 The citrate molecule in urine is thought to protect against formation of calcium stones. This thought began as reasoning from chemistry, and culminated in clinical trials which substantiate the idea. As a result manufacturers produce citrate products for medicinal use, and doctors prescribe the medicine.
The citrate molecule in urine is thought to protect against formation of calcium stones. This thought began as reasoning from chemistry, and culminated in clinical trials which substantiate the idea. As a result manufacturers produce citrate products for medicinal use, and doctors prescribe the medicine.
All this is a wonderful success story, a kind of perfection of the paradigm of translational science: From science to a treatment for patients that reduces illness from kidney stone disease.
But what, exactly, is the science? Can scientists not enjoy the story of such a success, physicians derive from it a deeper understanding of the drug they so regularly dispense and patients the comfort that a perfected knowledge support the rightness of their prescribed treatment?
Citrate
The Molecule
As usual in such diagrams the carbon atoms are simply angles or kinks. Reading from left to right, there is a carbon atom bound (the solid lines) to two oxygen atoms (‘O’), one with a single and the other with a double bond. These bonds represent sharing of electrons by the atoms.
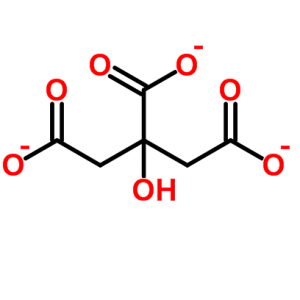 The single bonded oxygen has an extra electron in its outer shell, so it carries a negative charge (-). Calcium is an atom with two positive charges, so the idea of calcium and citrate binding to each other comes naturally as one thinks about opposites attracting one another.
The single bonded oxygen has an extra electron in its outer shell, so it carries a negative charge (-). Calcium is an atom with two positive charges, so the idea of calcium and citrate binding to each other comes naturally as one thinks about opposites attracting one another.
Next in line is another carbon atom; the kink means the carbon is linked to the carbons on its left and right and to two hydrogen atoms. The hydrogen atoms are necessary because every carbon atom makes four bonds.
After that, is the third carbon which is very occupied. It has an oxygen which is itself bound to a hydrogen – a hydroxyl molecule, really 2/3 of a water molecule – and another carbon bound to two oxygens, one of which has a negative charge. To the right of this busy carbon the molecule repeats itself as in a mirror.
How beautiful nature is, how powerful its symmetries and suggestive its forms!
It is as though some great sculptress were taken with an image of perfection that a string of carbons might take the perfect form to mate with calcium, tiny in comparison, and doubly charged positive.
But how? How would the mating occur? If you do not look down, could you have imagined it?
Binding Constants
Calculation and experimental determination of calcium binding by citrate is complex. Partly, all 3 oxygens can accept a proton, so 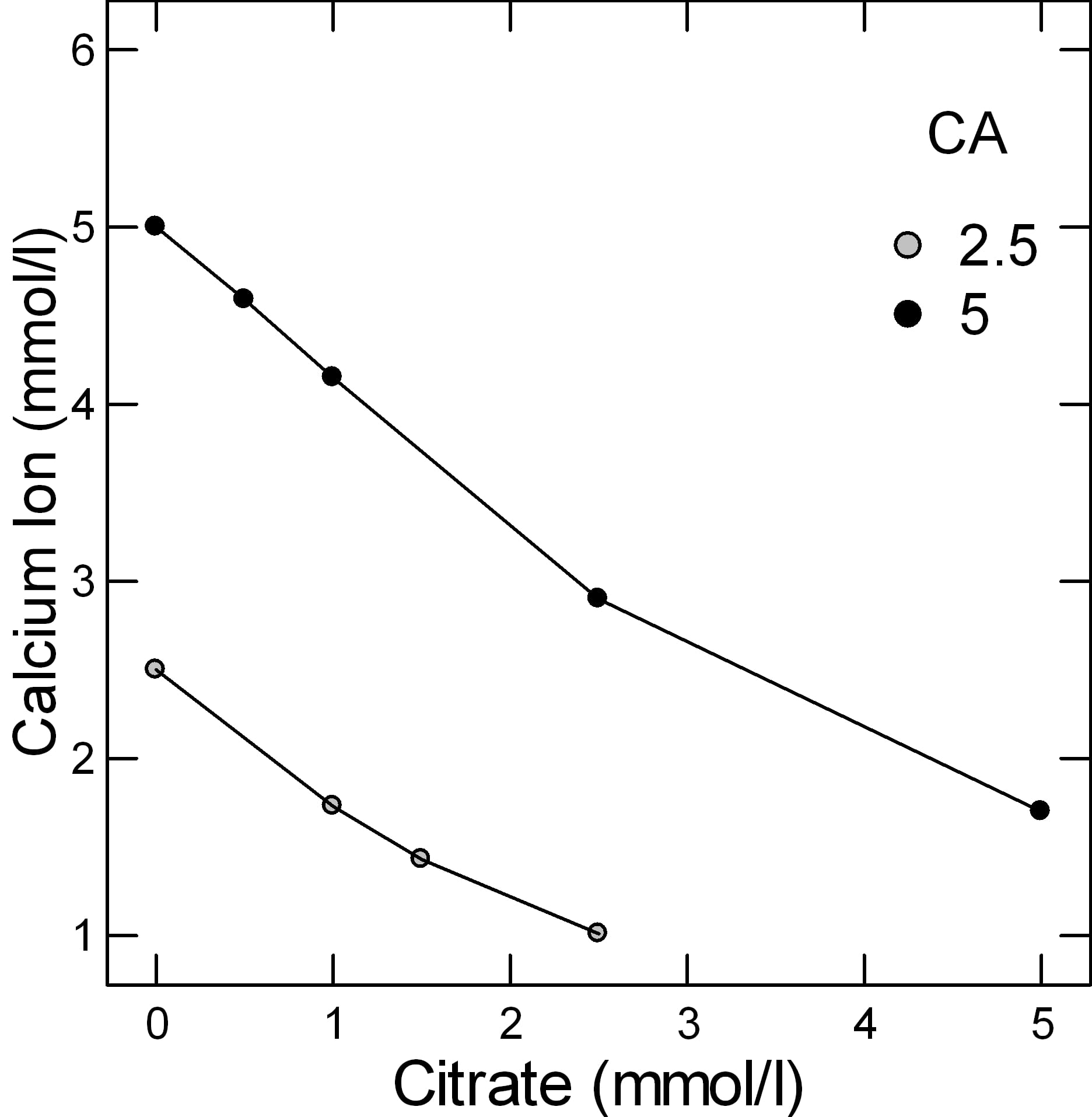 the acidity of the solution – urine in our case – matters. Partly, binding is complex. As shown in the section below, it involves forming a ring structure and a bi-molecular structure. In general calculations are performed using computer programs.
the acidity of the solution – urine in our case – matters. Partly, binding is complex. As shown in the section below, it involves forming a ring structure and a bi-molecular structure. In general calculations are performed using computer programs.
But simple experiments give a reasonable gauge of the power of citrate to bind calcium. In the figure adapted from Table 2 of the reference at the top of this section, when the molarity of calcium and citrate are equal, at both 2.5 and 5 mmol/l of total calcium (2.5 – grey circles and 5 – black circles in the legend), a common range in urine, only about 1 mmol of calcium is unbound and therefore a free ion that can combine with oxalate or phosphate to make a stone. From the shapes of the graphs, citrate is a powerful binder as the calcium ion falls almost linearly with citrate molarity.
