But you need 24 hour urine testing to be sure the 24 hour average is at least pH 6. Regards, Fred Coe
I am confused about the dosage. How many mg of potassium citrate can I safely take per day in order to dissolve a kidney stone? And do I have to divide the dosage?
Hi J.T., If you have uric acid stones, your goal is a 24 hour average pH above 6, ideally between 6.1 and 6.5. Usual dosage is 40 – 60 mEq of potassium citrate (1080 mg = 10 mEq) taken 2 to 3 times a day. Stones may not dissolve if admixed with calcium crystals, or very large. You need to be sure about other stone risks, for if urine calcium is high raising urine pH much above 6.3 chronically could cause calcium phosphate crystals to form. Your physicians know about this, and have to help you chose a proper treatment regimen. Regards, Fred Coe
If the grafts show that you want a pH over 7.5 to keep from forming stones, then why do you recommend keeping urine between 6-6.5? Don’t you want it closer to 8?
Hi Rachel, the graphs do not show that. By pH 6, SS with uric acid will be less than 1 in almost everybody. Raising pH much higher raises risk of calcium phosphate stones. Regards, Fred Coe
Hello Dr. Coe, thank you for such thorough write-up on Uric Acid stones. It means a lot to us that are stone formers.
I had my 1st Kidney stone attack in March of 2019, at age 71, and having been very healthy practically all my life, needless to say it was a very painful experience. My GP doctor sent me to ER, I was given morphine injection. I passed the stone at the ER urinal before finding out my diagnosis so no tests were done, few days later I did pass another smaller one, 4-5mm, had it tested, it was uric acid stone. My doctor put me on Allopurinol and all was fine until few months later when I developed bad stomach cramps and a lot of gas. I stopped taking Allopurinol and started to drink a lot of water instead to dilute the urine concentration. That worked fine. Then in August of 2020 I was diagnosed with colon cancer, had colectomy and as of now I am cancer free and being monitored by oncologist. This June during my annual general check-up they discovered blood in my urine so my GP sent me to urologist. He started me on Potassium Citrate 10 mEq 3 times daily. What I am curious about is how long does it take for the Potassium Citrate to start to show some improvement in my urine pH? I measure my urine pH about 6 times a day, first morning readings and most of the day are 5.5, then towards the evening it starts going up to 5.45-6 and even higher 6.25 late in evening. Is this daily difference somewhat normal or am I doing something wrong? Does the Potassium Citrate take a while to stabilize the pH level throughout the day?
Thank you so much
Mike
Hi Mike, the combination of your low urine pH before the colectomy (uric acid stones then) and the fluid losses from a colectomy probably require a lot more K citrate. You may be able to use sodium bicarbonate, incidentally as colectomy often causes sodium losses – the extra sodium as bicarbonate will not matter for blood pressure. You need 24 hour urine collection to find out and I would suggest you do that. Regards, Fred Coe
Thank you Dr. Coe, I ordered the 24 hr urine test on friday through my urologist, and I was just looking for the Sodium Bicarbonate on Amazon when I got your reply.
4 days after I saw my urologist I passed 6 mm stone and next day 2-3 mm stone, I’ve been passing little gravel for a while, and every now and then maybe 2 mm stone, not too often.
I’ve had 3 CT scans ordered by my oncologist but I never really looked at the reports, my mistake, I finally asked my urologist to send me the latest CT scan report and I found out I have 1.04 cm stone on my left kidney and 8 mm stone on my right kidney, my hope is that with the start of taking 30 mEq of K citrate daily plus increasing the water intake to 4 L+ per day and now adding maybe 20 or 30 rg Sodium Bicarbonate some of the large 1.04 cm uric acid stone will dissolve or break up.
I have always asked for the copy of the CT scan on CD and I can look at them now and see how my UA stones increased in size every 3 months even tho I was without any symptoms or pain, thinking I was doing great.
