 If the lives of the Tudor nobility were luxurious, they were dangerous in equal proportions, for the King who bestowed their riches could in a moment wipe them, and those who possessed them, out. So intimacy with the person of the King and with the whole Royal Family was prized and feared. They lived, these powerful and dangerous people, in their Royal Palaces, to which you must go or have no influence. Even worse, King and Consorts made Royal Progresses, staying here and there as guests of the high nobility. Imagine that, the King as your houseguest. A person, like any other, and yet not at all like any other: glamorous, dangerous, and involved with high concerns.
If the lives of the Tudor nobility were luxurious, they were dangerous in equal proportions, for the King who bestowed their riches could in a moment wipe them, and those who possessed them, out. So intimacy with the person of the King and with the whole Royal Family was prized and feared. They lived, these powerful and dangerous people, in their Royal Palaces, to which you must go or have no influence. Even worse, King and Consorts made Royal Progresses, staying here and there as guests of the high nobility. Imagine that, the King as your houseguest. A person, like any other, and yet not at all like any other: glamorous, dangerous, and involved with high concerns.
You could say this is a silly preface to my common discourse on citrate, but not so. I have written before about its powers in our little domain: It binds calcium, it inhibits crystals, giving it reduces stones. But I have not said how it gets into the urine.
It comes as a royal visitor to some Duke or Marquess, Earl, Viscount, or Baron.
For this molecule has high purposes. It is noble and powerful. What it does in urine is but a tiny fraction of its many actions and probably not one of the more important ones. But what we do when we take citrate calls into play a vast biology. For all our lives we eat a diet that imposes an acid load on our kidneys, our bones, and elsewhere. Our kidneys, especially, adapt to that acid load, so what we call our ‘normal’ state is actually at one extreme. The pills, being alkali, reverse this lifelong adaptation and thereby profoundly alter the physiology of the kidneys and bone. In general one might say the alterations are for the better.
This is a long article but one worth reading for those who prescribe or take potassium citrate pills.
I want to acknowledge the expert error checking of Dr Yangming Cao (UCSF – Fresno) in the section ‘Why are Potassium Citrate Pills an Alkali Load?’ He corrected a significant error in the original article.
A Picture of the Kidney
Many of you are physicians or scientists who know about the kidney, but a few reminders are always worthwhile. Others are neither and we need to have names in common. Human 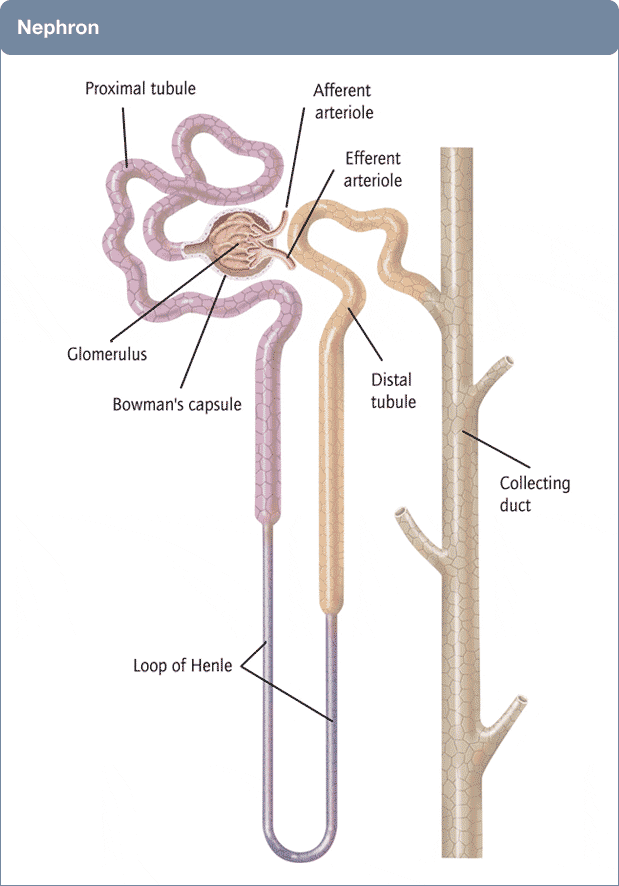 kidneys are made of about one million individual nephron units. The renal process begins with filtration of small molecules like citrate, or atoms like sodium and calcium, through the glomerulus, which is a complex of capillaries whose filtration pressure arises from the heart not a foot away.
kidneys are made of about one million individual nephron units. The renal process begins with filtration of small molecules like citrate, or atoms like sodium and calcium, through the glomerulus, which is a complex of capillaries whose filtration pressure arises from the heart not a foot away.
A majority of the filtered water, salts, and molecules is reabsorbed in the proximal tubule. The distal tubule (highly simplified here) performs tightly regulated absorption or secretion, so as to produce a final urine and maintain blood concentrations in their normal ranges.
These loops will come up again and again on this site so I should comment on the thin and thick portions. The long thin loops of Henle (Henle was the scientist who is credited with describing this part of the kidney) extract water specially well.The thick portions just below the ‘Distal tubule’ notation are called, appropriately enough, the Thick Ascending Limbs of the Loop of Henle. The thick limbs reabsorb NaCl, but not water, and in doing that entrain a marvelous system for – of all things – retaining water! In an article so long as this one, and concerned with citrate, I cannot pause longer here. But we will be back, someday.
Citrate is in the Blood
Kidneys Filter and Reabsorb Citrate
In one published study, concentration of citrate in blood is about 80 – 170 micromolar. A recent review places it at 120 micromoles/liter. If we use 120 micromoles/liter as a reasonable average, and a common value for glomerular filtration of 120 milliliters/minute, the filtration of citrate is about 21 millimoles a day. Of this about 1 – 4 millimoles appear in the urine, the rest being reabsorbed by the kidney cells. So the fraction of filtered citrate excreted is about 5 to 20%, and regulation of this fraction controls the amount of citrate in the urine.
Citrate in Blood Binds Calcium
The concentration in blood of calcium not bound with proteins is about 1 millimole/liter. Citrate concentration is about 0.12 mmol/liter, so in principle about 10% of non -protein bound – calcium can be bound by citrate. Because in calcium citrate crystals 2 citrate molecules can bind 3 calcium atoms, the the figure would seem to rise to to 15%. But in solutions like blood, other materials compete with calcium for a place on citrate – magnesium is one example. So the actual fraction is difficult to estimate. Normally blood citrate level is stable, so although significant, citrate binding of calcium is not likely to influence calcium metabolism by, for example, altering regulation of parathyroid hormone secretion.
Citrate has Signalling Roles
My purposes here are humble purposes, so all I wish to do is put here a tiny list of known effects of citrate on systems throughout the body without pursuing the details. Citrate concentration regulates lipid metabolism via malonyl-CoA. Citrate is sensed by the hypothalamus and thereby affects glucose intake and glucose metabolism by liver. To do these things citrate must enter the relevant cells, and it can do this only via a transporter that takes it across cell membranes.
The Citrate Transporters
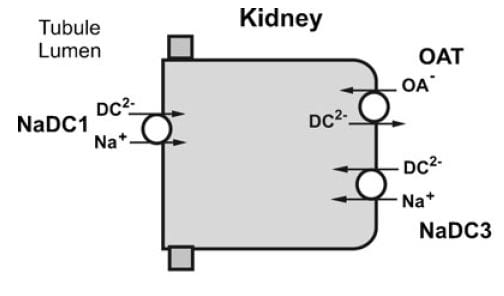
NaDC1 and NaDC3
NaDC1 is on the apical membranes of the proximal tubule cells of the kidney – the surface facing into the tubule fluid – and regulates the rate of reabsorption of the citrate that has been filtered. Its gene is named SLC13A2. This same transporter is on the food side of the small intestine cells and permits absorption of citrate from foods. The featured image for this article shows the structure of the transporter.
The citrate that enters the renal cells can be used for metabolism, or transported out the other side – called the basolateral side, facing the blood – via another transporter called the Organic Acid Transporter (OAT). Yet another transporter, NaDC3, permits citrate to enter kidney cells from blood. Because it appears to regulate urine citrate, my focus is on NaDC1.
The citrate transporter DC1 couples sodium and citrate movement. Since not everyone who reads this will know, let me mention an almost universal property of living cells: they pump sodium out of themselves and pump potassium in. Because they do this, sodium will tend to move into cells if given an opportunity – a hole. DC1 and DC3 can be thought of as sophisticated holes, or channels, through which sodium atoms can move if they have a citrate molecules with them. The actual proportions are 3 sodium atoms move with one citrate molecule, and the form of citrate which moves is one we have encountered before. Recall how citrate binds calcium because each molecule can have 2 or three negative charges on it. The doubly negative (divalent anionic) form of citrate is the one that traverse the channel.
They Transport More than Citrate
NaDC1 permits not only citrate to cross cell membranes but also succinate, alpha ketoglutarate, fumarate, malate, and a variety of less biologically relevant molecules. One might ask why, and I presume it is because the named molecules are all part of the citric acid cycle, which is the main engine of cell energy production. NaDC3 transports all of the same molecules as NaDC1, along with glutarate and a very long list of other molecules not in the citric acid cycle.
This cycle is at the center of that metabolism which uses oxygen to produce energy from food. The reference is to an excellent textbook review that is free online. Another chapter in that book finishes the story of how the cycle produces energy. The antiquity and centrality of the citric acid cycle will become apparent to you if you even browse these chapters. If you read them, you will encounter some of the most important aspects of living cells.
Why are Potassium Citrate Pills an Alkali Load?
In the citric acid cycle citrate is metabolized as citric acid, meaning that 3 protons are taken up from blood with each molecule. Removing protons is identical to adding alkali. Typical dosing is about 20 – 40 mEq of potassium salt daily, but the amount can vary widely.
Commercial potassium citrate contains 1080 mg of the compound in a 10 mEq pill. Typically the potassium citrate salts have a potassium on each of the three anion sites on the citrate molecule. The MW of citrate anion is 189.1. Urocit K, a common commercial version, is a crystalline monohydrate salt so it has a MW of 3×39 (for 3 potassium ions) + 189.1 (for citrate) + 18 for the one water molecule, or 324.1 in all. Given 324.1 for 3 mEq of base, the 10 mEq tablet contains 10/3 x 324.1 or 1080 mg.
The Flow of Citrate
In an earlier era organ physiology was popular and scientists often gathered together 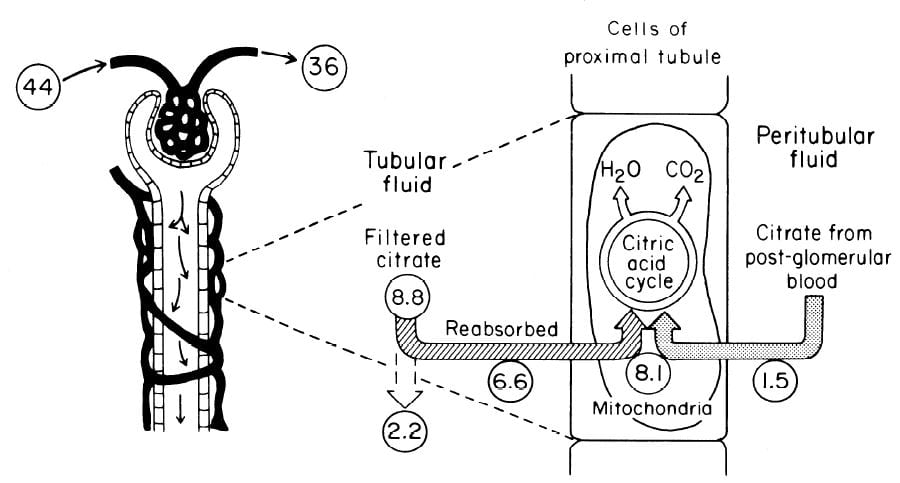 measurements to paint a picture of how things work overall. Here is such a picture from a wonderful review of renal citrate handling by Simpson. Values in small circles are micromoles (umol) per minute.
measurements to paint a picture of how things work overall. Here is such a picture from a wonderful review of renal citrate handling by Simpson. Values in small circles are micromoles (umol) per minute.
Citrate is presented to the glomerular filter at 44 umol/min, and 36 umol/min leaves the glomerulus (8.8 umol/min filtered) in blood what will pass by the blood side of the proximal tubules. From that 36 umol/min, 1.5 umol.min are taken up by renal proximal tubule cells and metabolized in the citric acid cycle. Of the 8.8 umol/min filtered, 6.6 umol/min are taken up on the urine side of the same cells making 8.1 umol/minute for metabolism. The remaining 2 umol/minute (3.17 mmol/day) are lost in the urine. NaDC1 and NaDC3 had not been cloned and sequenced at this early time, but physiologists knew the transporters were there and toted up what they did.
Urine Citrate Varies With Acid Base Status
Acid loads, such as high protein diets, will increase citrate uptake into the renal cells and thereby reduce urine citrate. Alkali loads such as diets high in fruits and vegetables or potassium alkali supplements reduce uptake and increase urine citrate.
Alkali
Clinical Response
In a trial, calcium stone formers with low urine citrate excretion eating a constant diet were given sodium bicarbonate or  potassium citrate, 20 mEq three times a day. Urine citrate rose with both treatments, as did the urine pH. Not relevant here, but in later articles, the sodium alkali did not change urine calcium, but the potassium alkali lowered urine calcium. Alkali itself lowers urine calcium, sodium raises it, and their antagonism is the reason for the differences.
potassium citrate, 20 mEq three times a day. Urine citrate rose with both treatments, as did the urine pH. Not relevant here, but in later articles, the sodium alkali did not change urine calcium, but the potassium alkali lowered urine calcium. Alkali itself lowers urine calcium, sodium raises it, and their antagonism is the reason for the differences.
Mechanism May be Increase of pH
If the citrate transporter is placed into test cells, the movement of citrate can be studied, and such a study shows how powerful is the effect of pH.
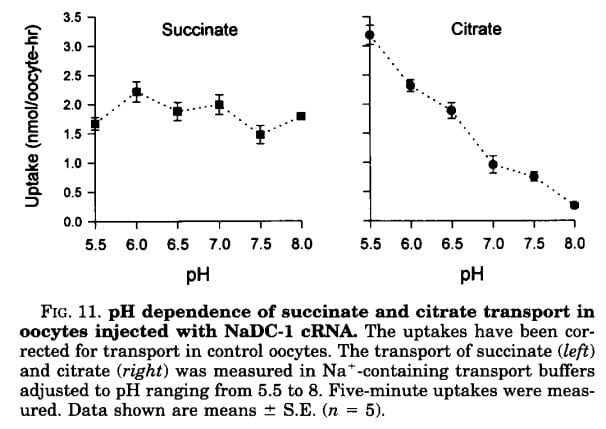 Succinate is a citric acid cycle intermediate like citrate, but its uptake by the citrate transporter is not affected by the acidity or alkalinity of the medium (pH). Citrate uptake is powerfully affected.