This graph is very approximate. Actual calculations of citrate binding effects have to consider pH, ionic strength, and many varieties of citrate calcium salts. These are part of how supersaturations are calculated. Yet for all its simplifications, this graph of ancient data suffices to show what citrate can do as a protection against urine crystallizations of calcium salts like calcium oxalate and calcium phosphate.
Calcium Citrate Crystal
But this is not a complete story. What if calcium and citrate combine to make a crystal which becomes yet another kind of stone? They can indeed form a crystal, but one which is so soluble it is never a stone risk. Even so, how the crystal forms is a way to show how the molecule binds calcium, which is in itself simply very interesting.
Creation of the Di-Citrate
I have made this structure the featured illustration for the article, but put a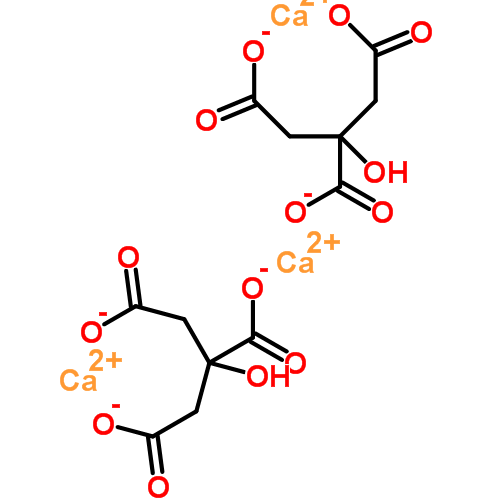 copy here for visual convenience. Would you have imagined the two ends of the molecule would bend around to hold the calcium, which makes what was linear into what is, now, a ring?
copy here for visual convenience. Would you have imagined the two ends of the molecule would bend around to hold the calcium, which makes what was linear into what is, now, a ring?
Just below is another ring, identical in character. Neither is a crystal, merely they are a pair of rings.
But, that odd outcropping of a carbon atom on the original molecule has its charged oxygen, and through another calcium, caught there, the two are linked.
Citrate has two ways to bind calcium. A single molecule can bind one calcium atom. Two citrate molecules together can bind one calcium atom. So the ratio is 1.5 calcium atoms per molecule of citrate (3 atoms/2 citrate molecules).
The Making of a Crystal
How these paired rings make a crystal is not an easy story to tell. Here is a good reference which I will explicate not as a crystallographer, which I am certainly not, but as a narrator telling a good story.
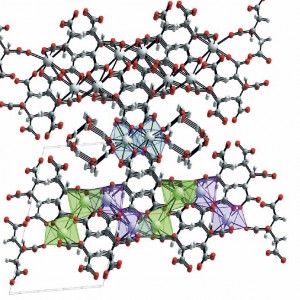 The calcium citrate crystal is built up out of repeating units, each of which is a pair of citrate molecules linked by a calcium atom. This ‘di-citrate’ unit has three calcium atoms in it, one in the center, which is unique, and one at each end which mirror each other. These are in the diagram just above.
The calcium citrate crystal is built up out of repeating units, each of which is a pair of citrate molecules linked by a calcium atom. This ‘di-citrate’ unit has three calcium atoms in it, one in the center, which is unique, and one at each end which mirror each other. These are in the diagram just above.
All three coordinate with 8 oxygen atoms. By this I mean that in addition to the 2 oxygens shown in the simple figure of the di-citrate molecule, oxygens are shared that belong to di-citrate molecules ‘above; and ‘below’ and to the sides so as to make a set of plates like the floors of a parking building.
This complex macrame shows all three calcium atoms. The middle one – calcium 1, at the center of this drawing – relates to 6 oxygens bound to carbon atoms, and to two belonging to water molecules that are pulled into the final structure. The second – at the left lower corner – is coordinated by 8 oxygens bound to carbons, and the third – at the right lower corner, is coordinated with 7 oxygens bound to carbon and one hydroxyl (OH) bound to a carbon (see the simple di-citrate drawing).
This crystal, calcium citrate, is actually a medicinal product (‘Citracal’™) which when swallowed in a pill form dissolves in the gastrointestinal tract to donate calcium and citrate that can be absorbed into the blood. The crystal itself is not found in urine. The medication is of no immediate interest concerning stone disease because one does not use it as a treatment for stones but rather as a supplement for bones. Whether or not it might be helpful in preventing stones would require a trial.
The citrate used for stones is potassium citrate, which is simply the single citrate molecule with its 3 negative charges satisfied by potassium ions or protons.
Why, then, have I troubled you with the elaborate business of citrate calcium binding and crystal formation?
Because it is one way that citrate protects against stones. The molecule binds calcium which is therefore no longer free to combine with oxalate or phosphate to form kidney stones. The crystal calcium and citrate forms does not make stones because it is very soluble. If it were not, citrate would not be a protection against stones but merely the substrate for yet another calcium type stone.
Solubility of Calcium Citrate
What, then, is the evidence for this statement – that the calcium citrate crystal is so soluble that it does not make stones? I have said it several times but have provided you with no proof.
The three oxygens of citrate are partially occupied by protons, and when 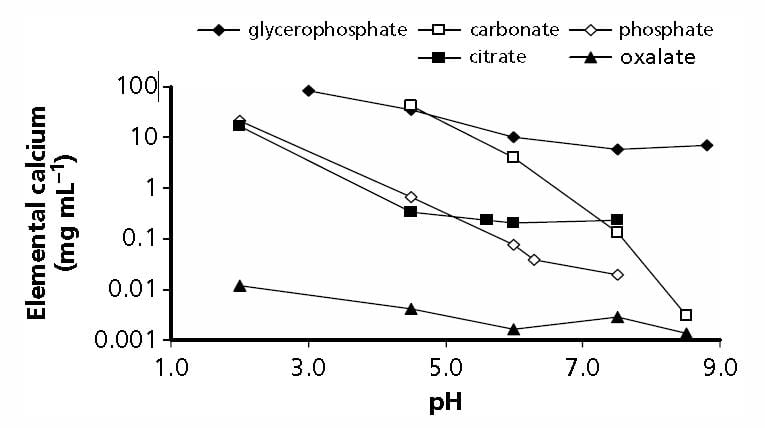 they are they are not available to coordinate with calcium to make the di-citrate and its crystal. Therefore the solubility of citrate will be influenced by the concentration of protons, the acidity of the urine, represented here by pH.
they are they are not available to coordinate with calcium to make the di-citrate and its crystal. Therefore the solubility of citrate will be influenced by the concentration of protons, the acidity of the urine, represented here by pH.
The experiment is done by adding crystals to a simple salt solution, letting them equilibrate with the solution at a constant temperature, and measuring, in this case, the concentration of calcium that is in the solution, having left the crystals as they dissolve.
Two of the three oxygens are ‘weak’ acids which are half saturated with protons at pH values of about 3 and 4, meaning that throughout the range of urine acidity – pH 4.5 to 8 – both are free to bind with calcium. The third is at pH 6.4. One might expect an increase in calcium binding as pH rises above this point, but there is no obvious change in the solubility of the crystal between 5 and above 7.
At pH of 6, the mean for normal urine, the concentration of calcium in solution is about 0.2 mg/ml or 200 mg/liter. Given the atomic weight of calcium – 40- this is 5 mmol/liter. Calcium oxalate crystals dissolved in the same way yield a calcium concentration below 0.005 mg/ml or 5 mg/liter which is about 0.05 mmol/liter. Calcium phosphate crystals give a value of 0.08 mg calcium/ml. 80 mg/liter or 2 mmol/l, less than half of the citrate.
