Diagnosis of MSK is Increasing
Urologists and nephrologists specializing in the treatment of kidney stones seem to encounter patients with medullary sponge kidney (MSK) far more often than one would predict given the fact that this disease affects less than 0.5-1% of the general population.
One potential explanation is the high frequency of stones in such patients, nearly 70%. Stones call attention to themselves. Even so, referrals for MSK seem to be occurring at ever increasing rates, out of proportion to the prevalence of the disease.
Another explanation is that we can see more because of dramatic improvements in CT scanners and endoscopic equipment we use for stone removal.
Latest generation, thin slice CT scans can isolate tiny stones as small as a millimeter in size. Meanwhile, modern flexible endoscopes not only visualize every crevice of the renal collecting system but are able to do so in high definition. Consequently we can identify previously unrecognized variations in the appearance of stone forming kidneys, of particular interest here nephrocalcinosis on CT and tubular plugging on endoscopy.
But diagnostic capabilities may be progressing faster than our ability to comprehend the significance of what we see. As a result, one can misclassify patients as having MSK when the correct diagnosis is another more common finding such as nephrocalcinosis or tubule plugging.
For example, a urological surgeon performing ureteroscopy with a modern high resolution digital instrument notices “hundreds of tiny stones,”, “abnormal papillary architecture” or “stones located under the urothelium” and proceeds to label the patient with MSK. Or, a patient with urologic symptoms such as renal colic, recurrent urinary tract infections, or microhematuria has a CT scan showing ‘nephrocalcinosis’ and is labeled as having MSK.
In both instances, the true likelihood of actually having MSK is, by a recent small study, only 4/15 (25%), but physicians are not generally aware of the differences between MSK, nephrocalcinosis, and tubule plugging because these are new areas of knowledge which have not been proliferated widely.
This article is one way we hope to make the diagnosis of MSK, a unique and complex disorder of renal development, clearer for physicians and their patients.
What Is MSK?
Our collective understanding regarding the development and pathophysiology of MSK is rather sparse even though G. Lenarduzzi first described it in 1939.
The Cause of MSK
The exact mechanisms that produce MSK are unknown. It is believed to be a result of abnormal renal development in utero. More specifically, scientists believe the ureteric bud – which will give rise to the ureters – interacts abnormally with the metanephric blastema tissue in the embryo which will produce much of the kidney substance.
There appears to be a genetic component to the disease. Recent evidence is that about half of patients diagnosed with MSK will have at least one relative with some degree of similar affliction. This kind of familial clustering can suggest an autosomal dominant gene expression or the actions of multiple genes giving that impression. The review of the above link is an excellent recent treatment of the matter of development and genetics which we highly recommend.
The Anatomy of MSK
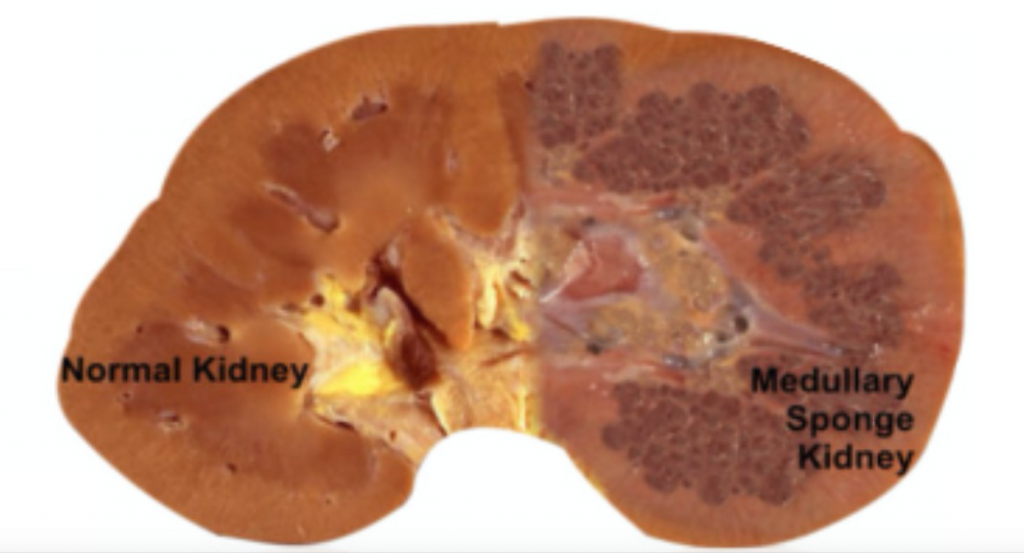
MSK, as its name implies, is characterized by sponge like, cavitary regions within one or both kidneys (Figure 1).