Thank you again Dr. Coe for your wonderful research on UA stones and sharing all the information for us.
Mike
Hi Mike, Be sure and check 24 hour urines for a while to get average pH and SS for uric acid. The average is more important than spot measurements. Fred
Hi Dr. Coe, I have received my 24hr urine test results, my SS was 0.60 and pH 5.763, not high enough and that is after taking 30 mEq of Potassium Citrate and 30 gr of Sodium Bicarbonate daily. I guess my pH is still too low so I am going to ask my urologist to increase my Potassium Citrate to maybe 40 mEq/day and I am also considering increasing my Sodium citrate to 40-50 gr/day.
Other than the pH level everything else was within acceptable range. Good news is that I do not have any gravel stones or small stones in my urine since I started to take K-citrate, so I hope I am on a road to recovery and hoping the large stones in my kidneys will slowly dissolve.
Again, Thank you Dr. Coe for your wonderful write-up on UA stones and all your great research, it was huge help and also mental support for me understanding what is happening with my body.
Greetings, Mike
Hi Mike, You are perfectly correct. pH needs to be about 6 and more K citrate or sodium bicarbonate will achieve that goal. Fred
Good Day,
I had a CT scan a few weeks ago and found out that i have around 9 stones on my right kindey and 4 on my left kidney, 4 of these are a bit big but dissolvable according to my urologist. Im on allupurinol and Propan mist pot cit complex. Im also on about 3L of water a day. Was wondering how long would it take to dissolve all these stones
Hi Gift, I take it your physicians know the stones are uric acid, and indeed raising urine pH to about 6 will – can – lead to dissolution. Allopurinol is irrelevant in treating uric acid stones, but potassium citrate in adequate dose to raise urine pH is very effective. You would generally require about 20 to 30 mEq of the agent twice a day. Regards, Fred Coe
Thank you for this article. It has helped me understand my stones more and i will talk to my Dr about this treatment. 20 years ago stones grew and attached to my left kidney basically turning it to stone. I had to have it removed. I was fine until recently. I now am having large stones causing blockage, infection and kidney injury. Aug 13 I had stones lasered and removed Aug 27th I was in ER with a 10mm stone, worse infection and kidney function. Emergency surgeries, stents and bad infection, its like i am at war with these things lol Needless to say I have been researching to try to prevent this since I only have one kidney now. Your article was easy to understand. And has given me some hope. Thanks.
Hi Tanya, Are your stones actually uric acid? If so, raising urine pH with alkali is curative. You do not say what the stones are made of. Given only one kidney, it is an urgent matter to determine if the stones are uric acid and if so to use 24 hour testing to determine average urine pH and then raise it with suitable alkali salts – as in the article. Regards, Fred Coe
Good Afternoon Dr. Coe,
I have uric acid stones. Testing with a PH meter, my urine PH seems always to be between 5.0 and 5.4. I’m diabetic and have secondary hyperparathyroidism (high PTH and normal calcium). My vitamin D level is low (24), Is there any evidence that calcium citrate supplementation will raise my urine PH?. Is there any evidence that vitamin D supplementation, with the goal of lowering my PTH level, will raise my urine PH?. What about lemon water? Thanks..
Hi David, Diabetes lowers urine pH and leads to uric acid stones. Vitamin D nor calcium citrate are ideal given uric acid stones because the dose of alkali needs be rather hefty. Potassium citrate is the usual treatment and will prevent stones. The high PTH is presumably due to reduced kidney function – perhaps a bit from the low 25D level. There is no problem with using OTC vitamin D to raise serum vitamin D levels, and there is no reason that calcium citrate is not useful to get your normal daily calcium intake of 800 to 1000 mg. But I would not rely on the calcium citrate for uric acid stone prevention. Regards, Fred Coe
Thanks for the information, Dr. Coe. It was very helpful.