Since calcium and citrate are released from the crystal in proportions of 1.5 calcium per di-citrate, one presumes the equilibrium citrate molarity will be 66% of calcium or 3.3 mmol. Given the molecular weight of citrate is 192 mg/mmol, this amounts to 633 mg/liter of citrate. That is a high concentration of citrate, given that common excretion rates are rarely above 750 mg/day and urine volumes about 1.5 liters a day. Even so, some urine samples almost certainly achieve these concentrations of calcium and citrate on occasion. But equilibrium is not enough to create new crystals; one needs to achieve a higher value so that new crystal nuclei will form. That will be very unlikely for calcium citrate.
So citrate can combine with calcium to remove it from binding with oxalate and phosphate, and form a crystal of considerable solubility. Being very soluble, calcium citrate is rarely if ever found as a kidney stone.
Calcium and Citrate in Urine
One of two crucial issues about citrate in stone prevention is the relationship between the concentration of calcium and that of citrate. The higher the concentration of citrate compared to calcium, the lower the concentration of unbound calcium, which is free to combine with oxalate or phosphate to make kidney stones.
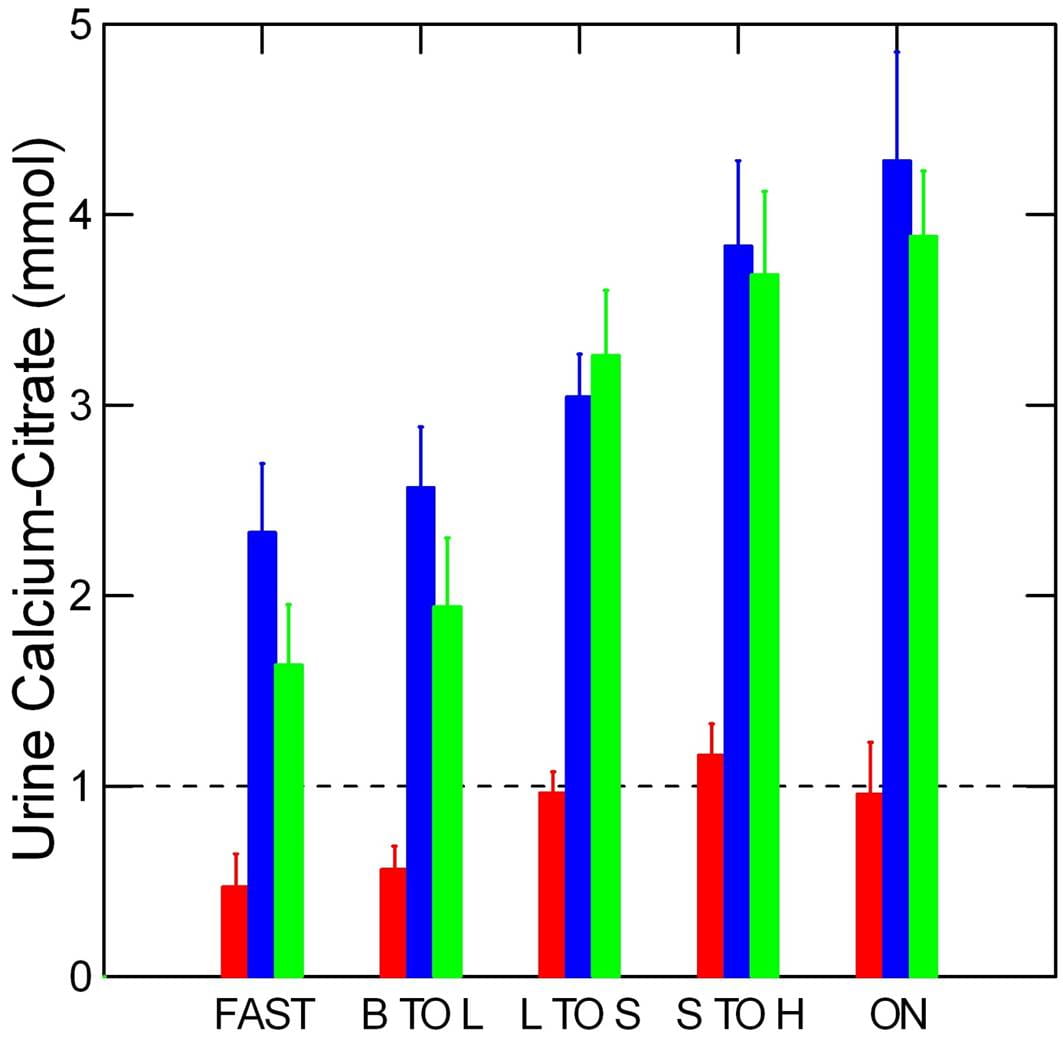
This graph from our research work shows the difference between urine calcium and urine citrate concentrations for normal people (red), and calcium phosphate (blue) and calcium oxalate (yellow) stone formers over the full day and overnight periods. These two kinds of stones have already been reviewed on this site.
Normal people have lower urine calcium excretion rates than stone forming patients, but about the same excretion rates for citrate, so the calcium – citrate difference is below 1 mmol/liter. In the graph above which shows free calcium ion at two concentrations of total calcium – 2.5 and 5 mmol/l vs. citrate concentration, when the calcium and citrate concentrations are equal the free calcium is below 2 mmol/liter. When citrate concentration exceeds that of calcium, the free calcium will be lower.
Both of the patient groups have much higher calcium excretions than normal people and because their urine citrate excretions are no higher, and perhaps even lower than among normals, the concentrations of free calcium are much higher, in the range of 2 – 3 mmol/liter.
It is this kind of information which has long made scientists believe that citrate is an important factor in the normal defense against calcium stone formation, and which led to the successful trials which proved that this believe is not unfounded.
What is the Real Science?
The citrate story illustrates all the three forms of scientific research.
Empirical science is what we would call the meticulous measurement of the binding constants between citrate and calcium, and the specific structure of the calcium citrate crystal. It is also what we would call the pretty graph of urine calcium – citrate differences in normal people and stone forming patients.
Applied science is the trials which showed that the intuition of citrate as a treatment was a true intuition. It is indeed a treatment, and that is a fact which time will not alter.
Basic science, however, is not so obvious here. Where in the story do we encounter the passion or curiosity to ask how citrate has come to be in urine.
Certainly citric acid plays a role in biology vastly – one might say infinitely – greater than that of stone prevention. It is the key molecule in the citric acid cycle which is so well known that I have only to reference it from Wikipedia. Known to schoolboys and schoolgirls everywhere, this cycle is used by all aerobic organisms to generate energy, and is of an extreme ancient origin.
Surely a molecule of such lineage and power is ruled little if at all by the problems of renal crystals. Yet it is handled by the kidneys with considerable finesse as if somehow important to the renal system or – perhaps – as if the renal systems were somehow important in the larger matters of maintaining serum levels of the molecule.
Here is imaginative science. Here is the place where a question of underlying cause comes into focus. Here is where nature presents issues of monumental consequence.

6/21/15 This is strange — For the past two days I’ve noticed that in my poop in the toilet are 2 undissolved potassium citrate pills for each day. They float. To me this does not seem normal. The pills are just going through me whole, so they are not doing any good. Have you ever heard of a situation like this? Please respond. Thank you – Judi Stratton
Hi Judi, yes I have heard about this frequently. The 10 mEq (1080 mg) large pills are wax matrix slow release, and the shell remains intact despite the contents leaving the pill and entering the bloodstream. I have noticed the 5 mEq pills – smaller and shiny surface – seem to be coated and may not release their contents. But follow up 24 hour testing, which is always appropriate when a medication has been prescribed, can tell what has happened. Compared to your initial evaluation, the 24 hour urine should show changes from the pills: increased urine potassium, increased urine pH, and a fall in urine ammonium ion as compared to the urine sulfate ion. Your physician knows to look for these changes as evidence of absorption of the active medication. The urine citrate itself is a less exacting sign because it may not increase even when the drug is fully absorbed. I am sure your physician has already made these observations and I would simply call and be sure the pills are working. Incidentally, you posted two nearly identical comments within several minutes, and I took the liberty of leaving only one on the site. Regards, Fred Coe
Hi Dr. Coe,
I have a client with kidney stones and am curious if this tx can be applied for her? She is only on coumadin for meds. She eats low oxalate foods but is frustrated with the list of options. I wondered if is works as a binder in hopes to increase her food choices. What do you think?