Figure 1 – A normal appearing kidney (left) compared to MSK kidney (right).
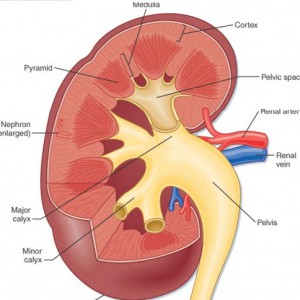
More specifically, the dilations occur in the inner medullary (precalyceal) collecting ducts. These collecting ducts are the terminal region of the nephrons, the individual functional units of the kidney.The inner medullary collecting ducts (IMCD) have the job of delivering urine to progressively larger ducts (ducts of Bellini) that deliver the final urine out of the renal papilla into the minor calyces (Figure 2) which connect into the renal pelvis and thence into the ureter to the bladder.
One way to think of the IMCD is as a collection of streams merging and forming a river (duct of Bellini) which leads to a delta (minor calyx) and then a lake (major calyx) and ultimately an ocean (renal pelvis) (Figure 2).
Figure 2 – Anatomic depiction of kidney and collecting system
The IMCDs are not visualized in Figure 2 but if you scan down to Figure 3 just below several human nephrons which were microdissected from an MSK kidney show what is really wrong with them.
In MSK, some IMCD are dilated markedly and have outpouchings (cysts): blind sacs which begin at the IMCD lumen but go nowhere – like a hallway someone walled off at one end. It is at the ends of these hallways one finds the stones, free floating and probably trouble. Other IMCD are not dilated and do not have cystic outpouchings.
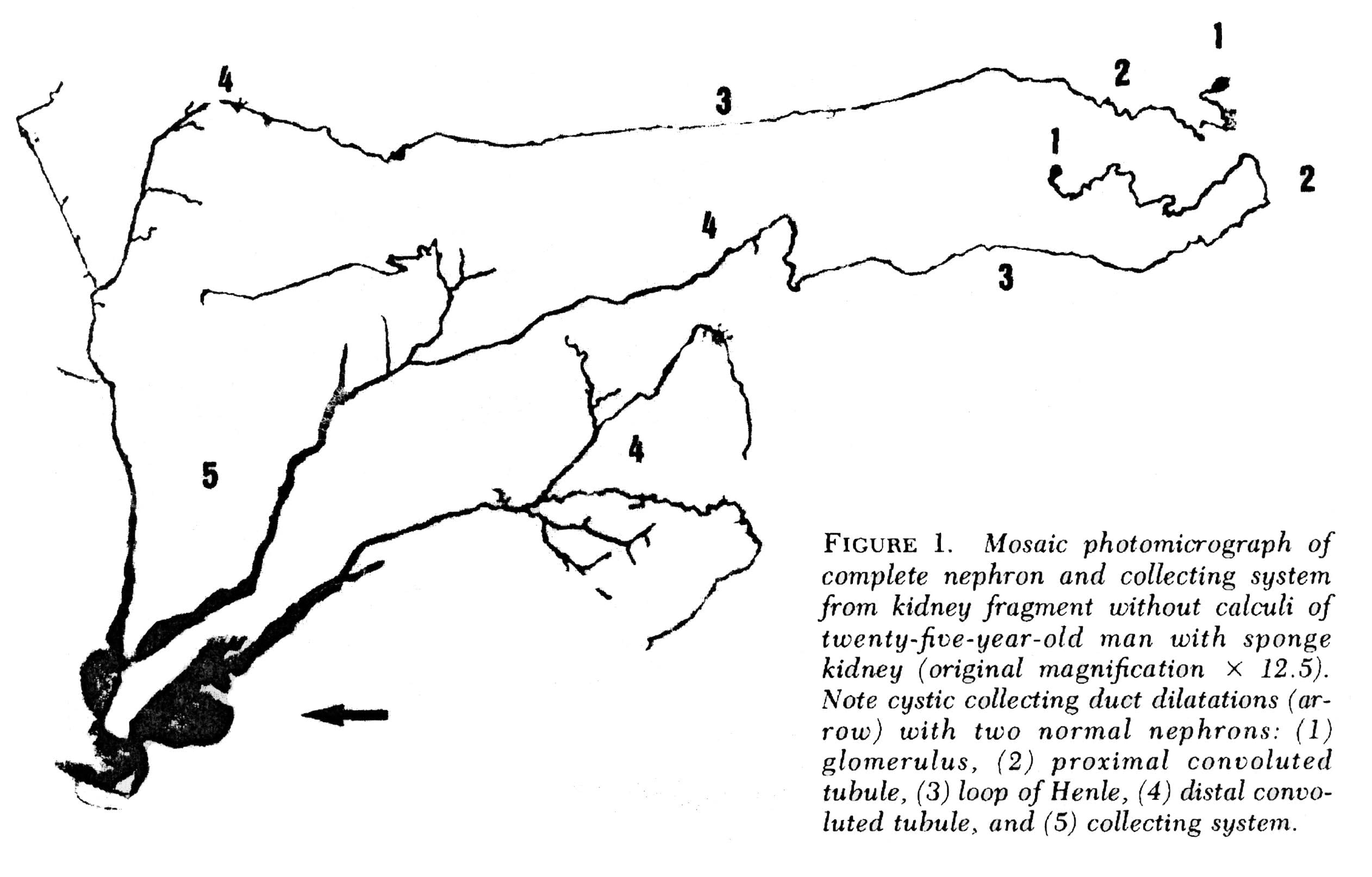 Figure 3 – Microdissection of complete nephrons from MSK. Irregular dilation of the IMCD is present. Cystic outpouchings are present, of varying sizes. The blind ends are obvious. It is in these cysts that tiny stones are found.
Figure 3 – Microdissection of complete nephrons from MSK. Irregular dilation of the IMCD is present. Cystic outpouchings are present, of varying sizes. The blind ends are obvious. It is in these cysts that tiny stones are found.
Quite apart from the IMCD dilatation and cysts, the kidneys of MSK have two other abnormalities which mark it as a specific disease. Those IMCD which are not dilated nor cystic have a multilayered epithelial lining, whereas a normal undilated IMCD lining is one cell layer thick. The interstitial cells of the renal papillum – the cells between the tubules and vessels – are more numerous than in normal kidneys, and have an immature appearance much like is seen in fetal kidneys.