Succinate is a citric acid cycle intermediate like citrate, but its uptake by the citrate transporter is not affected by the acidity or alkalinity of the medium (pH). Citrate uptake is powerfully affected.
We have encountered pH before and remind ourselves here that urine values vary from about 4.5 to just below 8. Likewise, citrate has three sites that can accept protons, the acid component of water systems. As I mentioned in the paragraphs just above this point, the charge on the citrate molecule rises with pH as protons are progressively removed, and the sequence of pH values (the pKa values for the dissociating sites for those of you who know about such matters) are 3.13, 4.76, and 6.40. Obviously, in urine, the divalent (2 open negative sites) form will predominate until urine pH rises above 6 and will fall to about 1/2 of the total at 6.4. At about 6.4 transport of citrate was indeed just about half of that at the lowest pH.
pH in the Proximal Tubule
But it is not urine pH which affects citrate transport, it is the pH of filtrate in the proximal tubule of the kidneys, and that pH is not the same as that of the urine. At the end of the proximal tubule, the pH is about 6.7 to 6.8, and at that pH more than half of citrate is in the trivalent form and not available for transport. With alkali loads, as in the experiment in the table, the pH will rise, and citrate transport fall below normal, so citrate appears in the urine.
Problems with the pH Idea
Strangely, modern sources do not mention an older literature which raises questions about this mechanism. Simpson, in an important review from late antiquity (1983), mentions that the drug acetazolamide, which raises pH inside the proximal tubule and lowers pH inside the renal cells raises urine citrate only slightly and at first, but shortly after administration urine citrate falls despite a continuously alkaline urine and presumably tubule fluid. This suggests that even a high tubule fluid pH is not enough to counter the effects of changes in pH within cells or perhaps the blood. So it is not only tubule fluid pH that matters, but perhaps the pH inside the renal proximal tubule cell.
Acid Loads
Those unfamiliar with the matter may not realize that the diet we eat in the US and most of the other first world countries imposes an acid load that must be excreted daily in the urine. So the urine citrate excretion we find in our clinics and in experiments on ‘normal’ diets are those consistent with an acid load. When we give potassium citrate or other alkali we often do little more than neutralize this acid load, yet urine citrate usually rises. Experiments about acid loads add to the diet acid an extra amount of acid.
Tubule Fluid pH
As for alkali loads, a lower proximal tubule fluid pH will increase the fraction of filtered citrate in the divalent form which is transported by NaDC1. The pH of the tubule fluid will fall with acid loads for several reasons. Acid loads – for example a high protein meal – are buffered on blood bicarbonate which lowers the concentration of bicarbonate, and therefore the pH of the filtrate. LIkewise, the tubule cells are stimulated to increase their reabsorption of filtered bicarbonate which further lowers pH. All of this implies that kidneys sense the acidity or alkalinity of the blood, which they surely do.
Transport Adaptation
Over time – many hours to days – the NaDC1 transporter and its gene (SLC13A2) increase 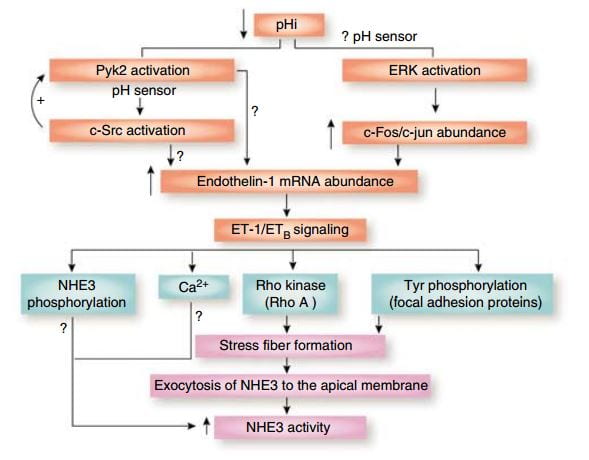 their abundances. This increase is mediated by endothelin – 1 (ET-1) through the endothelin B receptor (ETb).
their abundances. This increase is mediated by endothelin – 1 (ET-1) through the endothelin B receptor (ETb).
This figure from the above reference shows thinking about acid and endothelin as it was in 2007 and seems to be still. A fall in pH in proximal tubule cells can be sensed by a protein named Pyk2, which activates by adding a phosphate to one of its amino acids (tyrosine) and, interacting with another protein (c-Src), increases the abundance of the mRNA of ET – 1 which then signals through its ETb receptor to increase renal acid excretion – bicarbonate reabsorption – via NHE3, a transporter that reabsorbs sodium and secretes acid into the proximal tubule fluid.
This same ET -1 and its ETb receptor also signal increase of NaDC1 transport. Here, mice engineered to have (ETb+/+)or have not (ETb-/-) the receptor were challenged with an acid load. 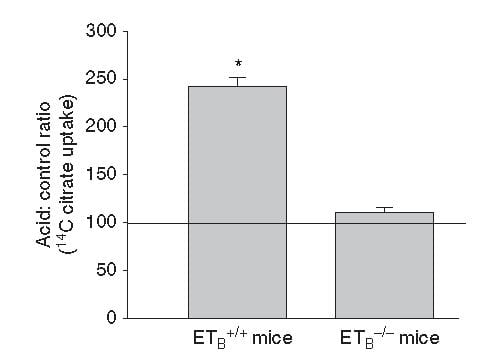 Citrate uptake by isolated NaDC1 transporters in the deficient mice do not respond to acid.
Citrate uptake by isolated NaDC1 transporters in the deficient mice do not respond to acid.
So one and the same effect, acid sensing and endothelin – 1 signalling increases acid excretion and citrate conservation.
But, you may ask, why am I grouping these two together?
It is because both concern acid base balance.
Citrate is metabolized as citric acid, taking up 3 protons per molecule metabolized, which is the same as saying it provides 3 molecules of alkali – like bicarbonate. Loss of citrate is therefore loss of potential alkali. NHE3 is a main driver of acid – protons – out of blood into proximal tubule fluid which reclaims filtered bicarbonate – conserving alkali.
So urine citrate, which we are interested in because it binds calcium and inhibits crystals, has a much larger role to play – part of the grand system which maintains a constant blood pH against the acid or base loads of diet.
Which pH?
I have spoken about pH of the proximal tubule fluid, of the blood, of the urine, but the one that is central to regulation of NaDC1 is the pH inside the proximal tubule cells. That pH appears to respond to acid or alkali loads, but the manner of its response is not simple. The signalling is through the Pyk-2 sensor already discussed and a parallel pathway via ERK (same diagram, above) which I did not discuss. But how sensing works, what is sensed, this remains very much an open research issues, and I will leave off here as this article was about urine citrate and the conversation has already taken us through many byways, beautiful if exhausting to follow.
Potassium
But – that awful word – one important fact remains to be uttered. Depletion of potassium lowers the pH inside kidney cells and lowers urine citrate. I will not pursue the details of this well worn story, except to point out its extreme clinical relevance. Diuretics that are used in stone prevention, or for hypertension, deplete cell potassium stores. It is the potassium citrate we give to patients.
Ammonium, and the Rest of the Story
How can I leave off without filling out the details of how kidney cells respond to acid challenge with production of ammonia that balances acid load with acid excretion?
Bicarbonate
A Better Buffer than Most
A buffer keeps pH relatively constant by taking up protons when they enter a solution and giving them up when alkali enters. It is a kind of shock absorber.
At the beginning, evolution favored bicarbonate. It is a buffer of considerable virtue in that it can take up protons or release them, like common buffers do, but has a special trait.
Bicarbonate is forever in equilibrium with carbon dioxide gas (CO2). When bicarbonate takes on a proton to become carbonic acid, much of that acid becomes carbon dioxide gas. When protons are taken out of blood, CO2 gas forms new carbonic acid which donates a new proton to the solution, and essentially bicarbonate appears in solution ‘out of thin air’. That it flows from solution into thin air and back makes bicarbonate a more stable buffer than those which live only in solution so it was an excellent choice.
What Kidneys do with Bicarbonate
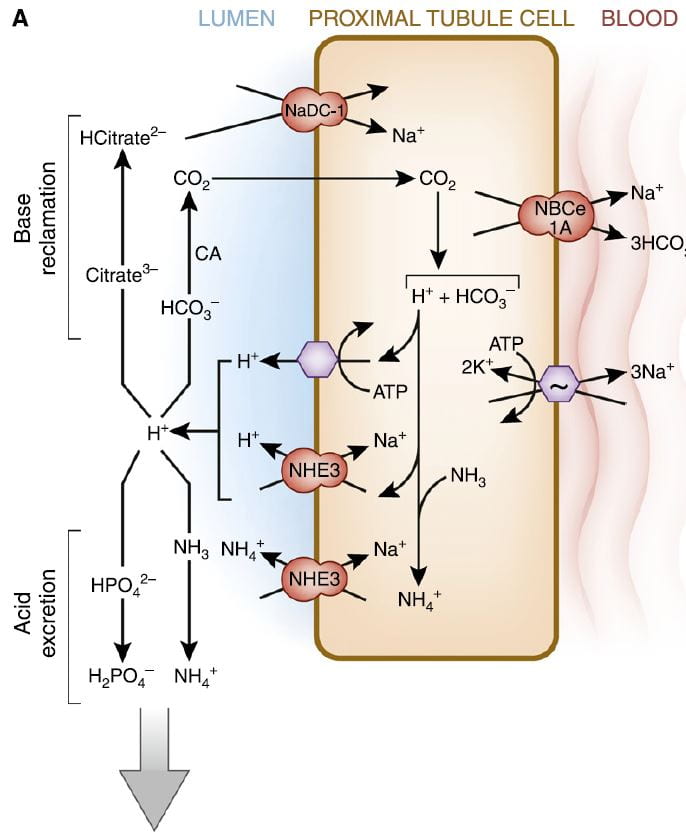 It is this very molecule, bicarbonate, which the kidneys traffic in when they respond to alkali or acid loads, and it is, of course, CO2 the lungs regulate in blood under the control of the brain.
It is this very molecule, bicarbonate, which the kidneys traffic in when they respond to alkali or acid loads, and it is, of course, CO2 the lungs regulate in blood under the control of the brain.
The figure is from the ‘A’ panel of a lovely drawing in a lively and engaging review. Being small, bicarbonate is filtered, and being the main buffer of the blood almost all of what is filtered must be reclaimed. So the proximal tubule cells, which do most of that reclamation, busy themselves forever with that task.
The way they do it is the simplest way. They add protons (H+) to bicarbonate in the tubule fluid, which becomes, as I have said, carbonic acid that transforms into carbon dioxide (CO2), which gas passes through the cell walls into the interior. Note, ‘CA’ is carbonic anhydrase an enzyme which speeds up the process of the transformation. In the cell, the CO2 becomes carbonic acid. Because protons are being pumped into the tubule fluid, protons are stripped off the carbonic acid so it becomes bicarbonate. The bicarbonate enters the blood with Na via the NBCe1A transporter.
There are two proton pumps. One uses ATP for energy to move the protons. The other (NHE3) uses the low Na in the cell as a gradient; sodium moves in through a channel like a revolving door, which makes one proton go out for every Na that moves in. At the blood side of the cell, the ancient ‘Great’ ATPase pumps Na out and potassium in, as it does in most cells that live on Earth. NHE3, the exchanger, is the molecule we met a few paragraphs above. It is increased by Endothelin 1 via the ET1b receptor.
At the top of the left side of the picture is citrate, our little slice of this massive structure. A few scraps of proton add to citrate so it has 2, not 3 negative sites, and can be reabsorbed. Its gene is regulated by endothelin 1 so when NHE3 is increased so is NaDC1.
Phosphate
Reclaiming bicarbonate is Sisyphean work. Nothing happens to get rid of acid loads from meals. But more protons are secreted than are needed to reclaim bicarbonate. Some are buffered on phosphate. But all the protons buffered on phosphate produce bicarbonate from carbonic acid inside the cell, and that bicarbonate enters the blood via NBCe1A.
Ammonium Ion
Ammonia is produced in the proximal tubule by removal of nitrogen from glutamine, pictured at left. As always, 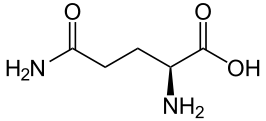 kinks are carbon atoms in this kind of drawing. The first one on the left has an oxygen and NH2 group, and a bond to the next carbon. Carbons typically form 4 bonds each. The next 2 carbons are merely linked to one another. The fourth has another NH2 and the final one at the right 2 oxygens. The left hand one is removed by an enzyme to produce NH3 and glutamic acid. The second one is removed to produce α-Ketogluteric acid which lacks any NH3. The 5 carbon skeleton remains unchanged.
kinks are carbon atoms in this kind of drawing. The first one on the left has an oxygen and NH2 group, and a bond to the next carbon. Carbons typically form 4 bonds each. The next 2 carbons are merely linked to one another. The fourth has another NH2 and the final one at the right 2 oxygens. The left hand one is removed by an enzyme to produce NH3 and glutamic acid. The second one is removed to produce α-Ketogluteric acid which lacks any NH3. The 5 carbon skeleton remains unchanged.
Ammonia (NH3) can tale up a proton to form NH4+, ammonium ion, which has a pKa of 9.3 meaning that at the pH of proximal tubules and cells, it is fully protonated. Loss of this ammonium ion in urine represents net acid excretion because the protons that were taken up came from carbonic acid which is converted to bicarbonate and transported into blood. Unlike titration of phosphate, excretion of ammonium ion does not increase urine pH because the pK is far above the pH of urine.
Under normal meal conditions, about 40 – 60 mmol/day of acid are excreted, of which about 2/3 is ammonium. Large acid loads, as for example, a ketogenic diet for weight loss, would induce a large increase in ammonia production so acid excretion can keep pace with acid production.
α-Ketogluterate
One might think this byproduct of glutamine metabolism, the 5 carbon skeleton, might be metabolized and done with, but no. A significant amount is metabolized. But some is not.