Thanks, Hope Hayes, RD
Hi Hope, the trials for stone prevention with potassium citrate emphasized calcium stone formers with reduced urine citrate excretions. They are summarized here. Coumadin would not be an issue. Low oxalate foods are reasonable if her urine oxalate excretion is high, but not otherwise. Regards, Fred Coe, MD
This is a great article. I notice quite a bit of reference to Potassium Citrate which, although is very soluble in water, doesn’t have me convinced as the preferred prescribed form of Citrate. Is it not true that while passing through the digestive tract it will first be blasted with HCl in the stomach then Sodium Bicarbonate in the Duodenum?
This would convert the very expensive potassium citrate to citric acid then convert it to sodium citrate. This being the case, wouldn’t the popular candies such as warheads (that contain up to 60% citric acid) be just as effective and a fraction of the cost of potassium citrate?
If you were to go a step further looking at the molar ratios of Potassium Citrate and Citric Acid you would get 1.59 times more citric acid per gram of Potassium Citrate (and enjoy a tasty candy). 🙂
Hi Chase, The citrate is metabolized in the Krebs cycle as citric acid, so it takes up a mole of protons per mole of citric acid metabolized. In blood citrate and citric acid are in equilibrium which greatly favors abundance of citrate, but since metabolism is only of citric acid the process consumes protons. These protons come from carbonic acid – the blood buffer is bicarbonate/Carbonic acid – and as they are taken up new bicarbonate is made from CO2 whose partial pressure is regulated by respiration. The GI tract effects are rather neutral as you point out. Citrate is protonated in the stomach to citric acid and regenerated in the duodenum by bicarbonate secretion, but the formulations are usually wax matrix and escape gastric dissolution anyway. If they did or did not the result on acid base balance is about neutral. Once absorbed as citrate, however, the molecule is metabolized as the acid. Giving citric acid itself predictably does nothing to urine citrate because it is simply metabolized as such. Thanks for the outstanding question. Regards, Fred Coe
Interested in your, Calcium binding by citrate, article, by Fredric Coe, MD. Could you run rainwater through mineralized, chalk like, porous rock, through adsorption, would the molecules now have a net positive charge and leave a negative charge behind in the chalk? Doubly reinforcing the water when it dissolves the chalk. Would the calcium carbonate molecule split, causing a calcium atom with a double positive charge(ion) to be washed away in the water. Would that leave negative ions in the water? Looking for ways to get negative ions for health? Thanks so much…Kim
Hi Kim, Things do not quite work that way. If you run pure water – I think that is what you are after in rainwater, through chalk – calcium carbonate, and if the rain is acidic as rain is these days, some of the carbonate will become bicarbonate and release its calcium. The calcium and the bicarbonate will enter the solution. The calcium has two positive charges, the bicarbonate one negative charge, and the water forms a hydronium ion with another negative charge. The charges are all balanced – the sum of the positive and negative charges is always 0. Charge separation is not possible under conditions of ordinary chemistry reactions like this one and only occurs at very high temperatures, or within high voltage electrical fields, neither condition one you might want to be around in. So you can get lots of negative ions anytime you want – dissolve baking soda in water, but you get an equal number of positive ones right in the same place to balance them. Regards, Fred Coe
hi. again, thanks for this service.
does citrate bind to calcium in the gut (as in a calcium citrate supplement) reducing the amount of calcium available to bind with oxalates? how about citric acid (as from lemonade)?
on a different question, does d3 in calcium supplements influence the binding of calcium and oxaltes?
Hi Terry, No, it does not because ingested citrate is absorbed and metabolized to bicarbonate, and that bicarbonate signals the kidneys to release citrate from blood into the urine. Citric acid is useless. Citrate is metabolized as citric acid so it takes up a proton, citric acid already has its protons and its metabolism does not lead to production of bicarbonate. Vitamin D is not known to affect urine oxalate. It may improve calcium absorption but this latter is rarely more than 15% and should be taken with a high calcium diet leaving ample to bind oxalate. Regards, Fred Coe
thanks again. i feel like the jester at the tudor court, trying to dance my way through the complexities of chemistry in your article re: potassium citrate. i am a lacto-ovo vegetarian and eat low oxalates. when i have a meal i suspect is higher that desired in oxalates, i take half a 600 mg. calcium carbonate tablet (without d3). would it be helpful to switch to a calcium citrate supplement, even though all of them seem to have the added d3? also, i enjoy lemon/lime in my water. no harm/no benefit from that? best, terry
Hi Terri, It is complex. What I can offer on a site is the general rule but real meal planning and supplements especially need to follow on what was found in your urine and be collaborative with your physicians. Here is a plan for you – if I have not already brought it. Be sure all the steps have been gone over with your physician. They should lead you properly. We have a new article on calcium in the diet, just put up. The invaluable thing your physician brings is an understanding of all this complexity so you can get what you need more easily. Regards, Fred Coe
You are going to smile doctor because from your articles I have been trying to solve my Shih tzu problem in developing stones! It is a breed prone to them. She was a rescue and has had two surgeries the first had Calicum and struvite stones and the second just Calicum as she is on a strict diet. Her urine is always very concentrated, she does not pee as much as other dogs I know. Her only option seems a third surgery I told my vet about potassium citrate which is available for dogs which he was not aware of and I recently bought it and have been giving it to her but I believe you said existing stones cannot dissolved correct?
Hi Deirdre, Dogs get stones and there are scientists who study the matter. The potassium citrate may help but it is hard to know because the urine risk factors are not calibrated for dogs. But if you get even a small sample it can be analysed by commercial vendors for supersaturations which will help. Struvite usually means infection, so that is a separate issue.
Hello doctor- thank you for this invaluable site, I’ve learned things here I’ve never heard anywhere else.
Do you have any thoughts on the recent discovery of Hydroxycitric Acid as a possible calcium stone reducer? I’m including a research link in the website field. Apparently it causes CaOx to break up even in a supersaturated solution. HCA is already on the shelves as a weight-loss drug (likely ineffective), and it seems that taken orally at the recommended daily amount a lot of HCA does survive and make it to urine. The initial discovery was only a few months ago and I believe they’re looking into trials to see if it does in fact work in humans. HCA does have complications with seratonin medications but seems pretty tolerable from what I’ve read.
Do you know anything about this? It sounds too good to be true but I hope they find a way!
Hi Damon, One of the authors is a close friend and long time collaborator of mine, so I do indeed know of this work. Since the material is open for public use and thought to be safe I can have no special concerns if it is used for stone prevention, but I also have no reason to believe it will actually stop stones from forming – it is funny how what seems inevitable is not once tested. I do not know if a trial will be done as the stuff is cheap and in public use. So, the matter is moot. I will forward your comment to Dr Asplin, my colleague, along with my response and see if he would add anything more. Regards, Fred Coe
Dr John Asplin replied with the following comment:
“Fred,
We are in the planning phase of a trial of hydroxycitrate in rats and a trial in humans (human trial will have an endpoint of changes in urine chemistries, not stone formation). At this time, I would not recommend people use the compound as a treatment for kidney stones as much is still needed to be studied. In addition, the supplements available in stores often contain other compounds which make it difficult to know what the net risk/benefit will be for stones. A few years ago, a weight loss supplement called “Hydroxcycut” had to be reformulated due to cases of liver disease. Whether the problem was hydroxycitrate or another component in the pills is not clear. It is clear that the government continues to allow products to be sold that contain hydroxycitrate but without better evidence for efficacy and without better control of what is in the various supplements, I don’t feel it should be used for stone prevention at this time.