Why Stones Form in MSK
Elsewhere on this site we have pointed out that supersaturation is a force, a source of energy directed at forming solid phases such as stone crystals. The kinetic retardants in urine, which include citrate, stave off crystallization but can never prevent it: A supersaturated solution will eventually collapse into two phases, crystals and a residual solution precisely at the solubility point, devoid of extra free energy.
The stagnant flow as a result of the dilated MSK IMCD, and particularly the static conditions in the fluid filled blind ended cysts, are the probable reason those innumerable tiny stones form which end up packing the ‘sponges’ with masses of crystals. How MSK patients produce larger stones, big enough to block the ureter is unknown. It is not true that MSK patients have remarkably high urine supersaturations or other physiologic abnormalities such as hypercalciuria and hypocitraturia. Possibly the tiny stones somehow leave their cysts and enter the urine where they act as nucleation centers.
Other Associations
Other common associations with MSK include urinary tract infection, microscopic and gross hematuria, and impaired renal function. Perhaps the stagnant flow in dilated IMCD and particularly in cysts, predisposes to infection. But since the papillae are abnormal in other ways it seems likely that these intrinsic abnormalities themselves must be clinically important, and more work needs to be done on the problem.
Making the Diagnosis of MSK
Of course, when we speak of what is wrong in the MSK kidneys, and how it affects people, we base everything on knowing that a given patient has MSK. If you have a kidney from such a patient and can show the dilated IMCD, the cysts, the tri-layered IMCD epithelium, and abnormal interstitial cells, diagnosis is certain. But what can we do when we are dealing with a patient?
Radiological Studies
Intravenous Urography
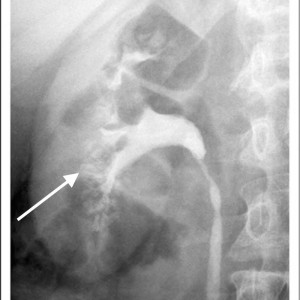
Historically, the diagnosis of MSK has been made on intravenous urography (IVU). Radiographic contrast is administered and x-rays of the kidneys, ureter, and bladder are taken periodically as the contrast is absorbed and later excreted by the kidneys through the urinary tract.