Hi Dr. Coe,
I recently had a utereroscopy. One of the stones was removed and an analysis revealed that it’s composition was 90% uric acid and 10% calcium oxalate. I’m diabetic and have secondary hyperparathyroidism. My urologist told me that my urine PH was too acidic. Is there something I can do or take to prevent new stones from forming? I was told pottasium citrate or sodium bicarbonate were options. Should I stop eating foods that contain oxalate?
Hi Paul, it is the low urine pH that causes your uric acid stones. The chapter you write on tells the basic story. Here is a more detailed one. The whole treatment is to raise 24 hour average pH above 6. Food oxalate is immaterial. Just do the one thing and all should be well. Potassium loads in diabetes require physician oversight. Sodium bicarbonate is safe and the sodium load from bicarbonate does not seem to raise blood pressure as much as sodium chloride. Regards, Fred Coe
Hi Dr. Coe,
Is potassium bicarbonate effective as a treatment for uric acid stones? They sell it over the counter. If it is and each capsule contains 510 mg of potassium, approximately how many capsules would be needed to raise the urine PH high enough to dissolve the stones and prevent new ones from forming? I’ve tried potassium citrate and sodium bicarbonate to treat my uric acid stones , but was, unfortunately, unable to tolerate a therapeutic dose. Thanks..
Hi Dave, If you are right and each tablet contains 510 mg of potassium (AW 40) giving 12.75 meq which is identical to the mEq of bicarbonate. So one tab is about 1/2 the alkali effect of a single K citrate tablet (1080 mg). I would guess that 2 tabs 3 times a day should do it but be sure by collecting a 24 hour urine. The effect is more immediate with bicarbonate than citrate (that must be metabolized to produce alkali) so it is best to spread the dosing out as much as practical to avoid low pH periods. Frankly the ideal is 2 at bedtime, the other 4 spread through the day – as a guess. Regards, Fred Coe
In my early twenties i was diagnosed with uric acid stones. I was prescribed alupurinoll and took it for several years before stopping it. Now I am 48 and lately i have been having right side pain off and on. My doctor said my urine test was ok and i didnt need to see a urologist. I noticed on my lab work that my GFR was 70. Should i see a urologist?
Hi Kevin, I would be sure neither kidney is obstructed by a stone – a CT is most ideal for this and modern machines have very low radiation dosage. Urine testing for uric acid stone risk is simple enough, and if urine pH is around 6 risk is nil. If there is no obstruction, your physicians should investigate the cause of the low eGFR, starting by a test for urine albumin or protein. Regards, Fred Coe
I have had Uris acid stones in the past
I feel like I may have them again with pain on and off
GFR is 70
Should I be on allupurinol?
Hi Kevin, You must use potassium alkali to raise urine pH above 6 as the article says. Allopurinol is ineffective against uric acid stones. GFR is often reduced as uric acid stones tend to form in people with high blood pressure, lipid disorders, and the like, so overall treatment is complex and multisystem. An obvious problem is obstruction, so be sure your kidneys drain properly – your urologist is expert in that matter. Regards, Fred Coe
How do you know what kind of kidney stone it is if you passed at the hospital after the cat scan it was 3.3 millimeter. How going to a urologist will know what kind of stone it is. Can they tell by the scan
Hi Mary, If you passed it in the hospital I presume it was collected and sent for analysis. The analysis will say what it is made of. The CT scan can tell a bit; your physician can measure the density of the stone on the CT (in HU); uric acid stones are rather low, about 400 or so, calcium stones a lot higher, 800 to 1200. Regards, Fred Coe
Hi Dr. Coe,
Before commenting, I wanted to let you know that I’ve posted a number of comments through this website and your responses have always been very helpful and informative.
I had a utereroscopy a couple months ago, my second in 2 years to remove a number of kidney stones in my left kidney, The largest was 1.6 cm. The surgeon, unfortunately, was unable able to remove all the stones, leaving behind a 4 mm and a few other smaller ones. The stones were 90% uric acid and 10% calcium oxalate.