John”
Hi Dr. Coe,
I am actually a patient of yours with a unique case to which none of my doctors have been able to really put me on any plan. My tests are all over the place. I really need to speak with you and have another appointment. Maybe also with a nutritionist that you could refer me to. Anyway, with this article, I am confused. If citrate binds with the calcium, then isn’t that doing the opposite of what needs to be done, which is for the calcium we eat to bind with the oxalates we eat and then be excreted? Can you explain this in less “medical/scientific”talk? Also, how do we get the oxalates/calcium to be excreted in our feces rather than the kidneys? Thank you for any help!
Hi Michelle, Citrate binding with calcium in the urine will have no effects on oxalate binding by calcium in the GI tract. Thanks for posting your comment on the site so others can share. But as you are my own patient you have direct access. Please call Kathleen at 773 702 1475 and tell her you want an appointment. Warm regards, Fred
Dr Coe – It has been nearly 50 years since my last organic chemistry course, so I am happy to have somewhat followed this discussion. Getting off the track slightly, are you familiar with Chanca Piedra? It apparently has had anecdotal success for some South Americans. Are you aware of any studies concerning its effectiveness on, as “they” claim, its ability to dissolve, reduce the size of, and facilitate passing of stones? BTW, it was a lot easier to read your and Harris’ article on following a low oxalate diet. I have a ‘bucket list’ item of learning to fly, but the FAA will not medically certify anyone carrying stones. Thanks for your help.
Hi Jim, I have made some inquiries and think it is nonsense. The only reference of consequence is to a report by a physician in the 50’s that was anecdote. There are huge numbers of what seem self referential and not honest ‘references’ but as for stone breaking no. I had planned to write an article on it and probably will but the topic is so poorly documented I will be bored. Someone is making money. Best, Fred Coe
Potassium citrate lowers urine calcium excretion
LINK is no longer active. Unable to open . . . not to be found
Hi Christine, I could find the link at all in this article. But the fact is detailed in other places. Here is where I put the main evidence. In this binding article I could not find a link to urine calcium, but if you can just copy the text and send it to me and I will repair the link. But this link here is ideal for your question. Thanks so much for thoughtful reading. Fred
Hi Fred, I have calcific rotator cuff tendonitis. Does taking Potassium Citrate help break down calcifications in the shoulder at all? Apparently, I’m excreting electrolytes in my urine. My doctor first said it was because I was taking D-mannose, but I haven’t been taking D-mannose for months and my urine electrolytes are still high. My doctor doesn’t have an answer as to why. Do some people excrete more potassium in their urine than others, and if so, why is that? Could excreting more potassium causes the body to be more prone to forming calcifications? Thank you, Susan
Hi Susan, No; it will not help calcified rotator cuff disease. Taking citrate will not influence blood citrate. Anything that lowered serum calcium ion so joint crystals dissolved would endanger life – the brain, heart, etc all require serum calcium levels be very constant. As to urine potassium it faithfully follows diet and drug potassium intake – it is an atom. Likewise for sodium. So, you must eat more potassium than is usual – fruits and veggies are the usual source. But be happy; on average our urine potassium runs 40 – 60 mEq/day. The US ideal is over 100 mEq/day. Potassium intake has not effect on joint calcium content. Regards, Fred Coe
Dear Dr. Coe,
The chelating of the divalent calcium ion by citrate anion to keep the calcium ion in solution rather than combining with oxalate or phosphate anions to form stones. Basically, as I understand it, this chelating effect prevents supersaturation of the combining of oxalate or phosphate with calcium to form stones.
What fruit juice would you recommend to supply the citrate anion to give the required chelating effect?
Anthony Perrotta (PhD Chicago, 1965)
Hi Anthony, indeed citrate complexes with urine calcium to form a very soluble salt. That reduces free calcium molarity. Oxygens on citrate also bind to surface calcium ions in calcium oxalate and calcium phosphate crystal nuclei competing against oxygens from phosphate and oxalate. This tends to disrupt orderly crystal growth and can provoke dissolution by disordering the surface charge array. Urine citrate is determined by the NaDC1 transporter that responds to pH of the proximal tubule fluid, so any alkali will raise urine citrate. Fruit juices are a miserable way to provide metabolizable anions because they collect the fructose from many fruits into a concentrate. Fructose – all sugars – raise urine calcium and fructose is a genuine health hazard. The best is some form of potassium citrate or else a diverse array of fruits – not the juices – and veggies that all contain abundant Krebs cycle precursors. Best, Fred (MD Chicago, 1961)
Dear Dr Coe
On a slightly different matter – citrate in blood – I do platelet apheresis and I expect you’ll know that citrate is used as an anticoagulant in this. Some is returned to the donor. Where I do this (in the UK) there’s concern about the citrate complexing blood Ca++ and so causing cardiac arrest. I usually get about 330 ml of 3% sodium citrate per session, and have none of the classical finger tingling or anything. I drink about two pints of milk in the 6 hours beforehand to keep up my Ca level. None of the nurses in charge can tell me how much Ca I should ingest; I calculated this amount of milk on the (incorrect) basis of one mol Ca reacting with one mol citrate. I’d be interested to know what your views on the use of this angicoagulant in apheresis. I think it’s safe as I survived 80 sessions with no effects. Many thanks, Vic Breeze
Hi Vic, Citrate is well known as a systemic anticoagulant, and your physicians are doing nothing I could criticise. Regards, Fred Coe
Dear Dr Coe,
What if you have a cat with chronic kidney disease and you feed a cat food with potassium citrate added. And you want to add a calcium carbonate binder to bind up phosphates in the food. Would calcium in the carbonate bind to the phosphates (what is wanted) or to the citrate, or both. This is a dilemma. Hoping you can advise me. And would using calcium acetate instead make a difference or would it be the same. What if you use AlOH instead. Thanks.
Hi Jules, Veterinary medicine is a speciality I know nothing about, and I would be wrong to offer advice. Cats do form calcium oxalate stones, but their treatment requires a real expert. Regards, Fred Coe
Good morning Dr.
How can abuse of vitamin C increases calcium oxalate stones , and how can citrate cause low PH of urine?
Hi Pierre, vitamin C can convert to oxalic acid. Citrate cannot lower urine pH – but citric acid can. Regards, Fred Coe
Dear Dr Coe. Is lemon juice effective in dissolving calcium oxalate stone ?
Hi Abhijnan, No; nothing dissolves calcium oxalate. You need prevention. Take a look here for a plan. Regards, Fred Coe
Dear Dr Coe,
Thank you so much for this informative and fascinating explanation of the role of citrate in inhibiting the formation of calcium stones.
I have an asymptomatic non-obstructing lower pole stone that is most likely a calcium oxalate stone, based on the analysis of a previous stone I passed. My current stone has been slowly growing for a number of years, and although I will no doubt eventually have to undergo a procedure to address this pesky little passenger, I’m very keen to delay that day — perhaps even for some years, considering the promising new treatment methods on the horizon (namely SonoMotion’s BreakWave and StoneClear focused ultrasound approaches, currently in development.)