What is not metabolized traverses the kidney to cells in the later nephron, the intercalated cells in the collecting ducts, which usually pump protons into the tubule fluid to create the final urine p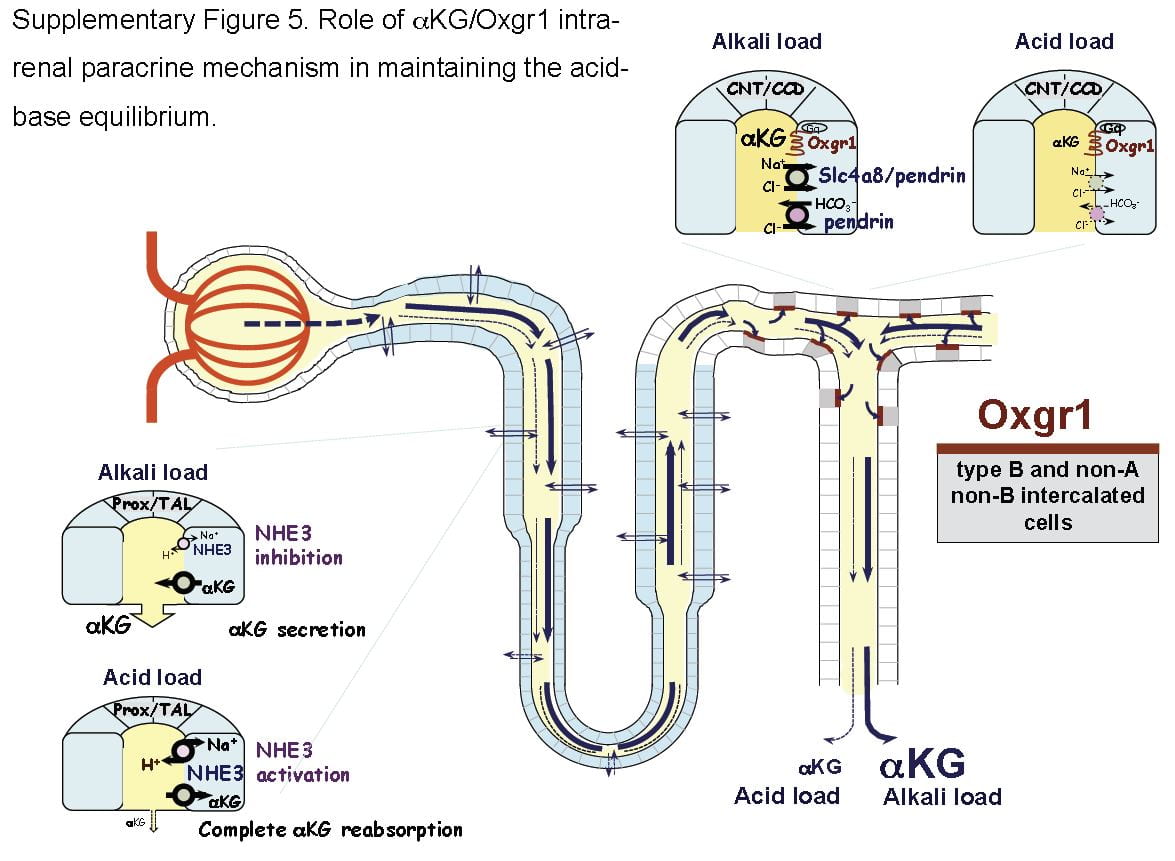 H which is critical to supersaturation and stone formation. But these same cells can reverse themselves and pump bicarbonate into the tubule fluid and protons into the blood, and they do this when confronted by an alkali load.
H which is critical to supersaturation and stone formation. But these same cells can reverse themselves and pump bicarbonate into the tubule fluid and protons into the blood, and they do this when confronted by an alkali load.
It turns out that α-Ketogluterate is itself filtered and reabsorbed in proximal tubule, and its reabsorption is profoundly reduced under alkali conditions so that more is delivered distally to a receptor (Oxgr1). When occupied by α-Ketogluterate this receptor signals the reversed intercalated cells (B and non-A cells) to increase their secretion of bicarbonate. The transporter for α-Ketogluterate is NaDC1. The net effect is to enhance bicarbonate – alkali – loss which offsets alkali loads.
The same receptor signalling stimulates pendrin, a complex exchanger which moves bicarbonate and Na together with chloride to effect NaCl and NaHCO3 reabsorption. Because acute acid challenge increases and acute base loading reduces proximal tubule NaCl reabsorption, this action would tend to maintain salt balance in that the intercalated cells would increase salt reabsorption as proximal tubule reduces salt reabsorption. Of note, although chronic acid challenge increases NHE3 abundance and activity, it reduces NaCl reabsorption via effects on other transporters. For these reasons the α-Ketogluterate – pendrin link is probably more important in minute to minute or hour to hour regulation than in adaptation to acid or base loading diets or treatments.
Citrate and Oxalate
You would think I had exhausted the topic by now, but no. NaDC1 and slc26a6, the citrate transporter and the anion 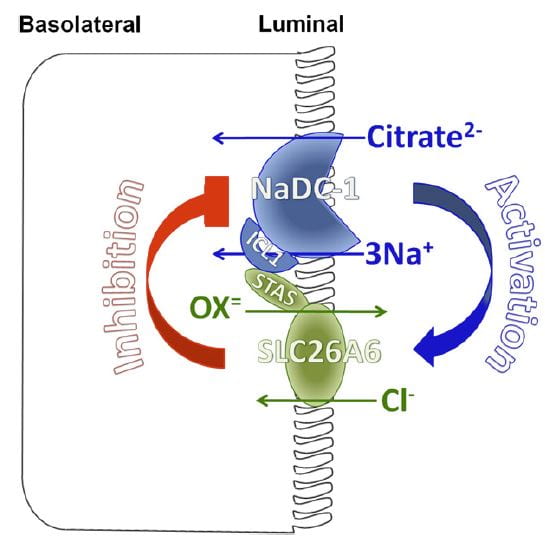 transporter (oxalate is an anion it can transport) which disengages NaCl transport from NHE3, themselves interact in relation to kidney stone formation.
transporter (oxalate is an anion it can transport) which disengages NaCl transport from NHE3, themselves interact in relation to kidney stone formation.
At least in animals and in cell experiments, the two transporters – which are present in a complex within the renal cell membrane – interact as in the figure. Slc26a6 inhibits NaDC1, so that when actively transporting oxalate into tubule fluid citrate reabsorption is reduced, urine citrate rises, and binds urine calcium to reduce risk of calcium oxalate stones. When oxalate secretion is minimal, NaDC1 increases to salvage citrate.
These animal and cell experiments imply that in human urine citrate and oxalate excretions should show parallel changes; this has not been tested.
Putting it All Together
Just Diet
Our urine citrate is an outcome of our biologies, which are variable, and our diets. Most of us eat a diet that imposes a net acid load, so our kidneys tend to conserve citrate and α-Ketogluterate, our intercalated cells pump protons not bicarbonate in to the final urine, our proximal tubules produce considerable ammonia and our urine pH is about 5 – 6.
Some of us, vegetarians whose diets do not have a proper balance of protein, very massive fruit eaters, as examples, have low citrate reabsorptions and high distal deliveries of α-Ketogluterate; our intercalated cells are reversed and stimulated to put bicarbonate into the final urine, our proximal tubules do not make much ammonia.
But the words ‘most’ and ‘some’ are misleading. In the US, certainly, chronic acid loading is the overwhelming rule, and the same throughout Europe and considerable parts of urban Asia. So our ‘normal’ poise centers on adaptations to acid load. It is not that we live in a neutral acid base condition, demanding from our kidneys little excretion of acid or of alkali. Life long we demand acid excretion. That is where we start. It is to that task our kidneys – and our bones, as I shall someday speak about – apply themselves all the days of our lives. However it is, for good or for evil, that lifelong adaptation to acid load affects us, that is our state, our permanent condition.
What Does Normal Mean?
When we give potassium citrate or any other alkali in doses of 40 to 60 mmol/day we neutralize a large fraction of diet acid. This is best considered not so much as an ‘alkali load’ as it is the removal of that acid load to which we have long been adapted.
Of course, urine citrate rises. Because we give alkali over months or even years, renal cells will adapt fully to the changes. But by ‘adapt’ I mean they give up the adaptations to acid loading. In the case where the dose of alkali just matches acid production one might best say the kidneys are relieved of their burdens in either direction, and reveal the way they would function if not driven to either extreme.
Like the small sailor plying the simple waters of a bay fills its sails sometimes southerly, sometimes northerly, making little way, dancing before a playful breeze, the cells shift their powerful machinery a bit here or there as one meal gives way to another. What shall I call this state of freedom? Why is this not the ‘normal’ from which point we register the responses to extra alkali or acid?
I have read where it was in Eden a condition of fruit, as the animals were not for them to eat. Perhaps I am wrong, and if Eden was as it says in our books the ‘normal’ state was alkali load. Perhaps Milton is wrong. After all he was not there, merely a poet making into life what he read in a holy book.
Potassium Citrate Pills
Raise Urine Citrate and pH
The expected changes are a decrease of proximal tubule reabsorption through reversal of the effects of chronic acid load. ET-1 signalling must fall, citrate reabsorption must fall because NaDC1 is no longer stimulated by ET-1 and because proximal tubule fluid pH will rise and with it the fraction of trivalent negative citrate.
Urine bicarbonate and urine pH will also rise. Partly, blood bicarbonate will rise and with it filtrate bicarbonate concentration and pH. NHE3 transport will be decreased vs. chronic diet acid loading, the baseline in the first world countries, and much of the proton secretion will be used in reclamation of bicarbonate. Naturally, NH3 production will be greatly reduced because the Pyk-2 sensing system will be signalling a higher pH.
Increases in Citrate and pH Vary Among People
But the biology is complex enough that in some people the main response will be citrate, and in other bicarbonate. Given all of the regulatory steps and signalling pathways involved a variety of responses is inevitable. Clinically this means one must measure and determine if the main effect is mainly increase of citrate excretion or of pH and therefore of CaP SS.
What is the Ideal Dose?
A nimble answer is enough to match net acid production – urine sulfate excretion is a decent index. I suspect that answer because of the problem of high urine pH in some people, and because as a clinician I never find it perfectly suits most patients. Yet it is a good starting dose because it aims at neutral acid base balance.
A Simple Pill with Powerful Effects
Physicians who treat kidney stones may well be the main ones who prescribe alkali loads to people with normal kidney function over months or even years or decades of life. This is indeed a remarkable physiological and clinical experiment, and that we do it makes the physiology and cell biology of acid base balance a central topic in clinical practice of stone prevention.
Likewise patients who take this humble medicine undergo what amounts to a reversal of cultural norm, which is a condition of chronic acid loading.
Thence, and for this reason, I have written a very long article about the topic, for physicians and their patients, and especially for scientists who know more about this topic than I do but may not see things from exactly the same view point.

Hi doc…
I was diagnosed having a 2-3mm calyceal nephrolithiasis and my doctor prescribed a potassium citrate with a brand name urecit is there a brandnamelike this urecit isearch on the internetand i always find potassium citrate urocit is thereany difference between the two brands?…if i may ask is it really effective treating a calyceal nephrolithiasis? And is it safe to consume 1080mg 3x a day…i have stomach upset and slight muscle cramp..and i experienceda little discomfort on my lower to witch i did not experience before taking this medicine…is ok with that…iam confusedand afraid pls give me an enlightenment about this..thanks
Elen
Hi Elen, Potassium citrate is just that; be sure of the dose which is usually 10 mEq (1080 mg) per tablet. Three daily is a common dose. But be sure you need it. Stones often gather in calyces, so the words mean stones in the kidneys. If your doctor said calyceal diverticulum then one does not treat those medically, but only with removal if necessary. It it means stones, the you need evaluation to know the actual cause – here is a good approach. Regards, Fred Coe
Dr, Coe,
Have been diagnosed with a 5-6mm calcium kidney stone, and have it lodged in my ureter for the past month, making slow headway to the bladder. No blood in urine per visits to doctor and ER.
The pain was strong in December, nothing for weeks and flared up again on January 8th, lasting three days. Pain free now and drinking lots of water.
The proposed next course is surgical removal. Would taking Potassium Citrate Pills act to dissolve the stone?
My brother was a chronic kidney stone producer and now, free of stones for a few years, swears by lemon juice to prevent, but will it dissolve and assist in flushing it out?
Hi Jerry, Potassium will not dissolve the stone unless it is uric acid. Even then if the stone does not pass it needs to be removed because of the danger of obstruction and kidney damage. Dissolving even a uric acid stone takes quite a while. When it is gone, get a proper evaluation to find out why you formed it, and seek rational prevention. Regards, Fred Coe
Thank you Dr. Coe.
Right after I posted my comment, I read further into you other responses and realized it was a false hope to dissolve my stone.
At this point I am still hoping to pass it, lots of water and .4 mg Tamsulosin at bedtime.
Not in any pain, but pretty sure it is still there. Nothing in the filter to prove otherwise.
Dreading having it surgically removed. wondering if anything, reasonable of course, else I can do to get it through the final few inches of my uterer.
Walking, jumping up and down, massaging the area, hanging inverted perhaps?
Hi Jerry, I cannot blame you for disliking surgery; who would not? Prevention is critical and I already pointed out some articles for you. Nothing you mention or I know of specially works. Sorry, Fred Coe
Dr, Coe,
After a 24 hr urine test, I had both very low urine citrate and urine pH. I drank lemon juice with water 3x a day and it brought the urine citrate level way up, but the urine pH is still very low. I don’t want to take a supplement, so I drank baking soda by itself and it raised my pH level. I’ve read that you can mix the two, baking soda and lemon juice, but when I did that, it seems like the juice was neutralized because there wasn’t that sour lemon taste. If those 2 things are combined, will the lemon juice still be effective in raising urine citrate, or does baking soda cancel that out? I can check the urine pH level with testing strips, but can’t test the urine citrate on my own. Thank you
Hi Lee, Low citrate and pH is not a common pattern and usually found in people with gastrointestinal problems. Mixing sodium bicarbonate with lemon juice is not necessarily a good idea because the sodium load may increase urine calcium. Before trying experiments, take a look at this approach to interpreting your lab report; note that the type of stone is critical, and may be uric acid in your case – thence the version in the link. Here is for calcium stones. The mixing approach you describe will not be either fully rational or effective in any event and should be discussed with your physicians. Regards, Fred Coe
Dr. Coe –
I have been on Klor Con (Potassium Chloride for a while due to Crohn’s and low blood potassium). Thanks to Crohn’s disease for over 30 years, I have developed more than 50 kidney stones. My urine citrate was extremely low (less than 31) and I am now on Potassium Citrate. However, in order for approval, I had to stop the Klor Con. Will the potassium citrate still supply the needed potassium to keep my blood levels in normal range??? Thanks!