I started taking 1,300 mg of sodium bicarbonate in pill form about a week ago, which has brought my urine ph from an average of 5.0 to an average of 5.5, according to the ph probe i’ve been using for the testing. It’s been my understanding that an average ph closer to 6 is needed to prevent active uric acid stones from growing and to prevent new ones from forming. Unfortunately, I’m unable to increase my bicarb dose. When I’ve tried, I’ve experienced some very unpleasant side effects. So, knowing that a urine ph closer 6.0 ph is the therapeutic dose, is there any benefit in my continuing to take the 1,300 mg of bicarb? I know potassium citrate is another medicine used to raise the PH. I’ve tried taking it on a couple of occations and, unfortunately, experiened heart palpitaions and dizziness on a very low does, so had to discontinue taking it.
Dave
Hi Dave, Try this; collect your own 24 hour urine to get an average and measure pH in it. As for the bicarbonate, what side effects? Perhaps I can help get around them if I knew what they were. Fred
Mostly, alot of nervousness and restlessnes.
Dear. Dr. Coe,
I am a stone former with first stone at age 15; 16 stones in one summer. I am 55 yo with a father who also produced stones. I recently had a 6mm stone made of 50% carbonate appatite and 50% calcium oxalate dihydrate. I currently have a ureteral stone for over 3 weeks which was not visible on KUB.
I take 15 of potassium citrate twice daily and still form these stones. Unlike the usual patient, I am thin, 5’4”, 113 lbs without diabetes. Often my stones do not show up on ER CT scans, and medical professionals are unkind until I pass them. How can I can explain their presence when they do not show up during an episode? And how can I prevent and treat these?
What is happening?
Thank you,
Dr. Beth Lamb
Hi Dr Lamb, You are forming phosphate stones and these usually arise from high urine pH and calcium, perhaps with reduced urine citrate. You do not mention your 24 hour urine results, and I wonder about the efficacy of additional alkali. IF you have 24 hour urine results, I could try to help more. Regards, Fred
Hi Dr. Coe, Is it possible to raise urine pH purely through diet? And if so, how long would it take to accomplish that if I’m starting out with pH values of 5.819 and 6.131 from a 48-hour urine test? Am I looking at days or months?
I had an 8mm kidney stone in January, and was not able to get it tested. I had a lithotripsy procedure and my urologist pulverized the stone so that there was basically nothing to analyze. There was a lot of orangish-brown sediment when the stent was removed, but nothing was analyzed for its content. Initially we were thinking that it was a calcium oxalate stone, because my diet was very high in oxalates, but my 48-hour urine tests (done two months after the stone was removed and after two months of diet changes) show normal oxalates, but UA 24 elevated at 0.901 and 0.776, and pH of 5.819 and 6.131. So maybe I didn’t have an oxalate problem at all, or maybe I was able to correct it before the urine test.
After getting the urine test back, I’m limiting purines. I’ve modified my diet in the last two weeks to include only about 3-4 oz. of meat or fish protein per day, and eat mostly vegetables, fruits, and an egg or two each day. Occasionally I’ll have yogurt or cheese, and over the last couple days I’ve added 8 oz. of milk to each meal. I’m trying to lower my pH as well as watch the purines. I’m using pH test strips throughout the day, and I haven’t seen much of a change in my pH. The results I’m getting range from 5.0 to 6.5 most of the time. Do I need to be more patient, or is a supplement of some sort necessary to get my pH up to where it’s not averaging around 6 or below.
Thank you. I’ve learned so much from your website articles!