To that end, I have not only significantly increased my daily water consumption, but have taken to drinking adding fresh lemon juice, having read several studies that suggest that the citrate in lemons may impede the formation of calcium oxalate stones. I understand it will have no effect on the existing stone. My goal is only to impede further growth.
After reading your article, I am greatly encouraged, as I had been wondering if consuming lemon juice was just wishful thinking on my part. Your clear explanation of the science behind the effect of citrate on calcium stone formation provides great motivation to continue my current course of action, with a follow up scan in 6-12 months to try to determine what degree of growth has occurred. I’m very hopeful that the stone will show little change.
Thanks again for taking the time to write such an illuminating article, and for sharing it here.
Kind regards,
Shaun Coleman
Hi Shaun, Raising urine citrate to offset stone growth is a good idea unless your 24 hour urine citrate is already adequate. If it is, you might just raise the urine pH and promote overgrowth of calcium phosphate salts. Be sure about your 24 hour urine stone risks before undertaking presumed treatments, because otherwise things might get worse. As for lemons, I despise their use. A generall array of fruits and veggies – 5 servings a day – is recommended for all Americans and will provide as much or more alkaline anions along with finer nutrition. Regards, Fred Coe
Thanks for your reply. I will talk to my doctor about getting a 24 hour urine sample analysis before getting too carried away with home treatments to inhibit stone growth. To be honest, I won’t be sorry to give the lemons a miss, although the plus side is that after nearly a week of lemon juice just about every other fruit tastes amazing in comparison. Many thanks for your advice, and for the wealth of information you have provided on this site.
Is there a difference between taking Calcium citrate and potassium citrate for kidney stones?
Hi Sandra, there is indeed. Calcium citrate is important for bone, and also used as a calcium supplement against oxalate absorption. Potassium citrate is an alkali load to offset uric acid stones, or raise urine citrate. I assume you form kidney stones, but what you do for prevention depends on exactly what is wrong with you as a cause of stones. Consider this as a good introduction, and this as a more advanced version. Regards, Fred Coe
As a Black Tea lover and Ideopathic Calcium Stone Former, I am looking for a way to incorporate my favorite beverage back into my diet. I have not passed a stone in 8 years after 15 stones in 12 years, the result of diet modification. Above all, I aim to not pass another.
This paper from 2002 (http://apjcn.nhri.org.tw/server/APJCN/11/4/298.pdf) analyses oxalate content of teas and also calculates (though doesn’t measure) the binding capacity of milk in tea (25% milk to 75% tea). Dr. Coe, I see you writing extensively on the effect of Potassium Citrate on urinary calcium output, and indirectly the concentration of urinary oxalate.
What about the use of Calcium Citrate dissolved in the steeping liquid for tea in order to bind with the oxalate before it can even be absorbed? My traditional two cups of milked tea with the addition of a typical supplement pill (Kirkland brand) of 500mg Calcium Citrate, one Cup water, one Cup milk, and about 4g of tea, would it not seem likely to bind a substantial amount of Oxalate? 4g of the highest Oxalate containing tea from the paper would contain 26.6 mg Oxalate (in line with the Harvard List’s 28mg for that amount of tea). Addition of this supplement had no affect on flavor.
Dr. Coe, any thoughts?
(joking) Anyone with an HPLC able to do a thorough study?
Hi Eric, Milk with tea should work, calcium citrate might ruin the flavor. But the general idea is sound. Do it and get a new 24 hour urine to be sure oxalate has not increased. Regards, Fred Coe
Hello Dr. Coe,
Can oxalate crystals form and settle all over the body causing joint pain and stiffness? Since I have IBS-C I have trouble eating foods with calcium or taking supplements. My recent blood work revealed these results. Sweet potatoes help my inflammation tremendously but have severe joint pain and stiffness. Am I riddled with these crystals and will adding the calcium citrate and potassium citrate help bind the stones and, hoping the calcium does not prevent elimination problems, excrete them? I am also weary of adding potassium because when eating a banana after exercising, I had a weird nerve sensation all over my body at night that would not allow me sit still without extreme discomfort. This happens with onions, sweet peas, corn but not with sweet potatoes. My appt with my primary doctor is not until June but here are my red flags from my recent blood work. I am hoping with your expertise you can give me some direction to help me understand what is going on.
Globulin = 1.7 g/dL. ( Low)
Albumin/ Globumin ratio = 2.6 g/dL (high)
Vitamin D 25-OH=12 mg. (Low)
calcium 8.7. (Put total for information)
Thank you for your attention and any insight you can offer me is greatly appreciated.
Hi Leslie, Absent severe kidney failure, oxalate crystals do not exist in the human body apart from the interior of the renal collecting systems. I do note you have vitamin D deficiency and a low blood calcium, and treatment with Vitamin D might be something your physician might want to undertake. If you do not have kidney stones I know of no reason to use potassium citrate. Regards, Fred Coe
Thank you Dr. Coe.
Perhaps the low Vitamin D and Calcium is the reason for my joint pain, swelling and stiffness. I hope it is not my kidneys.
Sincerely, Leslie
I would ask your physician if vitamin D might not be of value. Fred Coe
Thank you Dr. Coe.
I sincerely appreciate your insight.
Leslie
Hi Dr.Coe-
I am trying to understand the relationship between calcium, oxalate, and the use of proton pump inhibitors. PPIs decrease create a more basic gastric environment (and according to this medical school lecture) this leads to more calcium bound to oxalate → reduced calcium reabsorption → increased risk of fractures in the elderly. Can you please explain the mechanism behind why the lac of acid will favor calcium bring bound to oxalate?
Thank you so much and cant wait to read any insight you can give regarding this topic.
Hi Micah, The theory you mention, a less acid stomach interior would reduce separation of calcium from phosphate, as well as from oxalate, so it can be absorbed. I think there is more to it. Because the pH inside the stomach is normally so low – about 1-2, it can protonate oxalate disrupting its binding to calcium. But I have not personally studied this area and want to limit my remarks here and instead do an article on the subject that will be proper. Thanks for asking, it is provocative and worthwhile. Fred
Hi Dr. Coe,
I have flags on my urine test which I provided 3300 ml/24 hrs (since kidney stone surgery last month, i have consistently drank 90 oz of water daily for the last six weeks). I was taking a Vitamin D supplement once per month in the amount of Vitamin D2 50,000 1.25 mg for deficiency for the last 18 months. I was also eating an extremely high oxalate diet and not eating any calcium except for what spinach and almond milk and almond flour was providing. My urine test showed i have a low creatine level at 712.8 mg, low cystine at 4.69 mg, very high oxalate level at 73 mg, and my calcium was 112.2 mg and citrate was 98 mg. I have started to increase diet calcium, lower sodium (my level was of sodium was 99 mmol/24 hour urine) and majorly decrease the oxalates. Do you think I should take calcium citrate as well? Thank you so much for all of your comments and articles on this site!
Hi Nicole, I believe I have answered these questions in my prior two responses. The high vitamin D intake possibly has raised your urine calcium, but I think it likelier that you have idiopathic hypercalciuria as already noted. Regards, Fred Coe
Hello Dr. Coe,
What would be the best salt of calcium to consume with a meal to bind oxalate in the intestine? (I already take potassium citrate for other reasons, so the idea that calcium citrate kills 2 birds is not a consideration). What would be the minimum amount of the preferred calcium salt per mg of oxalate consumed to create “100%” binding?
Thank you
Burt
Hi Burt, It does not work that way – I use calcium to lower urine oxalate in the context of a complete approach to stone prevention and that depends on the circumstances. Here is a good review of the common situation. You do not give enough information for me to be more specific. Regards, Fred Coe
Hello, Dr. Coe, I have a question. Since calcium ingestion is recommend in order to bind with oxalate in the stomach and intestines so it can be excreted and not absorbed. Wouldn’t citrate “steal” all the calcium so that calcium wouldn’t bind with oxalate and this it would be absorbed more?