The delayed phase image is essential for diagnosis. This is when contrast material fills the masses of dilated IMCD and cysts which make up the sponge.
The masses of dilated IMCD filled with contrast material produce a characteristic ‘papillary blush’ which appears like flames on the outer edges of the papillae. When particularly large it can mimic a bouquet of flowers peripheral to the collecting system.
Figure 4 – IVU image of medullary sponge kidney. The arrow designates the papillary blush in the mass of dilated IMCD space.
CT Scans and Other Imaging
Over the past 10-15 years, noncontrast CT scans have replaced IVU as the imaging method of choice for stone formers. While these scans are more sensitive in detecting small stones, the lack of contrast limits the ability to accurately diagnose MSK and has raised concerns regarding the potential for under-diagnosis of this disease.
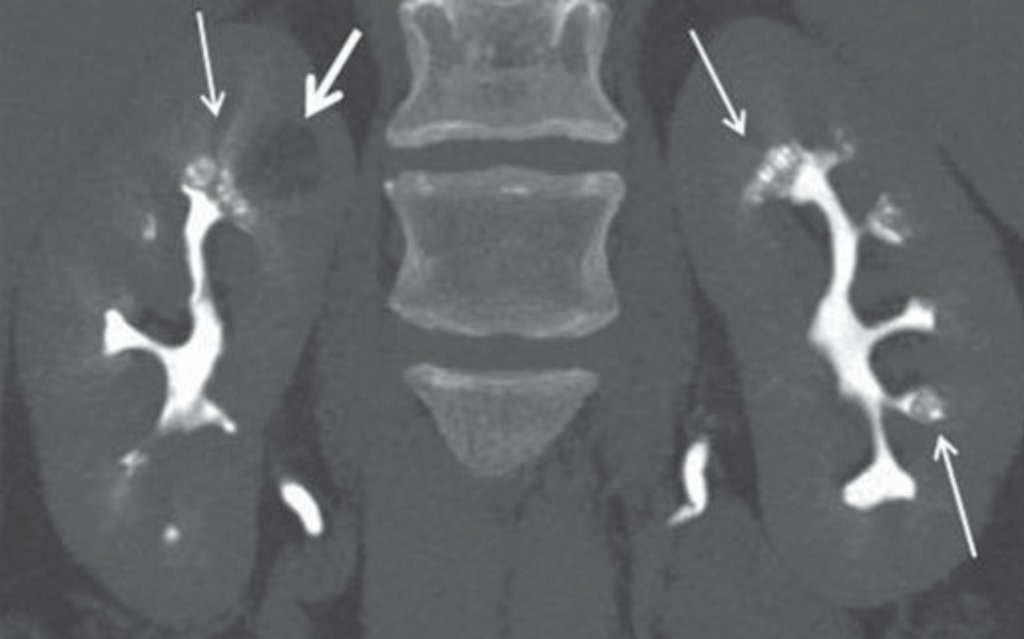
CT does have the capability of making this diagnosis when contrast is given and CT urography is performed; though this is not used as a first line choice for stone imaging and is generally reserved for specific indications such as hematuria and concern for urinary tract injuries.
Figure 5 – Demonstration of papillary blush (small arrows) on CT urography for patient with medullary sponge kidney.
Use of other imaging modalities have been investigated as well though they have proven suboptimal in their diagnostic capabilities. Ultrasound has poor sensitivity to detect dilation of the collecting ducts and MRI has the potential to delineate detailed renal anatomy but can not detect stones or calcification.
High Definition Endoscopy
State of the art high definition endoscopes have allowed investigators to make observations at the time of renal endoscopy correlating the appearance of the renal papillae and collecting system to specific types of diseases and metabolic derangements associated with stone formation. Because endoscopy is now being performed as a common and often preferred way to manage stones, this kind of detailed imaging of kidneys will be available more and more in the course of regular patient care and permit physicians to diagnose the exact kinds of disorders in the kidneys of stone formers.
Medullary sponge kidney in particular has an entirely unique appearance unlike any other type of stone related disease, making endoscopy a particularly exacting diagnostic procedure. Detailed anatomic descriptions from twelve such patients each with evidence of MSK on biopsy were recently described by Evan et al.
The Papillary Malformations Seen Via Endoscopy
In stone formers the papillae often appear abnormal, a concept that merits its own post and which we can only briefly summarize here.