Hi Dom, Klorcon is potassium chloride; Klorcon/EF is potassium bicarbonate. The low blood potassium can be raised with either but the chloride may be more efficient. Do plain KCl tablets not work?? I doubt your stones are due only to low blood potassium with low urine citrate – oxalate is usually a problem and low urine volume. I have not yet written the articles on the diseases, but I will soon. Regards, Fred Coe
Hi Dr. Coe,
Would you please help me understand the effect of potassium gluconate on urine pH compared to potassium citrate when taken to balance Chlorthalidone?
I realize that K gluconate does not have the alkalinizing effect of citrate, but it still increases K excretion. So it seems like it would increase pH to some extent as eating K-rich foods does.
Best regards, Al
Hi Al, Potassium rich foods have their potassium as the salts of metabolizable anions like citrate, malate, etc. But potassium gluconate will provide potassium and that is important in itself. Low potassium stores lower urine citrate, so any potassium supplement will help restore urine citrate which is an important defense against stones. Regards, Fred
Hi Dr. Coe, OK, that part makes sense now. I’m still trying to get my head around the effect of KCl and NaCl though.
First, I’ve read that KCl supplements acidify urine. Do they somehow impose an acid load on the body?
Second, you mentioned last October (protein/bone article comments), “The best course for you is to lower sodium intake as far as possible – I believe urine pH will fall.” Can you please help me understand how the mechanism?
(My 24hr tests don’t tell me whether this worked because I lowered meat and sodium at the same time, but the good news is that citrate is rising.)
Thank you again for your help and patience! –Al
Dear Dr. Coe.
I have kidney stone (7mm) in my right kidney which is clearly visible in the CT scan. I had an xray done as well, and for some reason the stone is not clearly visible. My local urologist said that it might be blocked by stool or gas or it could be a pure or partial uric acid stone. He has given me potassium citrate (1080 mg tablets 2 times a day), in case it is a uric acid stone.
I have scheduled a ureteroscopy with an out of town urologist (because he comes recommended by a friend) in about 9 weeks. My plan is to get a CT scan done in about 8 weeks to see if the stone has reduced in size before going ahead with the surgery. My initial questions are
1. Is 8 weeks of the above potassium citrate dosage enough to observe a noticeable reduction in the size of the stone?
2. is it really possible for stool or gas to block the stone in an xray? I would think since stool normally appears dark in an xray (meaning that it allows the xrays to pass through) , any calcium oxalate stone would appear light since it would block the xrays, even if the stool was in front of the stone blocking it.
Thanks
Nihal
Hi Nihil, A CT scan will show a stone that cannot be seen on a routine x ray. The difference in visibility does not establish it is a uric acid stone. More helpful would be the Hounsfield number – density – measured on the CT. Removal of the stone would be because of pain, obstruction, bleeding or infection, not just because it is there. The citrate is not likely to change even a uric acid stone is only a few weeks, but why is the surgery being done?? Whatever happens, if removed be sure the stone is analysed and you are evaluated to find the cause and get prevention. Here is a good plan. Regards, Fred Coe
Dear Dr. Coe,
Thank you for your quick response.
Fortunately there is no pain , non obstruction and no infection. I had blood in the urine after running, and that is what prompted the CT scan. After some time, I saw blood in the urine even without exercise, but it seems like after starting to take the potassium citrate, there is no visible blood (I see faint yellow streaks maybe). Maybe there are two stones very close to each other, located somewhat in between the renal pelvis and lowest calyx. The location is such that I feel if the stone gets dislodged, it would fall into the lower calyx, since it would have to jump over a hump to get to the ureter. The reason for contemplating surgery, is I would like to start running again and I am afraid if I do that it might for one cause bleeding, but more importantly the impact might cause it to get dislodged and jump over the hump and get to the ureter and block it.
I got the CT scan on a disk and after right clicking and doing a point analysis and moving the cursor over the smaller stone the HU numbers are between 300-354 HU. For the bigger stone (7mm) the highest point is 613 HU (everything else is below 600, few 500’s, mostly 400’s and 300s). I am doing this on the image WITH Contrast. I have to admit, I am somewhat clueless about what I am doing, but do those numbers tell anything?
If in fact this is a uric acid stone, how long do I need to take the potassium citrate to observe a noticeable reduction in stone size?
Do you think I am rushing to surgery?
Thanks
Nihal
Hi, The low values for the larger stone do suggest it contains uric acid. Potassium citrate might dissolve the uric acid, but I would think months would be needed. ALso, as it dissolves portions might separate off and pass. Regards, Fred Coe
Hi Dr. Coe,
When you say months would be needed to dissolve a uric acid stone, how many months is that? I have been given potassium citrate (1080 mg, 2 times a day). Is that the right dosage or should I ask for an increase? What is a reasonable time frame that I should take another CT scan to see if the medication has had any effect ?
Also I would like to know how potassium citrate would affect my kidneys since I am a type 2 diabetic. I ask this because my father was a diabetic and he eventually died of kidney failure, and at one point the doctors told him not to take any food that contains potassium. My microalbumin/creatinine ratio is 22 prior to starting the potassium citrate. It was only 10 slightly over a year ago.
Thanks for all your wisdom.
Nihal
Hi Nihal, You ask all the right questions but I am not your physician and one hesitates from this distance to go beyond the proper general statements. I do not know the real details of your medical situation. In general diabetics form uric acid stones because of a low urine pH, and the amount of potassium citrate needed is determined by sequential follow up 24 hour urine measurements. Months is a kind of order of magnitude estimate, so no one could become more precise. Potassium citrate will not itself damage kidneys. Diabetics can have disordered renal potassium handling and that is something your physicians need to determine as you use the potassium. The increase of microalbuminuria may reflect blood – be sure about whether the urine sample had any blood by dipstick. Sorry I cannot do more but I am sure you understand. Regards, Fred Coe
Hi Dr. Coe,
From what I have read, dietary NaCl, and also KCl supplements, impose a significant acid load and, in normal people, are expected to lower urine pH. However, you indicated that reducing dietary sodium may actually reduce high urine pH.
Can you please explain? E.g. are there other factors your are thinking of that may counteract the reduced acid load from lowering sodium intake and actually reduce urine pH?
Thanks & best regards, Al
Hi Al, THere is no acid load from sodium chloride or potassium chloride. Being salts of strong acids they do not donate or take up protons. I do not recall where I wrote that low sodium diet will lower urine pH; can you point me to it so I can understand the context? Regards, Fred
Hi Dr. Coe,
For context, please see my initial question and your first reply to my comment from October 13, 2016 in “Does Too Much Protein Increase Stones or Damage Bones?” (No need to go further since the discussion diverges.)
I’m really trying to understand the mechanisms and effects of diet & supplements on unwanted acid load and urine pH because these are so important for bone health and stone prevention.
Thanks again for your time and help. -Al
Dr.Coe,
Thank you for a most informative site/articles. My question pertains to a little understood condition called Citric Acid intolerance. There is very little medical information on the web and most sufferers are left to resources from Good Samaritan websites like this one – http://milind.com/2013/10/12/citric-acid-intolerance-acetic-acid-digestion-and-metabolism-disorder/.
My intolerance started with a (large) exposure to mercury through 13 mercury amalgams. A very interesting journey has ensued . The question is this – if one is intolerant of citric acid (in any form) but certainly when ingested as an additive, does it predispose one to the formation of stones – as you understand the underlying bio-chemistry, does this point to other metabolism issues that would place a burden on the kidneys and hence the bladder? Your input would be greatly valued.
Hi Jaya, A brief search of PubMed for (“citric acid”[MeSH Terms] OR (“citric”[All Fields] AND “acid”[All Fields]) OR “citric acid”[All Fields]) AND intolerance[All Fields] came up with nothing. One paper in rats found that giving extra citrate to mice when added to extra table sugar reduced glucose tolerance. But there are no papers at all on citric acid intolerance. The citric acid cycle in mitochondria is an ancient pathway for energy production and storage via fat. Abnormalities exist, so your question is apt but alas none so far fit your specific one. The web page you linked to has no reliable science, so I cannot evaluate it one way or another. Regards, Fred Coe
Thanks Dr.Coe! Yes citric acid intolerance turns up nothing but there are so many of us that suffer terribly from this particular intolerance. The symptoms range from acute gastric distress to headaches, malaise, and fatigue. The thing that seems to help many is a supplement of cofactors for boosting liver metabolism pathways.
If i came to you with citric acid intolerance and a case of urinary calculi in the bladder would you connect the dots as probable cause and effect? Or are there too many unknowns about the implications (not it’s cause) of this particular intolerance.
Hi Jaya, You are very astute and perhaps there is more than we know. If you did indeed present as you say I would analyse the stones and find out what they were made of and then try to figure out why they formed. But intolerance to citric acid would not occur to me as I know nothing about it and do not have a satisfactory source to turn to. Science is imperfect because what we cannot properly imagine is beyond science. What we can imagine may be beyond any present ways of testing in real life. I think citric acid intolerance is in the second group. Regards, Fred Coe
Dr. Coe…
I’ve been using Crystal Light for about a year, with mixed results. Mostly because I’m not 100% “compliant.” I find the stuff so very sweet, it’s hard to drink as much as I should. And so, I am still getting stones. Some rather big ones if I’ve let myself get dehydrated.
But that’s not why I’m back. My MD has suggested that I drink alkaline water. I know that there are a lot of crazy claims about alkaline diets and water etc… but I’m wondering 2 things.
1. Can it help raise the ph of my urine, and it turn help with my uric stones?
2. If I use it to make my Crystal Light, will it actually LOWER the citric acid of the lemonade and make it not work? Or will it add to the efficacy?
3. Okay, three questions. I am not investing in a $2k ionizer, but rather bought supplement drops to add to my water that contain: Potassium Lactate, Tripotassium phosphate, Potassium bicarbonate, Potassium citrate, Magnesium chloride, Zinc Lactate, Sodium selenite, and Calcium chloride.
Thank you for your time!
Hi Jennifer, Given you have uric acid stones all treatment hinges on raising urine pH. If Crystal light is just too sweet and you do not use it consistently how about potassium citrate to provide some fraction of the needed alkali and crystal light or possibly other of the beverages you mention for the rest. I do not know how much alkali there is in alkaline water and therefore if it can raise urine pH; THe additives you mention are also hard for me to know about because it is all about how much alkali you get in each day. That usually needs to be a lot – 40 mEq is usual – and uric acid stones can form and grow quickly while you experiment. So, I favor a simpler approach as noted, for safety. Regards, Fred Coe
Thank you for your reply!
When you say “How about potassium citrate” do you mean a pill like Uricit-K?
I cannot take that, I’ve tried 3x, and it has a very very bad effect on a heart arrhythmia I have (PVCs.) That’s why I’m trying to go with the Crystal Light.
The drops I have claim to raise water ph to 8. Test papers seem to show only 7ish. Is that enough to make a difference?
Also, if I make my Crystal Light with the alkaline water, will they “cancel each other out?” Meaning, will the acid lowering properties of the Alkaline drops take away the citric acid of the Crystal Light? (Wow, that even confused me!)
Hi Jennifer, The pH does not tell us much about the effects of the water. The buffer capacity is what matters and you cannot tell – the manufacturer has to say how many mEq of buffer are in a liter. You can use alkaline water with the Crystal Light and it will not affect the citrate. Regards, Fred Coe
Thank Dr. Coe. I truly appreciate the personal time you take to answer all questions. You are a good man.
Hi Dr. Coe,
This has got me puzzled. Small doses seem OK, but when we increased K supplements to 40 mEq, CA 24 increased a lot – like 75 or more. It seems like more than coincidence because it happened twice, a year apart. Once with K citrate, and then with K gluconate.
Grateful for any thoughts on what might possibly be the cause of this. Best regards, Al
Hi Al, K Cit lowers urine calcium for the prevailing urine – diet – sodium. Here is a link to the right article. It cannot raise urine calcium. Either your urine sodium rose, or your diet calcium rose. Regards, Fred Coe
Hi Dr. Coe,
My doctor noticed something odd that I had missed. Maybe coincidental, but maybe the answer to this mystery: A big jump in both Ca 24 and pH appeared at the same time I increased output from a bit over 3 to around 4 liters to try to lower SSs. Diet and Ca intake were quite constant.
Wondering if you may have any thoughts on a mechanism that might explain how increasing volume could potentially spike Ca 24 and pH?
And whether a rise in total Ca 24 (as opposed to Ca/liter) may actually be expected as a result of the volume increase? (Of course, I’m interested due to the implications for bone health too.)
Sorry for so many questions. Thanks again and best regards, Al
Hi Al, urine volume has no effect on urine calcium; but look at urine sodium, which has a huge effect. It may have gone up. As for pH, higher volumes have a small effect to raise pH because of buffer dilution. Regards, Fred Coe
Thank you very much! Just to close…
Despite your unimaginably generous and patient help, my nephrologist, her consultation with Dr. Bushinski, and my own research, this remains a bona-fide mystery.
Na 24 differed by only by one, and PCR was identical, yet Ca 24 surged by almost 100 and pH by 0.7. Not a hint of any systemic disease again this year.
I’ll keep working on this! Best, Al
Hi Al, so much can influence urine calcium: diet intake that day, protein intake that day, acid load that day, variation in the rate of absorption of diet calcium in the meals that day. In our clinical research center we see variability of the kind you mention when everyone is eating a fixed and standard diet. So I do not see the issue as directly suited to either clinical or research analysis, and doubt it has real clinical significance. My regards to Dr Bushinsky and your excellent nephrologist. Fred Coe
Dear Dr. Coe,
Since it seems like urine pH is very important (for forming and dissolving) uric acid stones, what is the best way to measure urine pH at home? You have mentioned pH strips but my experience has not been very good. I got HealthyWiser pH strips from amazon (it has probably the highest reviews) and since I had some doubts, bought a HealthyWiser digital pH meter as well. With some difficulty I was able to calibrate the digital pH meter to get close to the calibration solution. But when I used the pH strips to measure the same calibration solution (supposedly 6.86pH in one case), it showed me about 8 pH. Then there is the fact that the strips don’t have that high of a resolution. It shows it in increments of 0.25.