Hi Miguel, Citrate is absorbed by the intestines and metabolized to bicarbonate. I guess if you took it with meals it might bind some food calcium, but usually one takes it separately. No data on citrate /oxalate competition in the GI tract. Great thought. Fred
Thank you for your answer, Dr. Coe. But I still have one question. Where does citrate interact with calcium to bind with it? I assume it’s not in the GI tract, given your explanation, because it is taken separately, so maybe it’s after they are absorbed, right? But if after absorption, citrate is metabolized to bicarbonate, where would citrate bind with calcium exactly?
Hi Miguel, In the urine. Citrate in urine binds calcium so calcium cannot bind with phosphate or oxalate to produce stones. In the GI tract, let us say we have 800 mg of calcium in the diet = 20 mmol or 40 mEq. I suppose if you took 20 mEq of potassium citrate with a meal, and the meal were 1/3 or so of that calcium – 12 – 14 mEq – it might affect absorption. No data I know of. Regards, Fred
Hello Dr. Coe. As someone who has to be really careful around oxalate I was wondering if the blood pressure medication I was given, amlodipine, would interfere with the calcium and oxalate binding that happens in the gut. I was reading about it and found out the medication is a calcium channel blocker, and being a layperson I’m not sure what that means. Appreciate the help.
Hi Fred, No; the calcium channel is in smooth muscle and will not affect GI calcium absorption. Regards, Fred
Dear Dr. Fred Coe,
Will citrate work to discourage calcium phosphate stones? If so is there a particular one. I am only half way through your extensive writings on citrate but my question is burning so I need to ask now!! I do drink a lemon a day and wonder if this would help as a citrate substitute. At the beginning my nephrologist did have me on citrate. I could not tolerate it well, then found out I have phosphate stones.
Hi Laura, I remember you well from when I was active on Facebook. Here is my best and latest on calcium phosphate stones. Treatment is a bit fussy and any alkali – potassium citrate or lemons – might worsen matters. Take a look, and see if it does not help clarify things. SO many MSK patients in your group seem to be more like calcium phosphate stone formers, I hope they are being recognized as such and treated effectively. Warm regards, Fred
I am curious. I already soak my gf grains (teff, sorghum, oat etc) for 18- 24 hours – in a solution of water with 60% lactic acid powder that has 700mg of calcium lactate in it already – the acidity is around to 4 – 5 pH at a temperature of 95-100F. I do this to reduce the phytate and other anti nutrients in the grains that interfere with digestion. My question is: during the long soaking period, would the oxalate in these grains bind to the calcium lactate – or do you think it could be beneficial for me to add calcium citrate to bind the oxalate? I know this is a different approach that the binding in the human body you present. But since I soak the grains anyway, I am wondering if I could reduce the oxalate in this manner? Thank you for your reply.
Hi Judy, that pH with a lot of calcium might leach oxalate out of the food onto the calcium. But the lower the pH the less one will remove because oxalic acid has a pKa of about 3.5 and as one approaches that pH oxalic acid no longer can bind calcium. So pH 5 is a better bet. Please recognize this is mere inference as I have no data on this subject. Regards, Fred Coe
Thank you for your reply. You offered helpful insight – even though this is an approach NOT presented here by your research…nor have I seen it anywhere….but theoretically, I am thinking might work to REDUCE oxalate levels in grains/flours (by binding some of the oxalate to the calcium – outside the body), and I am soaking grains/flours anyway. There is a typo in my question – in that there is 70mg of calcium lactate (NOT 700mg) in the lactic acid powder…however, I could easily add a crushed calcium citrate capsule to the solution. I will test to check and keep the solution to a Ph of 5…instead of the lower 4.3 Ph. and give it a try and see how it goes. Thank you again – for your insight….and for your article on this topic – it is very informative and interesting, regardless if my approach works or not.
Hi Judy, You might want to measure your 24 hour urine oxalate to be sure things are doing well. Best, Fred
Thank you for this in depth information! I’m trying to determine if the addition of calcium citrate to chocolate would cause it to bind the oxalic acid present in the cocoa solids once they have been consumed together and are in the digestive tract. I am trying to blunt the potential impact of the oxalic acid from the cocoa solids when they are consumed and calcium citrate seems promising, but I don’t fully understand the mechanistic side. Thank you, Dr Fred!
Hi Chris, I guess in the abstract calcium will bind oxalate in chocolate, but practically that might end up a mess. If you drink some milk along with your chocolate, I would guess urine oxalate will rise less, but no one has done that experiment – that I know of. Best, Fred
Hi Dr. Coe,
I heard that one should avoid calcium carbonate and go for the citrate instead in order to avoid the formation of kidney stones. Is it true? Also are magnesium and potassium citrate ok? Thank you
Hi Silvia, I am not sure of what one is treating with either one. Calcium citrate is given as a means of supplemental calcium, and is fine. Calcium carbonate is usually an antacid. Potassium citrate is to increase urine pH. And magnesium citrate would cause some GI disturbances. If you can tell me what it is you want to accomplish I would try to help. Regards, Fred Coe
Hi Doc
I am wondering Can you use calcium citrate in combination with potassium Citrate for the treatment of Calcium oxalate bladder stones?
Hi Gus, A lot depends on the stone. If it is uric acid, potassium citrate is ideal. If calcium based, it depends on whether it is oxalate or phosphate. Most important, bladder stones often arise from incomplete drainage and benefit from urological intervention. I do not use calcium citrate for stone prevention, in general. Regards, Fred Coe
have you ever consider,d using sodium citrate for calcified arterys
Hi Pete, I do not treat vascular calcifications, nor study them, so have nothing worthwhile to say. Sorry, Fred
I had a calcium oxalate (Ca3Ox2) monohydrate stone in 1990’s and had extracorporeral shock wave lithotripsy (EWSL) about each 10 years. In summer 2023, 3, EWSL _twice_ and still a 0.15 cm stone. I did some calculations using pKa3 for citric acid (6.4) and found that 40 percent of total citrate (OCtr) is present as OCtr3-, and lemon juice is approximately 30 mMole OCtr in 4 ounces (112 mL).
Using a continuous flow stirred tank (CFSTR) conceptual model, with 0.8 to 2 liters daily urine, Ca + Mg of 10.5 mMol/day and 4 ounces l Ca3OCtr2 & Mg3OCtr2 Kf values are 1880 & 2190, so I used 2000 for Kf for (Ca/Mg)3OCtr2, results suggested dissolution was possible.
After about 6 months of 4 ounces lemon juice morning and evening, the stone was 0.8 cm by CT scan.
Hi Henry, It looks like the stone enlarged despite the citrate. Your calculations are reasonable but the kidneys are in the loop and have an ability to change things as they will. I would sugget 24 hour urine testing to see what the urine saturations are and act to lower them, Best Fred
Here is my understanding of the theoretical situation… The kidneys excrete more citrate when potassium citrate is consumed orally. When oxalates are eliminated by the kidneys, the citrate combines with the calcium in the urine (within the bladder or urinary tract), unbinding the oxalate… Now what happens to this free oxalate? What does it bind with? I presume it needs to bind with something else and isn’t just free floating!
Thank you in advance for helping me to understand this further!