Hi Michelle, given your urine chemistries I do not think you formed uric acid stones. The pH is too high. Even 900 mg/d of uric acid will not make a stone at pH 5.8 unless – careful here – your urine volume was low (a liter a day or so. Orange is usually uric acid. So I think (purest guess) you somehow were producing low volumes of urine for a while and at pH 5.8 concentrated things overly. Is this possible given your self knowledge?? I would not limit purines, as it is mere gesture, pH and volume control uric acid stones and low purine often means more breads grains and starches which are not very healthy for us all. As for alkali supplements, not ideal. Your urine pH is not low enough. Pay a lot of attention to your urine volume. Incidentally, if your surgeon looks at the preoperative CT scan of the stone she/he can measure the HU density of the stone. Uric acid stones density is low, below 600 HU or less, calcium stones usually 1000 or more. Regards, Fred Coe
Hi Dr. Coe,
I’ve had 2 utereroscopies over the past almost 3 years and the stones, analyzed after removal, consisted of 100% and 90% uric acid respectively. According to my last 24 hr urine test done back in March 2020, my 24 hr urine PH was 5.37 and the SS uric acid was 2.73. I test myself often with a good PH meter to see how diet is effecting my urine PH. Through testing, I’ve observed that almost always after eating a very low carb meal consisting mostly some type of annual protein, that my urine PH will rise considerably, sometimes into the low 6s (6.0, 6.1, 6.2) for a few to several hours. Why do you think this is happening? My understanding from reading a number of articles is that uric acid stone formers should eat less meat, as eating it is supposed to make the urine PH more acidic, not less.
Some other readings from the test:
Urine volume: 1.15 liters
SS CaOx: 6.76
Urine calcium: .73
Urine oxalate: 56
Urine citrate. .85
SS CaP. .22
Urine Uric Acid: .586
Thanks,
Dave
Hi Dave, The low pH of uric acid stone formers is not treatable with diet. One needs extra alkali. Potassium citrate 10 mEq tabs 2 three times a day is a common pattern but that is up to your physician. More detailed information about uric acid stones may be of interest to you. I do see a very low urine volume – it should be increased. But pH averaged over the 24 hours is most important – it needs to be above 6. Regards, Fred Coe
Dr Coe, my name is mutarr trawally I’m 29 years from The Gambia west Africa, this will be my third time writing I pray to get a response form you I’m diagnosed with kidney stones last year and went through surgery Ureteroscopy and the stone was broke into smaller pieces and I still have 4 bilateral stones, from your article I understand testing and knowing the kind of stone you have is crucial in treatment and prevention, i have no possibility of doing a 24 hour urine test because isn’t available in The Gambia and I can’t analyze my stones because I believe that too isn’t available here as I’ve tried calling all the labs in The Gambia I could find on google, from your article I learned that I could have a clue of my stone by checking the HU density from a CT scan which I did and the HU is in the low 200 post surgery, and I also learn Uric acid stones goes with a ph around 5 mine is 6.0 I was hopping if it was in the 5 I could take potassium citrate and watch my ph on weekly basis at the nearest pharmacy, but now I’ve no idea what to do? I also have high phosphate in my urine but my kidney function marker are all within the normal ranges, I would really appreciate your advice as it would make all the difference in the world for me, I feel lost and powerless because I don’t know how I can help my self,
Hi Mutarr, I already answered your prior note and suggested what you in fact did. The low HU, if correct, means uric acid or sodium hydrogen urate are the most likely main components. That a single urine pH was 6 does not dissuade me. Collect a 24 hour urine and measure pH in it. It may be a lot lower as pH varies over the day and night. If the stone has so low a HU and your 24 hour urine pH is really so high as 6 then another kind of organic stone is present and chemical analysis is very important. The urine phosphorus reflects diet intake. I mentioned the critical components in my earlier answer. Regards, Fred Coe
My family has a history of uric kidney stones and gout. I also had a kidney stone about 8 years ago. Now I’m feeling all those same pre-symptoms. Doc ordered a CT with contrast but nothing showed up. My urine is 5.5 ph. Is it possible to have a stone that wasn’t detected on the CT scan? And how much potassium citrate should I be taking?