Can you recommend a brand/model of pH strips or digital meter that is quite accurate and repeatable that can be used at home? If not is there some certification that I should look for when buying a pH meter/strips?
I am sorry if I am asking too many questions. I sincerely appreciate everything that you do.
Thanks
Nihal
Hi Nihal, No; you really do need to have some structured care by people who know this trade. I would not try ‘do it yourself’ anylonger because more stones may come. Regards, Fred Coe
Dear Dr. Coe,
Now you have me worried. When you say “more stones may come”, are you referring to Calcium phosphate stones forming due to pH being above 6.3, or is there some other mechanism that you are referring to?
I have responded to another thread asking for more information about getting some structured care from you.
Thanks
Nihal.
Hi Nihal, Merely this: You do not have an organized prevention program and while without one new stones are more likely than with one. Regards, Fred Coe
Hi Dr. Coe,
After initiating dietary changes, a recent metabolic follow-up for a history of calcium oxalate stones reveals blood potassium & sodium and urine sodium & citrate levels within normal range, but continued high levels of urine oxalate & calcium. With urine pH continuing to measuring between 5-5.5, my specialist has proposed taking 60mEq/day of potassium citrate.
Can this medication be of benefit where there is no indication of hypocitraturia?
Can efficacy be achieved at a lower dose or is there a minimum effective dose required?
Can pure lemon juice (eg. 85ml/day) impart equivalent benefits with less risk?
Kindly direct to your website should I have missed the answers therein. Thank you for your follow-up.
Hi Michael, The high oxalate and high calcium suggest you have genetic hypercalciuria and low calcium diet. Potassium citrate may not be ideal all by itself although it certainly is a fine treatment. You say your urine sodium is normal but normal is too wide – you want the urine sodium low, at the US optimal level of 1500 mg – 65 mEq/day – so as to allow high calcium diet yet maintain a modest urine calcium. The high diet calcium will lower your urine oxalate, and the low diet sodium control your urine calcium. Then, a drug like potassium citrate will work more efficiently to lower urine calcium further and raise urine pH a bit. Regards, Fred Coe
Dr. Coe:
I have endeavored to read most of your site, but I haven’t come across this issue: 2 years ago I had 2 stones removed uteroscopically. My urologist put me on potassium citrate ER 10 MEQ TB X 3 times per day. I have assiduously taken them. I get debilitating ocular migraines since then. I only recently questioned the connection and came across this: https://www.ncbi.nlm.nih.gov/pubmed/1555928
I have researched/tried many many other things, but the ocular migraines are increasing in frequency, to at least 1 per day and up to 3 per day. I am at desperation level. Just wondering if you have any comments or recommendations. Daily ocular migraine or occasional kidney stone? It’s getting to be a toss-up.
Hi Bill, I read the abstract. They refer to local potassium concentrations of 15 to 20 mmol/l, blood is 4 mmol/liter. If blood values rose to even 6 – 7 mmol/l the heart would stop. Given the massive cellular potassium pool and the relatively tiny amounts of potassium involved I doubt any relationships between the pills and migraines based on the abstract. Especially because the potassium pills are slow release, I doubt blood levels of potassium even rise. I might point out that US diet recommendations call for 4500 mg of potassium from fruits and veggies, which would be about 4 of the pills; you might try diet. Likewise beverages like Crystal light provide 20 mEq/liter of potassium citrate. Finally, do you really need this medication. Check this out and be sure you have been fully evaluated and that this medication is right. Regards, Fred Coe
Dr. Coe:
Thank you for your response. I have tried in the past 2 weeks to work up to drinking 2.5 liters of water/day. It is almost impossible and I usually don’t take in that much. I have now developed reflux all day. My urologist suggested reducing my water intake to about 1.5 liters/day. He also recommends I keep taking the potassium citrate, but I stopped taking it and have had no migraines since doing so. Now my dilemma is kidney stones or migraines or reflux – pick one. This is all so difficult.
Hi Bill, My rewsponse suggested you get a full evaluation to determine if you need potassium citrate at all. As for water, perhaps you might work your way up to 2.5 liters slowly. Low volumes such as 1500 ml/d will not prevent stones. Regards, Fred Coe
Hi There
Have you tried Sodium bicarbonate as a substitute?
Hi Bill. I have studied migraines quite extensively, due to many years of crippling migraines. My research has shown that *most* migraines are caused by low magnesium. Further, mangesium and potassium need to be in balance, or an increase in one will decrease the other- kind of like a teeter-totter. My theory then, is that your potassium pills are increasing your potassium enough to lower your magnesium, and this low magnesium is causing your migraines. A generic magnesium supplement taken daily can help prevent future migraines.
For any ladies reading this, your cycle causes hormonal changes, which make your magnesium dip, especially 2-3 days before you start. Taking extra mag that day can help prevent cycle-based migraines. Also, know that Milk chocolate lowers your magnesium, while dark chocolate brings it up- the darker the better. So if you just *have* to have some milk chocolate, take some magnesium with it to offset it. 20 years of cycle migraines here before I learned this, love the idea of helping someone else overcome migraines.
I have been taking urocit 10meq three times a day for 3 years to dissolve uric acid stones. A recent CT scan showed the stones have dissolved but incidentally I have bilateral adrenal adenomas.
Any connection between potassium citrate tabs and the occurrence of adrenal adenomas ?
Dr. Coe,
I’m working with your friends at the Cleveland Clinic (Dr. Manoj Monga) – and wanted your input on my test results. I was a bit confused during our conference call with Jill Harris yesterday (05/01/2017).
I have been forming calcium phosphate stones – last stone analysis was 90% calcium phosphate / 10% calcium oxalate (stone measured 27mm). Stone removed on 03/15/2017.
Litholink Results (04/02/2017):
24 hr calcium = 338H
24hr citrate = 357L
24hr oxalate = 29
Supersaturation CaOx = 2.83L
Supersaturation CaP = 1.37
Urine pH = 6.644H
Urine Volume = 4.33H
24hr Magnesium = 125H
24hr Phosphorus = 1.163
24hr Urea Nitrogen = 13.04
24hr Potassium = 88
24hr Sodium = 116
24hr Sulfate = 50
24hr Ammonium = 42
24hr Chloride = 129
Protein Catabolic Rate = 1.1
24hr Creatinine Per Kilogram Body Weight = 26.7H
24hr Calcium Per 24hr Creatinine = 146H
Based upon the course work and my findings – it seems like I have a case for hypercalciuria. I don’t believe that I will be able to make future dietary restrictions to bring the levels down further. I’ve read your articles about thiazide diuretics and also potassium citrate.
I’d like your input here… It would appear that I’m a candidate for a thiazide diuretic (perhaps 12.5mg of chlorthalidone?). What is your take on staggering this with potassium citrate? My urine pH is already elevated quite high… won’t potassium citrate push those numbers even higher?
If you think potassium citrate would be of benefit to my situation – what dosage would be appropriate?
Best Regards,
Cory Nutter
Hi Cory, You do indeed have hypercalciuria with calcium phosphate stones. Thiazide might well be ideal. But your urine sodium is too high – I would lower it to 1500 mg/day, or 65 mEq/day before using the drug. Given calcium phosphate stones potassium citrate is untested – it will raise urine pH with is bad but also may raise urine citrate which is good. No trial exists for this kind of stone. So, lower sodium intake more and then consider the drug you mentioned. But – Dr Monga is quite expert, and does not need my advice; perhaps he has a different view and if so I would go with it and ignore me as an outsider. Please send him my regards. Fred
Dr. Coe,
Thank you for your valuable input, much appreciated. It seems fairly odd that sodium came back elevated. I tracked my diet the day of the urine study and calculated a daily intake of 1170mg. Anyhow I’ve been tracking daily values and have been averaging somewhere around 1200mg/day. I’m currently seeking reopinion from Heidi Digennaro – I’d like to avoid calcium phosphate stones. I’ve taken your suggestion not to wait 6 months for follow up and am trying to be proactive with my prevention measures. I truly appreciate your expertise.
All the best to you,
Cory Nutter
Hi Cory, The urine sodium tracks well with intake if intake is reasonably constant. So if the urine is higher than your diet tracker says and this occurs regularly your tracker is not correct. Regards, Fred Coe
Hi Dr. Coe,
I was the one in Jill’s last class on Monday, May 1st, 2017, that has a permanent ileostomy, fat malabsorption, short gut syndrome, secretory/chronic diarrhea. My urine citrate in the 3/6/17 24 hour urine collection was <18; urine oxalate 32, urine pH 5.697; SS uric Acid 1.34, urine calcium 58, Na24 <12, Mg24 was 21. I have since increased my fluids/daily to 4.2 liters; calcium via food to 1200 mg., since doing this collection/test. I know you said Citrate Potassium supplements/meds will not increase my urine citrate; but to take 10 gr. sodium bicarbonate 2-3 times daily. I just want to make sure this is correct and I didn't misunderstand. Also, I am very concerned the extremely low urine citrate level will cause more kidney stones. I started the sodium bicarbonate on 5/1/17 and bought pedialyte to increase my glucose. Can I take the next 24 hr urine test on May 21 or 29th or is that too soon to see if the sodium bicarbonate is helping? (I have a fast exit system where food/meds are out in my appliance in 8-10 minutes and sometimes need more meds to be effective than other patients). Lastly, since purchased pedialyte has potassium citrate, but home made does not, can I start making mine at home to save money and hopefully calories? Lastly, how will the sodium bicarbonate raise my urine citrate enough? (I thought taking 5 ml-3 x daily of potassium citrate would do the trick, but evidently not!). Many, many thanks for all of your guidance and expertise. Rachel Penney
Hi Rachel, Ileostomy is a special problem of potassium and sodium and alkali depletion. SOdium bicarbonate is absorbed in the upper intestine and will work. The tablet and dose are as you wrote them. You need followup in a few weeks because it should work right away. You can indeed make your own high glucose high sodium drinks at home. But I want to emphasize that your treatment needs immediate monitoring by your physicians as ileostomy management is complex. Be sure. Regards, Fred Coe
Thank you so much for your fast response, Dr. Coe. Two more questions. (1). I am on Potassium Citrate liquid 5 ml three times daily, basically at my request, since I was and still am concerned re. my very low urine citrate level. Now that I am on sodium bicarbonate, should I still stay on Potassium Citrate or get off of it? My new understanding is that I don’t need any potassium citrate supplement since I am on sodium bicarbonate? Is this correct or am I wrong? My doctors are more than willing to go by your opinion. (2). I am closely monitored by my doctors re. blood tests, etc. Are you referring to blood tests (CBC/Basic Metabolic Profile) when you say about monitoring? I did have a blood test done a month ago but of course b/f the sodium bicarbonate was added. That is what I had to get years ago every so often when on sodium bicarbonate. Fortunately, the doctors finally have most of my dehydration under control, even with a very high output, unless I get sick with kidney stones, intestinal obstruction, acute pancreatitis, stomach virus, etc. I ordered my 24 hr. urine collection kit from Litholink and it should arrive next week. Again, many thanks for all of your help and expertise! Rachel
Hi Rachel, Sodium bicarbonate and potassium citrate are alike in action both producing alkali. I suggested the former because your urine sodium is remarkably low and you are safer with sodium. You can take both and the net alkali load will be the sum of the two. As for blood testing it is about potassium, and bicarbonate – total CO2 – as your physicians well know. The goal is to maintain urine pH, prevent dangerous sodium depletion, and raise citrate if possible. So use both if you can tolerate the potassium. Regards, Fred Coe
Hi Dr. Coe, Thank you for getting back to me. I saw my GI doctor today and he will monitor my blood levels closely re. potassium, bicarbonate, calcium levels etc. He is very pleased you put me on Sodium Bicarbonate. I have no issues yet with Potassium Citrate Liquid. He has agreed if I get any signs of dehydration/stomach virus, etc., that he will arrange for me to go to the hospital immediately for IV’s to protect my kidney function. He is very pleased you are helping with my kidneys as he said you are the expert and no one can come near you. He will gladly make sure I have whatever I need to take care of my kidneys/prevent stones. Thank you for being so patient with me and answering all of my questions! You rock, Dr. Coe! Take care of yourself. With appreciation and respect, Rachel
Hi Dr. Coe: My blood tests on Monday show my Sodium is 134; potassium 3.9; chloride 94; calcium 9.2. No dehydration. 🙂 Just got my latest 24 hr urine collection results today. Urine volume increased rom 1.19 to 3.18; CaOx went from 6.20 down to 1.11; urine calcium went from 58 up to 67; urine oxalate went from 32 down to 25; urine citrate went up from <18 to 326; SS CaP was 0.38 to 0.64; urine pH from 5.697 up to 7.333; SS uric acid from 1.34 to 0.02; urine uric acid went up from 0.459 to 0.603. In addition, my Na 24 went from <12 to 39; Mg 24 from 21 up to 89. I don't know if you need more info. Please let me know if you do! I have been on Potassium Citrate, liquid, 5 ml 3 times daily with no trouble whatsoever since March 31st; and on sodium bicarbonate 10 gr., 3 times daily since May 1st. Should I increase my potassium citrate, and if so, to what please? And should I increase my sodium bicarbonate, and if so, to what please? I still need to get my urine citrate up! How to I lower my urine ph? What else can I do to help prevent those stones from returning? (I have the ileostomy; fat malabsorption/ short gut/ very rapid output.. . .that's why I have to take daily higher doses of meds than other folks as a lot goes out in my bag!) Thank you for all of your help! Have a wonderful Memorial Day weekend/Monday. I am so grateful for all of your help, expertise and knowledge! You are a godsend, Dr. Coe! Sincerely, Rachel Penney
Hi Rachel, I am happy for your excellent results. Please, however, check everything you do with your physicians as I am far away and not medically responsible for your care. When we get to dosing of meds your physician has all responsibility and I am acting under the understanding that he/she agrees with and approves of whatever you do with what I say. In general all of your changes are chemically good and stone risk has fallen. Your urine pH is already very high and I am not sure you need more alkali. If any it would be potassium. Regards, Fred Coe
Thank you, Dr. Coe. My local doctors have the results and do approve completely with the sodium bicarbonate. Will see my urologist in several weeks and see if he wants me on the potassium citrate at the same dose or not. At least I’m on the right path. 🙂 Thank you for everything!
Hi,
I had Cushing’s and have had a trapped kidney stone in my right kidney for years. Though we don’t know the composition of the stone, it does not appear on xrays and Cushing’s patients often have a higher instance of uric acid stone.
I’m on blood thinners and experience recurrent hematuria while exercising (running, long walks). The urologist thinks the stone has changed position and has given me Potassium Citrate to dissolve it.