Plaque
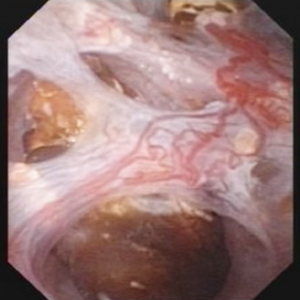
The renal 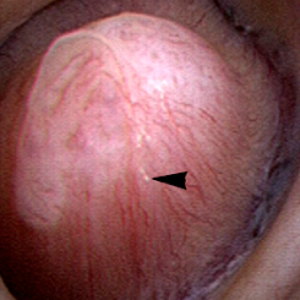 papilla is the anatomic subunit of the kidney where the IMCD merge together into the terminal Bellini ducts which empty into the minor calyx. Normally, it should have the appearance of a smooth walled mound or mountain without much if any calcification (plaque) along its surface. The link is to a detailed post about plaque by Dr Andrew Evan.
papilla is the anatomic subunit of the kidney where the IMCD merge together into the terminal Bellini ducts which empty into the minor calyx. Normally, it should have the appearance of a smooth walled mound or mountain without much if any calcification (plaque) along its surface. The link is to a detailed post about plaque by Dr Andrew Evan.
Figure 6 – Healthy appearing renal papilla with a minimal amount of Randall’s plaque (arrowhead).
Ductal Plugging
Another common abnormality identified in many papillae at the time of endoscopy are plugged ducts. The physiologic mechanisms for this process are currently unclear; 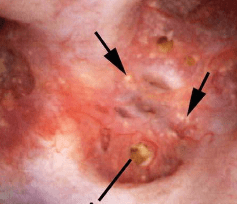 however, we believe it is a reflection of injury that begins a potentially disastrous chain of events for stone formers.
however, we believe it is a reflection of injury that begins a potentially disastrous chain of events for stone formers.
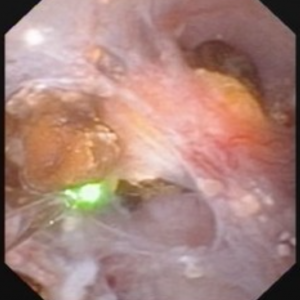
Figure 7 – Abnormal papilla in a patient with severe ductal plugging. Yellow mineral deposits (arrows) can be seen protruding from dilated ducts of Bellini.
Our present assumptions are that once crystals begin to form within a duct, they damage the lining cells and the duct loses its ability to make acidic urine. This in turn raises the local pH level and leads to the growth of more calcium phosphate mineral deposits which are favored by high pH.
Unclear is what begins this process. Since these ducts contain fluid which is very close in composition to the final urine, we suspect it is high supersaturation with respect to calcium phosphate. This occurs in those patients with both high urine calcium excretions (hypercalciuria) and higher urine pH levels – above 6.2. Such patients often form stones high in calcium phosphate composition and plugging is strongly associated with formation of such stones.
The mineral deposits subsequently grow and we believe can even act as a nidus for stone formation. The corresponding papillae can look markedly abnormal and the dilated ducts are easily evident. Remnant dilated ducts left behind after the mineral is spit out or surgically removed (Figure 8) show dilation without the mineral plug.
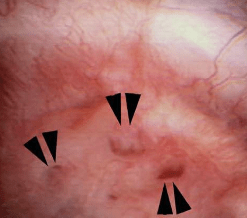 Figure 8 – Evidence of abnormally dilated ducts (arrowheads) at the surface of a papilla.
Figure 8 – Evidence of abnormally dilated ducts (arrowheads) at the surface of a papilla.
MSK
The findings in MSK are comparable in some ways to ductal plugging; however, rather than the papillae having one or several abnormally dilated ducts, the entirety of the papillum is markedly abnormal.
Therefore MSK and plugging type papillae look remarkably different.
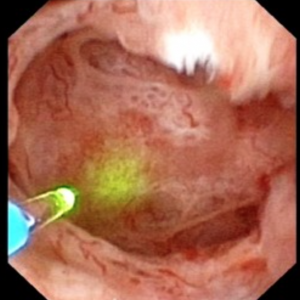
The MSK papillae are excessively round, enlarged, and billowy (Figure 9).