Hi Amy, uric acid crystals can cause pain and bleeding. As the article indicates the best bet is supplemental alkali to raise urine pH and prevent such crystals from forming. I would do that. Your physician can easily offer many alternatives. Here are some OTC products I reviewed – I do not advocate for any product. Regards, Fred Coe
Hi Dr. Coe,
I understand the importance of hydration in the prevention of stone formation. Is hydration as important as potassium citrate (Kcitrate) when it comes to uric acid stones? Or is it the other way around? I probably just averaged around 1.5 L /day. CT scan, 2 weeks ago, showed no more stones. I have been on Kcitrate for a year and a half. Is it fair to say then that hydration has a lesser role than Kcitrate when it comes to uric acid stone prevention?
I take Kcitrate 15 meq qid and my urine ph goes over 7.3 most of the time. I suspect this is the reason my brushite is 2.1 and ph is 7.3 (phos 953) in my last 24h urine. To prevent possible build up of brushite stones, I tried to modify the way I scheduled my med. I have been doing this for just a week now. I would check my urine ph before taking a 15 meq and if it is almost close to ph 6, I took another 15 meq tab. Doing this method, I only consumed 3 tabs a day instead of 4. Would you recommend this method? What other suggestions can you share to prevent possible brushite build up?
Thank you very much.
Hi Tony, Your goal is a 24 hour urine pH around 6 with will make uric acid stones impossible. More than that is to increase risk for no good reason. Hydration is pretty weak as a defense when urine pH is much below 5.3. Fred
Dr Coe,
Pls help me understand what is happening.
I take 15 meq of potassium citrate 3x a day for uric acid stones. I check my urine ph regularly throughout the day using urine ph test paper strips. Lately, 3 hrs and 6 hrs post ingestion of my med, the strips stayed under ph 5.8. This worries me. I am a bit confused as to what and why is this happening. I opened a brand new paper strip thinking there is something wrong with the current ph paper but results remained the same. Does this have to do with my metabolism or med? I still consume the same amount of fluids everyday. Can I just assume that potassium citrate is still working even if I do not see any color change with the ph paper? I know I have to get a ph meter soon for more reliability. Thank you
Hi Gina, I presume you take the K Citrate for uric acid stone prevention. The entire prevention arises from increased urine pH so I understand your problem. Clearly the dose is not quite adequate. I would collect a 24 hour urine and find out the mean pH that way before changing anything, for it is the only measure correlated with stone outcomes – and that is itself not ideal as mostly observational. Regards, Fred Coe
Hi Dr. Coe, My latest 24 hr urine results showed a ph of 7.0 (7.1 previous), brushite 2.1 (2.0 previous), phos 726 & citrate 1297. I take 15 meq potassium citrate 3 x a day for uric acid stones. Would you say 45 meq a day is a bit much basing on these numbers? This brings me to worry of potential brushite stones formation.
In spite of the high citrate and ph level, my urine ph drops below 6 (using ph strips) between 5 – 6 hrs after ingestion of a 15 meq dose. There is a 2-3 hour window for uric acid stones to potentially form since I take my dose every 8 hrs. Should this be a concern then? What do you suggest?
Just a side question to satisfy my curiosity- Does 24h urine collection result reflect ONLY the past 24h of bodily functions . . . or perhaps may include few days prior? (like A1c that evaluates glucose for the past 90 days).
Again, I thank you.
Hi Gina, as we treat uric acid stones with alkali we generally collect 24 hour urines to be sure about secondary risks as you note. I would suggest that approach for you. As for 24 hour urines, your question is wonderful. For sodium, calcium, ammonia, a 24 hour urine averages the prior 3-4 days. For volume, oxalate, pH, phosphate, the day of collection. Water is even faster, urine flow varies hour by hour with intake, as you already probably know from experience. It is this difference in timing that makes fluid a weak prevention against stones. Best, Fred
Hi Dr. Coe,
I’m a uric acid stone former who’s has 2 laser Lithotripsies in the last 4 years and is facing a 3rd procedure now to remove a newly discovered 2.3 cm, non obstructive stone. They also found a number of smaller stones as well. I’m, unfortunately, unable to tolerate either the potassium citrate or sodium bicarb.My urologist suggested I try Allopurinol, saying that although it wouldn’t raise my urine PH or disolve my current stones, it might prevent new stones from forming and my current stones from growing.Your thoughts?