My urine pH has been < 6 for years and is now in the 6.5 – 7.0 range. How long does a 1 cm stone take to dissolve? Do they generally just dissolve or do they dissolve and pass? How much water should I drink?
I probably should have said the cystography, CT, and cytology were all negative, except for the stone.
Hi Marion, uric acid stones will dissolve in urine of pH >6, but it may take a long time. Your physicians can determine the density of the stone on your CT; uric acid has a very low density. Be sure you have been fully evaluated – medical practice is better without too many assumptions. Regards, Fred Coe
Hello, Dr. Coe!
I’ve stopped in to re-read this article several times, and I appreciate it so much! It’s been very informative for me.
I had kidney stones during a couple of my pregnancies, but no problems with obstruction, and no determination of composition at those times. Last year, I did a high protein diet (150 g a day) for about 6 months. I’m an ultrasound tech, and noticed after about 6 months of doing this, I was developing diffuse nephrocalcinosis. I was also taking no vitamin D during that time – I’m a hypothyroid (Hashimoto’s) patient and had previously been low in vitamin D. I started urinating brown flakes.
After cystoscope w/ retrograde pyelogram, CT scan, and lithotripsy, they determined I have medullary nephrocalcinosis and the stone I passed was 100% calcium phosphate. My urine pH was 6.8, and citrate was 334. I saw urology and nephrology. They said I likely have some sort of acidifying problem, but not a full RTA, and I’m being treated with 15 mEq of Potassium Citrate twice a day.
My questions are this: 1) I am pregnant now and am told to continue the Potassium Citrate, but it’s a Category C. Do you agree? 2) My endocrinologist started me on Vitamin D again (my level was 23), and said this could have contributed to the calcium deposition. I’m on 4,400 IU a day 3) Because I had no nephrocalcinosis at all before, and my kidney function is now a bit less (GFR 83), I am worried this will cause permanent damage if it worsens. Is there anything else I should be doing for treatment? They recommended lots of water and low sodium.
Thanks so much for your time. I can give more information on 24 h urine results if helpful at all!
Another question I had: my PH rose to 7.33 after treatment with Potassium Citrate. My urine calcium, and supersaturations were all pretty normal to begin with. With the second 24 hour urine (showing pH of 7.33), my SSCaP was only 0.47, when prior to it had been 1.66 (still normal, however). Do you still agree this is necessary treatment given that the SS weren’t high to begin with? I’m worried the rise in pH will hurt more than help.
Hi Joyce, as I mentioned potassium citrate for CaP stones is moot and a bit fraught. Keep the CaP SS below 1 and after your pregnancy test again and see what is wrong. Regards, Fred Coe
Hi Joyce, WHen you say ‘developing’ nephrocalcinosis so you mean you had a normal scan and then began to see calcifications? If so, things went very fast indeed. High protein intake raises urine calcium, sometimes a lot, and that can cause considerable calcium deposition. The high protein should have lowered urine pH and causes calcium oxalate not calcium phosphate stones so something kept your urine pH up. A search for Hashimoto’s and renal tubular acidosis in PubMed yielded five papers but all patients had Sjogren syndrome, a known cause of RTA. I suspect your urine calcium is too high and CaP SS above 1. As for potassium citrate, no trials support its use as yet. I have written about this problem and begged people to do this trial. Perhaps you might best benefit from low sodium diet and thiazide – I prefer chlorthalidone, perhaps 12.5 mg/day after your pregnancy. Do not make 24 hour urine measurements while pregnant as hypercalciuria is placental driven and of no long term interest. Regards, Fred Coe
Thank you for your reply! Yes, I have read that there have been no trials for CaPhos formers and Potassium Citrate. I am taking the Potassium Citrate, however, as my Nephrologist and OB/GYN both said the 15 mEq/ twice a day, is safe and hopefully of benefit. It did raise my Citrate from 334 to 436, so it makes sense that it would be beneficial.
Yes- the nephrocalcinosis was VERY fast. I have been scanning my kidneys for years (as like I said- I am an ultrasound tech). Within 6 months – they went from completely normal (minus a couple small stones, which I had developed during pregnancies over the last 6 years, and had been stable) to macroscopic nephrocalcinosis. Like I said, my protein intake was VERY high, and I have no idea what my urine would have looked like at this time- I didn’t have a 24 h urine test until several months after I had stopped this diet.
I have reason to believe that the CaPhos stone that they broke up with Litho, and that I passed, was an old stone developed during a previous pregnancy. I believe it was one I had seen for years. So, then I guess the nephrocalcinosis could likely have been caused by my high protein and calcium intake and lack of vitamin D during those several months (and could have indeed been CAox deposition from high protein).
Do you believe a low Vit D could indeed cause rapid nephrocalcinosis? Like I said, I had previously been taking Vit D and stopped for several months when this developed. My endo seems to think this may have contributed.
I just want to make sure I am doing everything I can to protect my kidneys from further deposition, and potential loss of function. I am only 30, and otherwise healthy. My BMI is normal and I exercise daily.
Thank you!
And as for the Thiazide, I feel I would be a terrible candidate, as my BP runs very low.
Hi Joyce, Protein loading will increase urine calcium and that may have done you in. Vitamin D should not do much. Nephrocalcinosis from CaOx is very uncommon – such stones form on papillae but I suppose with only ultrasound many tiny CaOx stones on papillae could look like the sort of nephrocalcinosis we see on CT from calcium phosphate plugging. As for what now, be sure you have really been properly evaluated. Lower supersaturations with all available modalities if you are just an idiopathic CaOx stone former. But most important don’t assume too much. For example perhaps a CT scan would be helpful to really see your stones – ultrasound is really less resolved an image. Be very careful you do not have a systemic cause of stones. Regards, Fred Coe
Thanks, Dr. Coe. They also saw the stones and nephrocalcinosis when I had a CT. On my last 24 h urine, both CaOx and CaPhos supersaturation were extremely low, as was calcium. So maybe I’ll be okay now. I’ll be sure to remember that about the Calcium and Protein. Thank you!
How is it determined if you have a “systemic” cause of stones?
Hi Joyce, Physicians do this. This article has a reasonable list of diagnoses and their usual presentation in serum and urine testing. But there are subtleties to this – your physician is responsible for the outcome. Regards, Fred Coe
If your citrate s are Low, try 4 ounces of Lemon juice per day. I squeeze fresh lemons each morning to raise my Citrate levels. Have a blood test. I had Calcium Oxalate stones.. Had 7mms….now down to 3 mms.
Hi Dr. Coe,
Can you please tell me, in cases where Potassium Citrate would be problematic due to pH in the high 7’s and CaP content in stones, might Citric Acid supplementation make sense to try to raise Cit 24? (A few hundred points would be ideal, but any amount would help.)
If so, (1) what might be a suitable range of doses to try, and (2) what effect on urine pH would be expected?
Thanks & Best Regards, Al
Hi Al, Use of potassium citrate for stone prevention needs to be based on what trial data we have. Take a close look at this article that gives a strategy for how to use it in the context of a treatment plan. Warm regards, Fred
Hi Dr. Coe,
Your articles are a wealth of helpful information, and of course I’m working closely with a good nephrologist on treatment, diet, and fluid plans.
That said, I’m struggling to understand the implications of what you explain above about potassium citrate presenting an alkali load because it takes up two protons from the blood as it is metabolized into citric acid. I realize that is just one step in the citric acid cycle, and the rest is likely important as well since only a small part is excreted in urine.
So if one ingests Citric Acid capsules *instead* of K citrate, does that mean the Citric Acid already has the two protons and therefore won’t act as an alkali load? Or do other factors still end up alkalinizing urine? Grateful for all your help. -Al
Hi AL, Yes; citric acid has its protons and will not take any up. So it does nothing. Regards, Fred Coe
Sorry, one last part!
My understanding is Potassium Gluconate is *not* expected to raise urinary pH like K Citrate does. Perhaps this is an over simplification, but it seems like K Gluconte would also take up two protons in return for the potassium, and thereby present an alkali load.
So wouldn’t K Gluconate and Citrate both raise urine ph? (Multiple 24 hour urines seem to show this.)
Thanks so much! -Al
Hi Al, Gluconic acid has one proton site so I presume two gluconic residues bind one calcium. It is indeed an acid salt and if metabolized as the acid would take up a proton. I cannot find any articles on its metabolism when given as a salt. A recent article is the first to actually describe the enzyme that initializes its metabolism in humans. I presume its metabolism will produce bicarbonate but have written to the senior author and asked. The paper is difficult to read. Regards, Fred Coe
Hi Dr. Coe,
I can’t thank you enough for your help! I didn’t know where to start, and this is very significant question because we sacrificed the benefits of citrate by switching to K gluconate – in part to try to avoid raising pH even higher. I will talk more with my Dr. and keep reading.
You were so right when you wrote about the importance of using 24 hr urines to confirm the effectiveness of treatment. I’ve also found it useful to do regression analysis on the data in Excel to help isolate the changes that are helping the most. -Al
I’m on Lemon therapy in lieu of Potassium citrate.
My 7mms stone has diminished in size to a 3 mms on my last CT scan within one years time. 4 oz of freshly squeezed lemon juice per day..my citrate levels are raised.
I had a percutaneous procedure to remove a 2cm stone in left kidney 14 months ago. 6 months after procedure kub showed another 1.8cm stone and 10 months after procedure cat scan verified a 2cm. by 10mm. midpole stone. My urologist wants me to take Sodium Bicarbonate and Pottasium Citrate. Can these meds effectively dissolve a stone of this size?
Hi Rick, from the pace and size I gather these were uric acid stones. Alkali as you describe can prevent them and dissolve them. Be sure you know what they are. Regards, Fred Coe
on the subject of uric acid stones, i started having them when i went on the atkins diet. i am now a chronic uric acid kidney stone producer. i average a stone a month. doc wants me on 40meq of potassium citrate. he does not seem to care about the fact that i suffer from intermittent atrial fibrillation, pvc and pacs of my heart rhythm. so i refused to take them but found your article about crystal light and have graduallly increased my crystal lite intake to 3 liters a day. however at this much i am experience increase in irregular heart rhythms. what is a heart patient suppose to do when they cant take potassium citrate? i am on allpurinol but on a low dose due to severe dizziness as a side effect. i feel helpless and scared. i have been like this for a year and a half and am worried i will die from kidney failure or sepsis if i keep getting them. sometimes i pee out 5 or more stones like sand or a little bigger. the biggest stone has been 5mm. will i eventually have bigger stones that need surgery? thank you for your time.
Hi Mary, IN general potassium stabilizes heart rhythm not the other way. Allopurinol has no value for uric acid stone prevention that depends on urine pH more or less alone. You need alkali and it should be potassium not sodium based. COnsult your cardiologist and do not continue forming these stones. They can be numerous and grow large. Regards, Fred Coe
Wow, sounds complicated. I have calcium oxalate stones…My Nephrologist checked my blood, seems I’m Low in Citrate…..
“Citrate prevents stone formation!” I juice 4oz of LEMONS per day……my
7mms stone is NOW 3mms at last CT scan
Look up LEMONtherapy vs. Potassium Citrate…NO
SIDE EFFECTS….it’s natural. Good Luck
Hi Kathy, I have approved all of your comments but do need to comment. No data support lemons or their juice in kidney stone prevention. Potassium citrate has several trials. Perhaps fruits will do what the pills to, but probably no one fruit will in fact the the right approach. In the absence of trials, I cannot agree with you or endorse what you have said as valid prevention. The ideal kidney stone diet contains an abundance of fruits and veggies as well as high calcium and low sodium; in this it mirrors the current US diet recommendations. If one needs potassium citrate, and one wants a non pill substitute some commercial beverages have more than juices. Regards, Fred Coe
Hi all,
I have been suffering from kidney stones for over 15 years. I (or did) pass about 1 every other month ( 5 or 6 a year ). Had to have three broke up with Lithotripsy ( one about the size of a dime ). I met someone who’s husband had them for years also. She told me he started drinking lemon juice about a year and a half before I met her and has had no stones since then. I researched it on the web. Decided to try it. *** GLAD *** I did, have had no stones in about 2 years. It works for me. I drink one large glass of lemon juice a little before bed time ( I believe when you sleep is when they form the most ) and one or two during the day. My wife makes it very strong without sugar ( we use a sugar substitute ). I am starting to take a small amount of potassium citrate also. The lemon juice has saved me a lot of pain. If my urologist would have told me this from the start it could have saved me a lot of time and money. Don’t know if this will work for everyone but lemon juice has helped me. The pain from stones will make you try almost anything. I will eat whole lemons if it will stop the pain. I hope this helps someone.
Thanks,
Dean
Hi Dean, The lemon juice presumably provides some potassium citrate. You could also use multiple other beverages that will do the same. Best, Fred Coe
Hi Dr. Coe,
I take magnesium supplements anyway for health reasons other than kidney stones.
Now I see from this article that magnesium can compete with calcium to bind with citrate. That doesn’t sound good.
Any thoughts on if or when magnesium excretion (e.g. 100, 200, or 300 mg/day) or a particular Ca/Mg ratio actually may to increase calcium stone risk by “wasting” valuable citrate?
Thanks!
Hi Kenny, Little fear; calcium competes against magnesium enough that your citrate will be fine. Also, magnesium binds oxalate. I know of no magnesium risk for new stones. Be sure you have been fully evaluated and need potassium citrate. Regards, Fred Coe
Hi Dr. Coe,
I have a 6mm stone in my left kidney. It was detected on an ultrasound but was not visible on KUB.
My urologist thinks it’s a Uric Acid stone. I also recently passed some grit – that was 90% calcium oxalate and 10% struvite.
Is it possible to have two different types of stones like this? Are calcium oxalate stones ever invisible on xray? thanks
Hi John, The lack of visualization on flat plate is only fair evidence – here is my uric acid article. The grit suggests common calcium oxalate stones with infection stone material – struvite. One can have multiple kinds of stones. Here is my suggestion; read the link and likewise chapter three of the book and plan our an evaluation for yourself. You will find the right answer with an orderly approach. Regards, Fred Coe
Hi Dr. Coe. I have been on potassium citrate since February to see if it will shrink a 22mm uric acid staghorn. It was 25mm in February but gone down to 22mm in June. I have been feeling particles in various areas of my back and can feel like stones rubbing in my spine like a porcupine. Could I be experiencing effects from the potassium citrate working on my stone and it breaking down? My back/butt feels inflamed. I have to wait till October for surgery if the potassium does not work. Any recommendations besides lots of water if this is what I am dealing with? Many thanks!