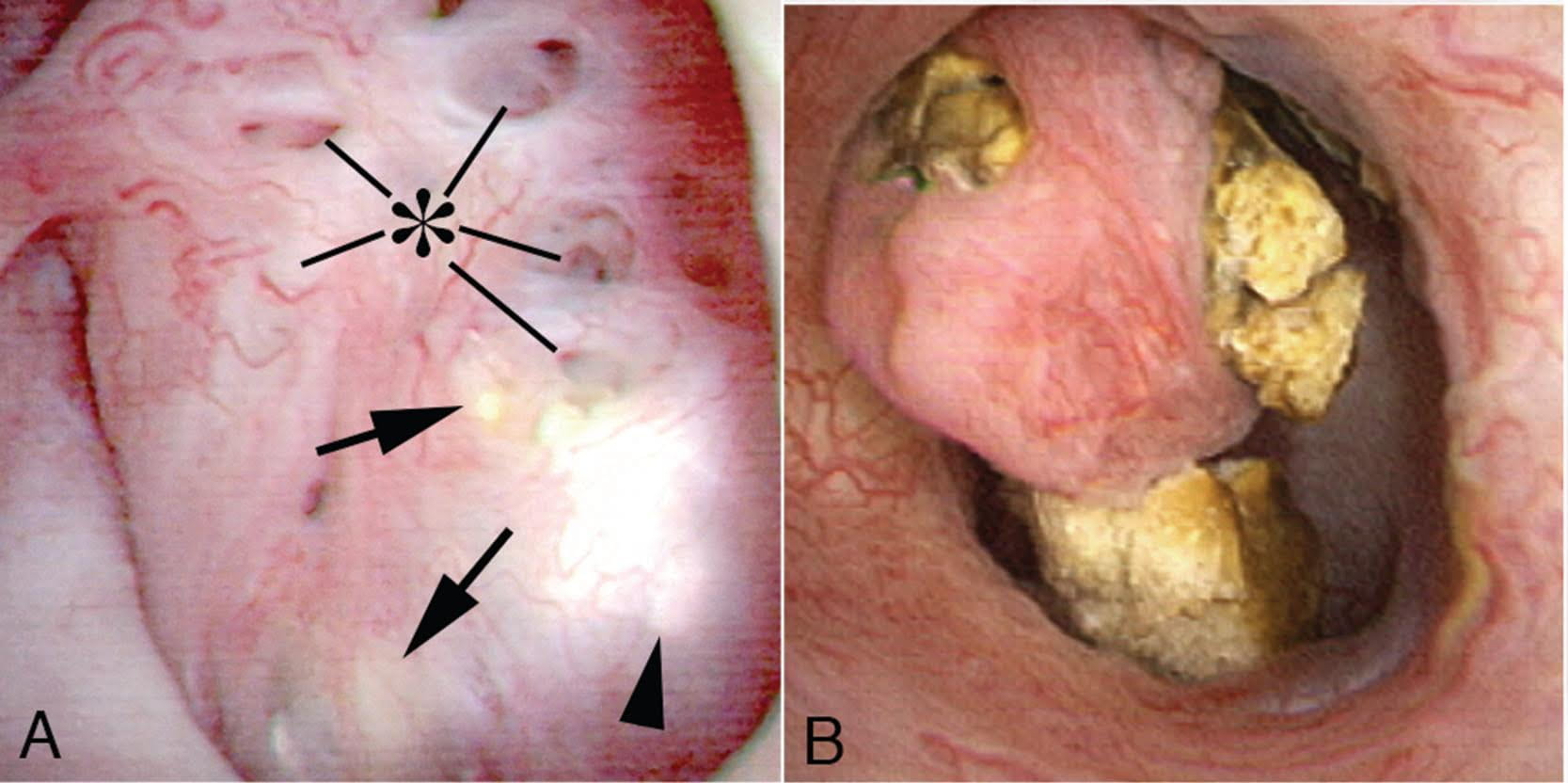 Figure 9 – A papillum in a patient with medullary sponge kidney. The papillae are rounded and enlarged with a billowy appearance. The papillary tips are blunted. No other kind of papillary disease is known to present this appearance which is therefore pathognomonic of MSK. Panel a shows dilated Bellini duct openings at asterisk; the arrows show yellow plaque – plugging of IMCD completely separate from the stones which fill dilated IMCD, the arrowheads show traces of white plaque. Panel b shows the billowy papillum with a blunted tip surrounded by calyceal stones.