Thanks..
Hi Dave, No. Allopurinol cannot help and your need to find some form of alkali you can tolerate. Sodium bicarbonate is usually benign, being present normally in blood at high levels. Here are a number of OTC alkali, perhaps one of them will work for you. Your pharmacist may have many alternative forms of alkali as well. You need at least 40 mEq/d of alkali, and I would not stop until I found a way to get it. Best, Fred Coe
Thank you, Dr. Coe.
Please correct me if I’m wrong, but one of the products I’ve seen you recommend in other posts is the formulations of Crystal Light Lemonade that contain potassium citrate. I’ve noticed in some of the companies newer products, that they’ve replaced the potassium citrate with sodium citrate.
Ingredients:
SUGAR, CITRIC ACID, SODIUM CITRATE, STEVIA LEAF EXTRACT (SWEETENER), CONTAINS LESS THAN 2% OF NATURAL FLAVOR, MAGNESIUM OXIDE, SOY LECITHIN, TURMERIC OLEORESIN (FOR COLOR).
Is sodium citrate effective at raising urine PH as well? If it is, would you know how much of the product I would I have to drink to raise my urine PH to a therapeudic level?
Thanks..
Hi Dave, Much thanks for noticing. For uric acid stones, citrate is citrate and will work the same. But for calcium stone formers the extra sodium is a drawback. Regards, Fred Coe
Thanks, Dr. Coe.
I do have and form uric acid stones and was wondering how much of the sodium citrate I would need to take to raise my urine ph to a therapeudic level. Would you know approximately how much in milligrams or teaspoons? I test my urine regularly with a PH meter and currently the ph has been averaging about 5.2. One can buy sodium citrate In powder form online and I’m thinking I could add it to a beverage. Tx
Hi Dave, in general 20 mEq twice a day suffices. But people vary. I would start there. Measuring spot urines is alright but I would advise collecting a 24 hour sample and measuring mean pH. Uric acid is rather good at forming and dissolving, and the average over the day – being the integrated net force for crystallization – seems a better index than individual measurements. As for using powdered K citrate, be sure to let your physician know and that she/he approves. Potassium can be unsafe and I do not know your medical details. Best, Fred Coe
Thanks, Dr. Coe,
Unfortunately, I’m not able to tolerate K citrate. I’ve tried it twice and, unfortunately, experienced disturbing (what felt like) heart palpitations that just wouldn ‘t go away.
With regards to sodium citrate, one can find it online in powdered form. The types I see are labeled as “food grade”. Can this type of sodium citrate, be used to raise a persons urine PH, and if yes, how much would one need to take to raise it to a therapeutic level? Tx.
Hi Dave, trisodium citrate has a MW of 258 mg/mmol. Having 3 proton acceptor sites, it is 258 mg for 3 mEq of alkali or 86 mg/mEq. We Need about 20 mEq 2 times a day which would be about 20 x 86 mg or 1720 mg twice a day. Be sure your physician knows what you are doing and approves. Regards, Fred Coe
Thank you, Dr. Coe. I’ve been testing with a PH meter and the sodium citrate has definitely been raising my urine PH to a therapeutic level.
This could of course be coincidental, but after mixing a quarter teaspoon of the sodium citrate (about 1500 mg) in an 8 oz cup of low carb fruit juice and taking it twice a day for 2 weeks, I passed a very painful, obstructive 2 cm x .5 cm kidney stone. It’s currently with my Urologist for analysis.