Hi Lynda, alkali will dissolve uric acid stones but slowly! Little particles can come off and cause pain. Water and lots of alkali is the best, as it is shrinking. If you cannot stand the particles there is surgery but you could possibly avoid it. Also the smaller the stone the better. Regards, Fred Coe
Hey,
i actually visited doctor for UTI and found that i have two stone left (2.4mm)and right kidney(3 mm), now i am taking
medicine. problem is when i take medicine like urocit-K potassium citrate, i start experiencing pain and its in lower back and flank area if i stop it goes away. any guide line
thanks
Hi Nouman, possibly the stones are uric acid and fragment when urine becomes more alkaline. Possibly when urine becomes more alkaline you form calcium phosphate crystals. I cannot tell from here but your physicians can. The alkali itself does not cause kidney pain. Regards, Fred Coe
Hi, iam suffering from kidney stone, i have 1.8 cm in my left kidney with some few calculi, can you please suggest any oral recommendation. since Dr. has suggested me to go for PCNL.
Hi Saad, I can only suggest you get proper testing and find out what is wrong that is causing stones. Taking remedies without a proper evaluation is not helpful for you. As for PCNL the stone is large, and the choice seems reasonable. But you should figure out the cause first so new stones do not just grow back. Regards, Fred Coe
Thank you so much for this brilliant article. It contains so much detailed information that I simply could not find elsewhere, assembled so eloquently. I would like to ask your advice, if you don’t mind. I do not have kidney stones, but I take 400 mg of zonisamide and 200 mg of topiramate a day. These are for migraines, as well as effective mood stabilizers for my bipolar disorder (valproate and carbamazepine both literally poisoned me). Per the research I can find, both of these medications can cause acidosis in about 40% of patients, and stones in a significant minority. I have not found anything about the combination of the two, but I can only assume it is grim. Therefore, I have been taking 30-40 meq of potassium citrate in 3-4 divided doses a day as prophylaxis. I currently lack insurance and am unable to afford the proper prescription medication Urocit-K, so I am taking a dietary supplement, hence the rough measurement. My physician, wonderful as he is, has no expertise in this area, and I have not been able to talk to him about this. Am I on the right track? Am I wasting my time? Maybe there is a lab test I should order to see if I should increase or decrease my dosage? I would greatly appreciate any advice you might have about my unusual use of this medicine.
Thank you very much for your time.
Another thing I meant to ask. Apologies for the multiple posts. Can I take simple citric acid for the same effect? Or at least perhaps a mixture of potassium citrate and citric acid? The potassium citrate causes some gastric upset, and it is not always reasonable for me to take it with a meal. I can live with it. But if I could mix it with citric acid, that possibly would be helpful. Thank you!
Hi Alfred, citric acid has no purpose – merely lost in the urine. Potassium chloride would be reasonable if your blood potassium falls. But for both of my comments: This is complex stuff and you need your physician to guide everything. The drugs produce a serious acid base disorder and I am a voice from another planet. Best, Fred Coe
Hi ALfred, Both medications inhibit carbonic anhydrase and cause a form of renal tubular acidosis. Potassium citrate is not an intelligent treatment against stones as it will merely raise urine pH more. It is not the low blood bicarbonate but the persistently alkaline urine that matters and that is not fixable. Very high fluid intake might stave off stones. Low sodium diet, perhaps. Ideally, some other kind of medication. If your blood potassium goes down, mere potassium chloride would be preferable to the citrate. Sorry I cannot do better. Regards, Fred Coe
Thank you for your prompt response! My physician thinks that this in general would be a good idea, but he is not knowledgeable of the details. His idea comes from studies such as this one:
https://www.ncbi.nlm.nih.gov/pubmed/20888032
So I am confused why potassium citrate would not be recommended? It will 1) increase urinary citrate and 2) increase serum bicarb (to a lesser degree), correct?
Thank you
Hi ALfred, Lacking any trials we are into a kind of philosophical spiral. Acidosis – low blood bicarbonate – stimulates renal citrate reabsorption and metabolism, so none gets into the urine. The drug raises urine pH and perhaps urine calcium. Potassium citrate will raise urine citrate but raise urine pH. What crystals see is supersaturation with respect to calcium phosphate which rises with pH and falls with citrate binding of calcium. Citrate also inhibits crystallization. Potassium depletion from the drug worsens citrate loss – less in urine. So, that the potassium citrate may raise urine citrate is good, but balanced as noted. So long as you and your physician are clear about the balance, there is no reason to not try the k citrate, but monitor urine supersaturation and stone growth. Regards, Fred Coe
Thank you so much for your responses, doctor. You do a great service here. We do not even have a nephrologist in 100 miles of this small town that my physician or I could consult. I was reading another of your articles on “Incomplete Distal Renal Tubular Acidosis.” Great stuff. Based on all the topiramate and zonisamide-specific research I’ve seen, this condition closely mirrors what the topiramate and zonisamide cause (calcium phosphate stones, high urine pH, low urine citrate, low serum pH). You say at the end of the article that the potassium citrate is sort of an unknown in efficacy for condition, more testing is needed. It makes sense to me to test….at least it helps two of the main issues. Urine citrate and serum pH, that is. Urine pH will be alkaline already, nothing to be done there it seems. I am only a sample size of one, but so far so good. I have regular lab work taken, and a great relationship with my physician, so if anything starts to go wrong we can correct it quickly. Thank you again so much for the wealth of knowledge here.
Hi Alfred, the drug produces real renal tubular acidosis – blood bicarbonate falls a lot. It is proximal tubule RTA. My comments about treatment are as in my prior note. I wish we had a real trial or that you could do with other drugs. Best, Fred Coe
Hello doctor, I have one last small question regarding the citrate. I hope I am not a bother! Are the effervescent solutions such as Effer-K, that contain potassium bicarbonate and citric acid, truly fully converted to K-citrate? In that case, they should be equally effective as a regular potassium citrate, correct?
Thank you
Hi Alfred, Yes; potassium bicarbonate and citrate both end up as bicarbonate. Best, Fred Coe
Hello Sir, Last year i had a kidney stone lasered, too big to pass. After the follow up, i asked the doctor what I needed to do to stop getting these stone (2nd stone, I’m 50 yrs old) He said to take Potassium Cit 1080 MG or drink a tablespoon of lemon juice every day, but I didn’t follow his recommendations (i think the potassium hurt my stomach, i have IBS). Now I’m experiencing discomfort in my lower back again, just like the last time. I have scheduled a follow up with him in 2 wks, what should I do to stop this from happening again and to help with discomfort until my appointment. Thank You in Advance
Hi Michael, about the present stone – if there is a new one, I can say little. But about prevention, I have a lot to offer. Just offering one remedy rarely works. Here is a real plan for prevention. Discuss it with your physicians. Regards, Fred Coe
Wonderful article, so refreshing to read real evidence. Helpful and totally informative.
Dr Coe
I’d like to personally thank you for your great work in this field.
It has taken me since the mid 80’s to finally have some one explain how this mechanism actually works. No MD has ever sat down to tell me anything close to this. Kidney problems since 1984 with high uric acid and high protein in the urine since then and now gout and kidney stones a problem.
Potassium Citrate and some diet modifications ( Reduce acid load) might be a naturally start to address the uric acid issues.
Thanks again for your great work and await for some more of your articles.
Hi Robert, I am glad the article was useful. Potassium citrate to raise urine pH is specific against uric acid. Diet acid load does not matter much as against the supplement. REgards, Fred Coe
So what does drinking a lot of pure fizzy water do?
What does eating lots of meat do?
What does consuming lots of fruit do. Like oranges?
Consuming a glass with bicarbonate of soda?
Lemon juice?
Hot water vs cold water when there is loads of calcium and fluoride I that water?
Thanks,
Hi Anita, some fizzy water contains a lot of sodium – that will raise urine calcium losses. Meat above 1 gm/kg/day raises urine calcium and stone risk – assuming you have calcium stones. Lots of fruit and veggies are healthy and part of a good diet. Take a look at the kidney stone diet – it matches the ideal US diet. SOdium bicarbonate is a sodium load and raises urine pH – do not use it unless you have some special reason. Lemon juice is just another fruit – a poor way to eat fruit because the fiber is all gone and all you get is a sugar load. Sugar loads raise urine calcium. Never drink from your hot water lines – serious risks. Heating your water makes no difference. Fluoride has no stone effects. Calcium likewise in that most people never get enough calcium in their diets. Regards, Fred Coe
Sir:
Thanks for the information. I am a 100% cysteinuric. I’ve been on potassium & captopril for years. Helped tremendously. Question. I have very serious vomiting and illness when I take many antibiotics… as well as morphine, demoral, flexoril etc. I’ve been told it is the sulfpher since I am a cysteinuric, I can’t tolerate the sulpher, sulpha, sulphite etc. in the drugs…. here is my question… what is it that I can’t tolerate? I’ve read several articles, but I just don’t understand what I shouldn’t take. I’ve recently been given a medicine with sulpha in it, I don’t know if I should take it… Can you clarify for me? I think I understand that I can’t take sulfa (sulfonamide) and sulfate drugs… Since the prescribing doctor is NOT a urologist, I am trying to find a correct way of explaining whether I am allergic to drugs with sulpher…. sulpha… etc.
Hi Pat, Your captopril is a sulfur containing medication, so it is not the sulfur atom itself. I am sure your urine is filled with sulfate as a breakdown product of proteins you eat. You seem to be describing intolerance to antibiotics, and perhaps to sulfa drugs. The latter have a specific ring structure that can create allergies. I do not think anything links your cystinuria to your allergies, especially as you fortunately can tolerate captopril. Your drug sensitivities are like those other kinds of patients develop and need to be managed on a drug by drug basis. Regards, Fred Coe
I’ve had 2 kidney stones in the last year, the last one was 11cm and required lithotripsy to break it up. I’ve had multiple blood and urine tests and the urologist has prescribed 2 medications. One is potassium citrate, my question is when I have a bowel movement the pills come out in the stool whole. Is that normal?
The wax matrix is what you see, the drug was absorbed. Regards, Fred Coe
Hi Dr. Coe,
An inexpensive substitute for Urocit-K is bulk food grade potassium citrate, readily available on the web for ~$0.02/gram. It is commonly available as the monohydrate.
Hi Glenn, You are right but I always caution that measuring out one gram at a time 1080 mg to be exact is essential as overdoses can be dangerous. Regards, Fred Coe
Hi Glenn, You are right but I always caution that measuring out one gram at a time 1080 mg to be exact is essential as overdoses can be dangerous. Regards, Fred Coe
Dr. Coe, no kidney stones ….. yet…. but I have oxalates in my lungs along with 2 toes that swell when I eat oxalates. I have been taking magnesium citrate and calcium citrate with my low oxalate meals. I may be over reaching with my question but you are so incredibly knowledgeable, here it goes…. Is there anything you would recommend to deoxalate lungs besides what I am doing? I have also been trying some probiotics that are known to help with oxalate absorption but at this time, my lung capacity is very low and nothing has made a difference. Any words of wisdom will be deeply appreciated. Thank you, christine madden
Hi Christine, If you mean calcium oxalate crystals in your lungs, actual crystals there, that condition is very rare and the crystals usually are phosphate not oxalate. There exists a very rare disorder of phosphate transport with lung crystallizations. I have found one report of lung calcium oxalate crystals arising from Aspergillus Niger infection. Lymphomas have been known to cause lung crystals because their cells store the crystals. Cystic fibrosis can cause lung calcium phosphate crystals. This large review includes diffuse pulmonary crystallization due to hereditary defect in a key phosphate transporter. Perhaps your physicians might want to consider these possibilities and also that you may not have actual crystals in your lungs. Absent crystals, oxalate will not be in lung tissue. Regards, Fred Coe
Hi Dr. Coe,
I have read that large acid loads can lead to the body taking Ca from bone to neutralize the acid in the blood.
Can you please tell me whether naturally occurring bone resorption would also have an alkalinizing effect as it releases Ca compounds into the blood?
Appreciate your time. Best regards, Al
Hi Al, yes, acids remove bone mineral and removal of bone mineral adds alkali to blood. But in usual settings evidence for a benefit of medicinal alkali on bone mineral balance is scanty and controversial. Regards, Fred Coe
Hi Dr. Coe,
Thank you! You raised an interesting point. But actually, I’m coming at this from the other direction. Everything I’ve read indicates pH normally drops at night low due to slower respiration and body’s acid purge cycle while sleeping. But my already high pH rises all night. My doctors have run out of ideas. So I’m trying to learn about anything that might raise pH, andI read in Pubmed that bone resorption reportedly peaks at night.
Assuming healthy kidneys and lungs, but known bone loss, can you possibly offer any suggestions on how I may roughly estimate how much each 10 mg of net Ca lost from the bones daily could potentially raise urinary pH? Best Regards, Al
Hi Al, You are asking me for a level of detail I cannot possibly provide. In order to help your physicians sort out your high pH problem I would have to be part of your actual care, review all your records, and render a serious medical opinion. Otherwise I would be a distraction to them and no help to you. Regards, Fred
Hi Dr. Coe,
Thank you for your reply. I certainly respect that. That said, perhaps I wasn’t clear. It certainly may be more complicated than I realize, but I think what I’m looking for is help with a general chemistry question…
I’ve read about how much a given amount of potassium citrate is expected to raise urine pH. I’m trying to calculate an estimate of the corresponding alkalizing effect of 10 mEq of calcium compounds in the form released by bone. But I’m not sure what those compounds are or how many protons they can accept. So I don’t know where to start. Best regards, Al
Hi Al, bone is calcium phosphate and the phosphate can take up two protons per mole. The calcium to phosphate mass ratio is 2.2. This permits you to calculate moles of phosphate alkali per amount of calcium released. Fred
Hi Dr Coe- any clue how long it takes for potassium citrate to alkaline the urine. I am a Urologist and I have always wondered why potassium citrate is not a part of medical expulsion therapy. Currently we only Flomax to relax the ureter but why don’t we use K-Cit to try to weaken the stone and thus help with eventual passage or even stone free rate after surgery? K-cit is a big part of preventing metabolic stones but can you see a potential role in assisting treatment (spontaneous passage or at the time of surgery)?
Thank you for this great article!