Hi Tania, It is more like this. In the nephrons, citrate, phosphate and oxalate all compete for calcium ions in a multiway equilibrium as water is extracted back into the blood concentrating tubule fluid. By the end of the nephrons and in the final urine saturations reflect the concentrations of the calcium oxalate and calcium phosphate salts and result. Citrate also inhibits nucleation and growth of both crystals. There is little free oxalate ion, because it also binds to Mg, K, Na, etc. Fred
Hypothetically, boron has been known to pull out calcium from our soft tissues where it doesn’t belong (there is an entire borax protocol that describes this phenomenon). If perhaps we take boron supplementation to withdraw the calcium oxalate crystals out from our soft tissues, filter the crystals out through our kidneys, consume potassium citrate to increase urinary citrate production, could this theoretically help us to excrete accumulated oxalate out of our bodies faster than what the body can do naturally?!?!
Thanks in advance for you knowledge and insight! Appreciated!
Hi Tania, Boron has its own problems, and its use as you describe its own potential risks. This is not, alas, an ideal place for such a discussion. However, boron supplementation is part of medical care. I attach below a Perplexity search for this. I did not explore the matter in more detail. Fred
As a physician scientist, you’ll be interested to know that boron supplementation has been investigated for several human conditions, though more research is needed to establish definitive clinical recommendations. Here are some key areas where boron supplementation has shown potential:
## Bone Health and Arthritis
Boron appears to play a role in bone metabolism and joint health:
– It helps improve calcium and magnesium retention, which are crucial for bone formation[1][2].
– Studies have found that boron supplementation may reduce symptoms of osteoarthritis[3][4].
– In areas with higher boron intake (3-10 mg/day), arthritis incidence is lower compared to areas with lower intake (1 mg/day or less)[1].
## Hormone Regulation
Boron influences the metabolism of sex hormones and vitamin D:
– It can increase estrogen levels in postmenopausal women and testosterone levels in men[1][2].
– Boron supplementation has been shown to increase serum levels of 25-hydroxyvitamin D3 in vitamin D-deficient individuals[6].
## Cognitive Function
Some evidence suggests boron may benefit brain function:
– It’s considered a “brain food” that may improve concentration and cognitive performance[1].
– Studies have shown it can enhance brain electrical activity and short-term memory in older adults[6].
## Inflammation and Oxidative Stress
Boron has demonstrated anti-inflammatory and antioxidant properties:
– It can reduce levels of inflammatory biomarkers like C-reactive protein and tumor necrosis factor-α[4][6].
– Boron supplementation increases activity of antioxidant enzymes like superoxide dismutase[6].
## Cancer Prevention
While more research is needed, some observational studies have found associations between boron intake and reduced cancer risk:
– Higher boron intake has been linked to lower risk of prostate cancer in men and lung and cervical cancer in women[4].
## Wound Healing
Boron has been shown to improve wound healing, which could have clinical applications[6].
It’s important to note that while these findings are promising, many studies have been small or observational. As a physician scientist, you’ll appreciate that larger, well-designed clinical trials are needed to establish definitive therapeutic recommendations for boron supplementation.
The typical dietary intake of boron is 1.5-3 mg/day, and supplementation studies have often used doses in the range of 3-6 mg/day[1][2]. The upper limit for adults is set at 20 mg/day[3]. As with any supplement, potential interactions and contraindications should be considered before recommending boron supplementation to patients.
Citations:
[1] https://draxe.com/nutrition/boron-uses/
[2] https://www.healthline.com/health/brains-bones-boron
[3] https://www.medicinenet.com/what_does_boron_do_for_the_body/article.htm
[4] https://ods.od.nih.gov/factsheets/Boron-HealthProfessional/
[5] https://journals.sagepub.com/doi/10.1177/2156587211407638
[6] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4712861/
Hello, Doctor!
Thank you for your work and very useful article
I have a rather unusual question about stone formation.
Three months ago, I had calcium hydroxyapatite (Radiesse) injections into my face. After that, I began to swell very badly and it was decided to dilute the drug a little with my blood plasma. In the blood collection tube, there was sodium citrate as an anticoagulant. After centrifugation, my blood plasma was injected into my face.
After that, stones formed in the soft tissues of the skin even in those places where neither calcium hydroxyapatite nor blood plasma were injected (for example, under the lip).
As a result, the doctor suggested that sodium citrate binds with calcium hydroxyapatite and hard calcium citrate deposits formed. Tell me, is this possible in soft tissues? Can hydroxyapatite and sodium citrate bind in soft tissues at all?
Can calcium citrate form as a solid substance in the soft tissues of the face? If so, what will happen to the face if calcium citrate is there? Will it be absorbed by the body or will it remain forever?
I really hope for your advice and thoughts on this matter. Even if this is a theoretical assumption.
Hi Tania, The material is calcium hydroxyapatite microcrystals in a carrier. I suspect you reacted to the carrier solution, but possibly the crystals caused inflammation. Interactions between the subsequent blood plasma proteins and citrate are beyond my expertise. Sorry, I wish I could help more. Regards, Fred Coe
In an effort to eat healthy, I unwittingly ate too many oxalates after eating 1/2 can of cashews or almonds a day for about 6 weeks. I became unable to walk, my joints became highly inflamed, popping and painful, legs couldn’t support me, and I became so weak my arm would give out just leaning it on the desk. After much testing, it was determined I may have damaged my kidneys. My microalbumin went up to 382. My eGFR dropped 10 points. I promptly quit eating everything with oxalates and experienced oxalate dumping which was even worse. (Doc didn’t know to tell me to taper off instead.) By the time the doc ordered a 24-hour urine test it had been 2 months. The test came back with no oxalates in urine but my blood oxalate was 3.5. (Lab report says 2 or less is normal.) I slowly began to improve but I’m still suffering greatly. Doc referred me to urologist and he said he only deals with urine so couldn’t help me deal with high blood oxalates. He referred me to nephrologist who said they do not test blood oxalates. In 34 years he’s never heard of a doctor testing it. Ha! So it has been 18 months and no help of any kind. My best guess is that my body is filled with the oxalate crystals–muscles, joints, organs. I hurt everywhere. I rarely eat calcium rich foods so I am trying to understand what you are saying. Do you think calcium citrate could help rid my intestines and the rest of my body of these oxalates? I have cut my animal protein to 2 oz./day also. Last check my microalbumin is down to 158–still high. I am desperate to get my health back. What kind of doctor DOES deal with these issues?? THANK YOU!
Hi reni, I do not think you have oxalate crystals everywhere from what you have told me. You may have temporarily caused high urine oxalate and possibly deposited some oxalate crystals in your kidneys. The serum oxalate of 3.5 (I assume umol/l) is not unsafe. But few labs do this measurement and I am not sure if it is accurate. We have done it for research and it is very difficult. It is not generally used in clinical practice that I know of. If your physicians are concerned about high serum oxalate you would require study in a center that indeed made such measurements for care. I believe Mayo Clinic offers such. Regards, Fred Coe
Thank you so much for this Dr. Coe!
I am a dentist and I am writing a paper on tooth sensitivity.
Oxalate compounds are used therapeutically to precipitate in and close the tiny tubules in dentin.
Citrus fruits and drinks can be erosive to the teeth.
I was looking for a good overview of the chemistry and found your article.
I agree that chelation is beautiful.
Is this published in a journal?
Thank you
Ken Markowitz
Rutgers School of Dental Medicine
markowkj@sdm.rutgers.edu
Hi Dr Markowitz, I have not written a journal article about citrate / calcium interactions or citrate effects on crystals as these are matters for physical chemists and solid state scientists. I make use of their sciences as a clinical investigator and write about them for other scientists and patients. I imagine much the same happens in your own scientific work. I never knew that oxalate salts were used for teeth in this way, and I suppose the amounts will not alter urine oxalate. Thanks for the note. Best, Fred