Question… Will the potency or effectiveness of the sodium citrate decrease if left in a liquid (grape juice, for example) for an extended period of time or will it have no effect? Currently, I’ve been buying a 64 oz bottle of low carb grape juice, pouring 8 oz into a cup, mixing in a quarter teaspoon of the citrate and drinking it. If instead, I mixed an equivalent amount of citrate into the full 64 oz bottle (2 tsp or 1 quarter tsp per 8 oz) and stored it in the refrigerator, would the solution lose it’s therapeutic effectiveness over time? In other words, will sodium citrate eventually break down into elements or compounds that would make it ineffective if it remained in some sort of liquid, lets say grape juice, for an extended period of time? And if yes, for approximately how long would this take to happen?
Hi Dave, Fruit juices can contain considerable buffers and the pH of the buffer will determine the outcome for the Na Citrate. If the pH is low then the citrate will be titrated from its 3 valence to 2 or even 1 meaning it has taken up protons from the juice and has no capacity to take up protons from your blood buffers and thereby signal your kidneys to raise urine pH. If you drink the juice, you will metabolize most of these buffers as their acid form so they will not alter urine pH downward, but adding the Na Citrate to the juice omits metabolism. This is why you could drink the juice and take the Na cit and urine pH rises but add the nacit to the juice and pH will not rise. Best, Fred
Hi Dr. Coe, Thank you for all your informative articles. I have previously produced several pure uric acid stones and have not had any since starting allopurinol a few years ago. Unfortunately, I’m having some liver side effects that are potentially from the allopurinol so I’ll have to discontinue that and I’m worried about forming stones again. My urine citrate falls well above normal on my Litholink 24hr samples (normal >550 and I’ve been between 900-1400 on my last six 24hr samples.) Sul24 and UUN24 are above normal, so I know to reduce protein…seems like even a small amount of protein lowers my urine ph (I have been testing with a ph meter.) CA24 was previously high so I know to reduce sodium, although my Na24 is within normal range. My concern is if my citrate is normal, should I still use Potassium Citrate as an alternative to allopurinol or is there a more appropriate option for my situation? Thank you for any and all input – Lisa
Hi Lisa, low urine pH is THE CAUSE of urine acid stones, allopurinol is a very weak alternative. I am sure your physicians would agree the allopurinol can be dropped and K citrate taken to raise urine pH to around 6 which will prevent uric acid crystals for forming and might dissolve some already present. The article you write on shows the evidence for this. High citrate is commonplace in low pH uric acid stone formers. I would not bother changing diet protein unless urine calcium remains high and protein intake exceeds 1.5 gmkg/d. Best, Fred Coe
Thank you so much, Dr. Coe. I’ve ordered one of the Potassium Citrate brands you’ve previously reviewed. I so much appreciate your help and all of your article, both invaluable – Lisa
Hi Dr. Coe,
I was wondering if high uric acid can create an environment that can lead to the formation of CALCIUM OXALATE stones.
Test results have us confused: stone analysis came back calcium oxalate, but 24hr urine study showed normal oxalate and calcium levels. Only uric acid level was elevated. I would think that high uric acid would lead to uric acid stones, but maybe they can lead to oxalate stones as well?
We would really appreciate your help! Thanks for so generously sharing your knowledge and expertise with so many who suffer with kidney stones!
(Also, my apologies if you have already answered this question elsewhere. I thought I bookmarked a previous query, but now I can’t find it.)
Hi Jamie, I began my career working on the uric acid calcium oxalate link and believe(ed) it exists. Here is some evidence related to this issue. (look at the last figures). I mention it in my overview chapter as well. The urine uric acid is high because of diet and can be lowered by moderation of high purine foods – typically beef chicken or fish or perhaps a large amount of seed based veggies. Allopurinol was successful as a prevention but diet should suffice. Best, Fred Coe