Hi Dr Arzola, For calcium oxalate stones, raising pH will not change stone size. While it is true that citrate attacks the surface of calcium oxalate crystals stones are a complex crystal protein amalgam and the drug would have little effect except over long periods. For calcium phosphate stones, the higher pH could cause rapid enlargement. For uric acid stones raising pH with alkali would indeed cause dissolution and shrinkage, perhaps rapidly, and would be worth a trial. Warm regards, Fred
Would drinking an alkaline water help raise Ph to help in reduction of storm formation?
Hi Jason, Alkaline waters usually use a powdered alkali, but raising urine pH is not always a good idea. Instead of picking out a remedy here is a plan to follow that may work better. Regards, Fred Coe
Can I know how to get rid of kidney stone safely.
Hi Zakir, If you mean prevention of new stones, here is my best on it. If you mean a stone in the kidney or passing, only your surgeon can be really helpful. Regards, Fred Coe
It’s really good work done by you for humanity, indeed.
Hi Zakir, thank you for saying so. Regards, Fred
Dr. Coe,
Thanks for a great article. I have a question on the citrate and oxalate transport system pertaining to electrolyte balancing in the tubular fluid. If oxalate (-2 charge) secretion is increased and citrate (-2 charge) resorption is reduced, don’t you end up with an overload of anions in the urine? What am I missing?
Thanks,
Shannon
Hi Shannon, Good question but no. The electroneutrality of the exchangers is achieved mainly by chloride movement. Oxalate transport via A6 is part of a complex bicarbonate, chloride, oxalate exchange. Citrate is via sodium citrate cotransport. Best, Fred
Thanks! I knew I was missing something! I had a friend who, when he taught electrolyte balancing, would state, “As the good book says, an ion for an ion!” Have a great day!
I’m currently on two 1620 mg (15MEQ) tablets of potassium citrate twice daily. I’ve already undergone my 24 hr collection and it came back with me having very low urine citrate. My stones are calcium oxalate. I’m just concerned about the long term effects of taking this much of this medicine and I haven’t really been able to find any information on the long term effects. Any advice?
Hi Mathew, very low urine citrate is not common. Is there an underlying disease? Do you have an unusual diet – eg very high protein, as an example. Often, the proper intake of fruits and veggies – 5 servings a day will improve urine citrate so the medicinal use can be lessened. But I am suspicious there is some specific cause here – diet, bowel disease, use of diuretics?. Regards, Fred Coe
Hey doc sorry for the late reply.
I was eating a lot of spinach and nuts for a good year prior to my stone. I also increased exercise dramatically (long barbell training sessions ending with intense cardo and then running at least 2 miles every other day and also a labor job). I was also consuming large amounts of coffee and often large very sweet ice tea. My urologist said my kidneys were fine as well as blood work looked fine. This bloodwork was done back in 2017 after the stone of course. I have been taking the potassium citrate since with no further issues, but I am still concerned about the potential effects. Is it possible my low urine citrate was a result of excessive exercise and coffee consumption and then the stones were likely brought on by the oxalate in my diet?
Forgot to add I was also living in a very hot and humid area
Hi Matthew, Lots of what you say could have caused stones. Even so, be sure and have 24 hour urine testing to be sure about stone risks. Blood testing detects only a tiny few of the stone causes. Regards, Fred Coe
Hi Dr. Coe,
Wondering if you have any insight into treatment for a friend who has Medullary sponge kidney and possibly renal tubular acidosis. She also has been told that her stones are usually calcium phosphate stone with a high urine PH. Her chronic kidney stones have continually worsened over the last 10 years and she is now battling about one every week. She is drinking about 2.5 L of water per day and is on a diuretic with KCl replacement currently. Thank you!
Hi Heather, Of course this is a complex kind of problem and I am far away. But I suspect she has calcium phosphate stones not MSK and needs more thoughtful treatment to prevent them. Water and a diuretic are not uncommon but one needs perhaps other steps like low sodium diet. Above all, urine supersaturation with respect to calcium phosphate needs to come down below 1. Be sure she has been fully evaluated, and consider if her treatment is complete. Regards, Fred Coe
Hi Heather, Of course this is a complex kind of problem and I am far away. But I suspect she has calcium phosphate stones not MSK and needs more thoughtful treatment to prevent them. Water and a diuretic are not uncommon but one needs perhaps other steps like low sodium diet. Above all, urine supersaturation with respect to calcium phosphate needs to come down below 1. Be sure she has been fully evaluated, and consider if her treatment is complete. Regards, Fred Coe
I need help– the first time I bought generic zaditen from a pharmacy overseas it caused me urine retention and a UTI and my PH IS/was ERRATIC SINCE– UP to this point I have had over 7-12recurrent UTI- and the stupid medications in the U.S. do not work because they are generics vs the ones used in europe even in a third world the drugs of choice are brand names such as Fosfomycin… story short… anything I’m eating now that is acidic or sweet my PH will drop to 6.5 and i will start having a burning sensation when i pee, on my end too and constant need to urinate and low grade fever– tried D Mannose and every single supplement out there they would work for a while and my UTI comes back- i do not have prostatitis nor kidney problems but my PH IS ACIDIC so i tried potassium citrate and potassium carbonate which caused me stomach ache and urine retention rather than increase in urination then my UTI would come back- I’M in constant tug of war with this issue it’s been over 2 years and these doctors are the worst i have EVER seen useless and they never move away from things they read about or saw in books and the medications they use are even useless- levaquin caused me ligament tear, and bactrim too and cephalexin caused me sever cramps i could not walk and doxycycline works for a while but no extra oomph to get rid of IT… useless medications and the whole thing sucks in the medical care system in the U.S… Any new idea or real help to get rid of my UTI– did all the normal things for this problem and nothing helped, it keeps coming back after my appendectomy in this hospital things went down hill- another thing when i pee the first time in the morning the foam and bubbles are about 5 inches high and very thick and linger for ever, till i flush the toilet . I’m so depressed and sad and lost joy in life because of that shit
Hi Veronic, I gather you are finding urine pH values of 8.5, but those are not acidic – rather alkaline. If you are indeed having infections, there must be organisms from cultured. Do you know what they are? Alternatively, you may be forming crystals that can cause the kind of pain you describe but require a different kind of treatment. Perhaps when you have an episode your physicians might look at the urine under a microscope and determine if there are crystals present, and what kind. Regards, Fred Coe
Maam you should try checking the water you drink or use un the household, test it in rhe lab. To find out if it is one of the factors affecting your UTI.
Hello Dr. Coe:
I had an (unknown) calcium oxalate supersaturation level in my kidneys that got me into an emergency room/surgery ~ one year ago. I researched EVERYTHING that I could on this topic, after the catheter/ stent were taken out; for my CT-scan/ X-rays showed about 9-10 stones in my system ALONG WITH the ones stuck in my ureter/bladder. I changed my diet COMPLETELY; started drinking 3-4 qts. of water daily; began a chanca piedra-based tincture/ herb capsule regimen; et cetera. I pretty much slowed stone-formation, yet it wasn’t until I began a potassium citrate therapy that I Really Felt that ANY treatment Was Truly ‘turning a corner’, honestly. Was trying 30mEq daily; now doing a 20mEq dosage. Thanks, James
Hi James, I am glad you found something, but your approach is not ideal. What were the stones made of?? Do your urine tests show low urine citrate or pH. Stone prevention is reasonably well advanced, and perhaps you might benefit from a more nuanced approach. Here is a reasonable start. Regards, Fred Coe
Dr Coe,
Need to pick your brain a bit here if you don’t mind:
– Bi lateral stone former
– Stones are Calcium Oxalate
– 24Hr Urinalysis presented nothing remarkable other than low citrate levels.
– BUN/GFR/UA/CR all normal range
– Electrolytes relatively in check, with exception of slightly higher sodium
Should I consider taking citrate in the form of potassium, magnesium or calcium?
Also, when referring to potential root cause where should i be looing at low citrate levels?
Hi Paul, If there is nothing abnormal but urine citrate and you form calcium oxalate stones, potassium citrate is a good idea. Be sure the citrate goes up. Regards, Fred Coe
Hello
Sir Fredric this article is very useful. I am suffering from kidney stone problem. There are four stones in my kedny/bladder. This potassium citrate solution is helping me a lot. Thanks once again for this extraordinary article.
Thank you for such a detailed dissertation. I have a histamine intolerance and was advised to avoid citrates due to the modern practice of fermentation to extract citric acid. That said, would potasium bicarbonate offer equal benefit if the goal is to treat bladder and/or urethra stones? Thank you.
Hi Forrest, if the stones are uric acid it would be ideal. If calcium, not necessarily; it would depend on the 24 hour urine chemistries. As well, bladder stones almost always reflect some outflow obstruction, and that needs remediation if one wants to prevent more. Regards, Fred Coe
Hello Dr. Coe, I really enjoyed the article ( and others you have written) although I can’t say I understood all of it! I was diagnosed with kidney stones last August after an ultrasound for something else picked them up, one in the lower portion of each kidney measuring 6 mm, both nonobstructive. The urologist ordered an x-ray ( to see if I was a good candidate for blasting) where no stones were detected in either kidney, which led me to believe the research that they are most likely uric acid stones.
Fast forward to February, and the doctor ordered a repeat ultrasound to check on the stones. This ultrasound showed no kidney stones in the right kidney and “multiple stones” in the left kidney with the largest measuring 6 mm, all nonobstructive. The urologist recommended a CT scan ( I have been pushing it off until now because of the radiation And the fact that it would not have made a difference in the treatment ) to see exactly how many I have and also a urinalysis before my follow appointment next month, where we will decide if I need to have surgery/a procedure to remove them . From all I have read about potassium citrate I would like to start a regimen before my next appointment in the hopes it would shrink the stones that I have and prevent them from getting any bigger. Have also modified my diet and increase my water intake dramatically. I have tried Crystal light but to be honest cannot drink it. What would you recommend to start on the potassium citrate regimen (I am 110 lb)? Are there any OTC brand you prefer? I also purchased strips to test the urine pH. Thank you!
Hi Heather, things are confused. You may have uric acid stones or not. The CT can distinguish UA from calcium stones. I would indeed get it. If the stones have a low radiation density and if 24 hour urine pH is low one can certainly try potassium citrate. But you will need both the CT and proper blood and 24 hour testing to be sure you are not mistreating yourself. Rushing to remedy sans diagnosis is never a good idea. This article might be of some help. Regards, Fred Coe
Thanks Dr. Coe! I appreciate your prompt response. I will get all of the tests and w/b when I have the results.
Dr. Coe,
I have a question about potassium citrate vs. magnesium citrate. My last 24 hour urine test (Quest Diagnostics) showed my worst levels were Citrate at 349 mg/day (should be > 320) and Magnesium at 69 mg/day (should be > 60). My Potassium was 54 mEq/day, normal range 19-135. My Endocrinologist put me on two 10 mEq Potassium Citrate tablets per day. I am wondering if magnesium citrate would be as appropriate for me.
I have some Magnesium supplement by Natural Vitality, which is “A highly absorbable proprietary blend of magnesium carbonate and citric acid- which, in combination with water, creates ionic magnesium citrate.” The recommended dosage is 2 rounded teaspoons (4.5 g), Magnesium 350 mg.
I am still taking the potassium citrate, but saw your article about Crystal Light. I can’t use that because of the artificial sweetener. I am allergic to most of them, particularly Aspartame. One Diet Coke will make me so depressed I will go to bed for three days.
What would be the effect of the magnesium, compared to the potassium citrate, for me?
Rhonda Peach
Hi Rhonda, The magnesium product will not help a lot because the citric acid is simply lost in the urine – not as citrate and not helpful to you. The urine magnesium is fine – the Quest report is misleading and needs rewriting. I presume you make calcium oxalate stones and low urine citrate is your only problem. If so, use the potassium citrate. Regards, Fred Coe
Dr. Coe,
I forgot to mention that my stone (November 2017) was 90% calcium oxalate and 10% phosphate apatite.This is the first stone since 2002, when I had two stones. The values are from my last test, which was about two weeks after my last stone.
Rhonda Peach
Hi Rhonda, Sorry! I did not see this right away. So, use the potassium citrate. Regards, Fred Coe
I am a semi vegetarian who it’s some fish and on occasion chicken. History of extremely high blood pressure. I am trying to determine if taking 2400 mg of potassium would be helpful. I recently realized that I have only likely been getting 400-800 mg. Per day. I have a supplement here with it says “ 99 mg potassium (from 595 mg potassium gluconate). Wondering if I can take enough of those per day to reach 2400 mg per day RDA for HBP. If not what supplement would you recommend for getting the needed amount? Also wondering how long the potassium stays in the body? Can it be taken all at once and the body will work with it or does it eliminate in the urine like vitamin C? Thank you so much for your thoughts. Blessings.
Hi Julie, Potassium is best from foods and those are mainly fruits and veggies. Here is a good article on blood pressure management you might want to read. In it I have redacted the most recent blood pressure guidelines, which include all of the diet issues. It is tilted toward stone patients, but refers equally well to anyone with hypertension. As for potassium, most of it is inside our cells. As we take it in, the cells help themselves to what they want and let the rest go into the urine. If you are taking a diuretic, potassium depletion occurs often and should be repleted. Regards, Fred Coe
Thank you for your very interesting article, which I found researching a fix for my brother’s chronic kidney stone problem that’s been poorly treated by his doctors over the past 6-7 years. His urine is consistently in the 5.2-5.5 pH range. Stones were diagnosed as uric acid type. I’m trying to get him to stop eating so much meat and dairy as well as sugar and grains but I believe that he’d rather take pain killers and visit the doctor several times a year than start eating properly. He’s also pre-diabetic with high blood pressure, fatty liver and high triglycerides. Would potassium citrate be useful in correcting his kidney stone problem? I might be able to get him to take niacin to help reduce the triglyceride issue.
Hi Joe, indeed with uric acid stones potassium citrate is definitive treatment and nothing else much matters. He should stop refined sugars as they raise triglycerides. Regards, Fred Coe
Dr. Coe,
My blood Uric acid level is just above 7 mg/dL. I am suffering from frequent painful attacks of Gout. I drink about 2 Ltrs of water everyday and consume a non- vegetarian meal consisting of fish, chicken (do not consume red meat). Would you suggest that having Potassium Citrate will reduce the uric acid levels and save the kidneys from any uric acid crystallization?
Hi Jyothish, Potassium citrate has no role in gout. Sorry. You need other medications from your physicians. Regards, Fred Coe
DoesPotassium Citrate (Urocit K 1080) reduces the size of a bladder stone?
Hi Jake, if the stone has uric acid in it, the drug will do that. If not, no. Regards, Fred Coe