Figure 9 – A papillum in a patient with medullary sponge kidney. The papillae are rounded and enlarged with a billowy appearance. The papillary tips are blunted. No other kind of papillary disease is known to present this appearance which is therefore pathognomonic of MSK. Panel a shows dilated Bellini duct openings at asterisk; the arrows show yellow plaque – plugging of IMCD completely separate from the stones which fill dilated IMCD, the arrowheads show traces of white plaque. Panel b shows the billowy papillum with a blunted tip surrounded by calyceal stones.
In the majority of such cases, these changes are seen diffusely throughout each kidney, though segmental sponge findings are present in a minority of patients.
The differences in appearance are well demonstrated in the post by Dr. Evan and the following video. With ductal plugging the bulk architecture of the papilla is intact though many ductal plugs are seen. Note the way the ductal plugs are adherent to the lumen.
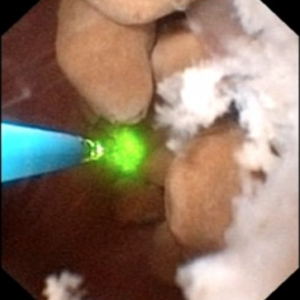
In MSK on the other hand, the papillary architecture has and unique, billowy appearance with massively dilated ducts and freely mobile stones within them. The following video is from a patient with MSK. A duct of Bellini is massively dilated and numerous tiny stones which are free floating pass out of it during the visualization as the opening is enlarged with a laser. You can see stones bouncing within the duct because of movement of the irrigation fluid.
Clearly neither papillum is “normal” and one can imagine the tendency to misdiagnose a plugging patient as a MSK patient unless the two patterns are clearly in one’s mind.
The ductal deposits themselves are an important clue. In all plugging diseases but cystinuria ductal mineral plugs are fixed within the ducts because the crystals adhere to the lining cells and often destroy them. As the lining cells are destroyed crystals fix themselves to the basement membranes, the collagen shell on which the lining cells once grew. Inflammation follows such injury with scarring and loss of the tubule segments. Deposits therefore never float free from a dilated duct except in cystinuria and in MSK. Cystinuria is usually diagnosed directly from stone analysis and urine cystine screening.
Perhaps because the tiny stones in MSK do not attach to the epithelium of IMCD there is no evidence of cell injury or inflammation, in marked contrast to all of the plugging diseases. Even in cystinuria injury occurs, not from the free floating distal cystine plugs but from calcium phosphate plugs which form in the IMCD.
Stones may cause pain in MSK despite the lack of ureteral obstruction and inflammation and cell injury. Possibly distention of the dilated ducts by masses of tiny stones could be a factor. Consequently, laser unroofing has been postulated as a potential treatment option in both disease states: those with plugging and MSK.
The video may not make it easy for everyone to visualize the stones and cavities of MSK. The 4 still pictures below show much the same thing for clarity.
Figure 10 (a-d) – Example of laser unroofing of sponge cavity full of freely floating stones. The massively dilated MSK bellini ducts are seen in Panel a; the white speckles at 9 o’clock are tiny stones in dilated IMCD or cysts. Panel b shows a holmium laser fiber being used to open dilated IMCD or cysts. Panel c shows masses of tiny stones free in dilated IMCD or cysts which float out as the surface is incised with the laser. In panel d, the remnant sponge cavity can be seen now free of stones.
Nephrocalcinosis and MSK
Strictly speaking, nephrocalcinosis refers to the presence of calcium deposits in the kidney tissue. Of course, this includes ductal plugging and the masses of tiny micro – stones inside cavities produced by numerous dilated IMCD in MSK. However, the word ‘nephrocalcinosis’ is also used as a radiological diagnosis which is far less specific.
Limitations of Radiology
When radiologists speak of nephrocalcinosis they can mean large numbers of calcifications within the collecting system or kidney tissue, because they cannot differentiate reliably between tissue calcifications and stones. When urologists speak of nephrocalcinosis seen during high resolution endoscopy they can specify if it refers to tissue calcifications, stones, or both, and will reserve the term for that component arising from tissue calcifications.
MSK is one of several disease states that is commonly associated with extensive nephrocalcinosis observed by radiologists. Other common conditions are stones caused by renal tubular acidosis and primary hyperparathyroidism. Calcium phosphate stone formers without any systemic disease can also produce sufficient combinations of ductal plugging and stones that nephrocalcinosis is diagnosed radiologically.
In a recent study of 67 idiopathic calcium stone forming patients undergoing percutaneous nephrolithotomy, rates of nephrocalcinosis ranged from 18-71% depending on the type of associated stone.
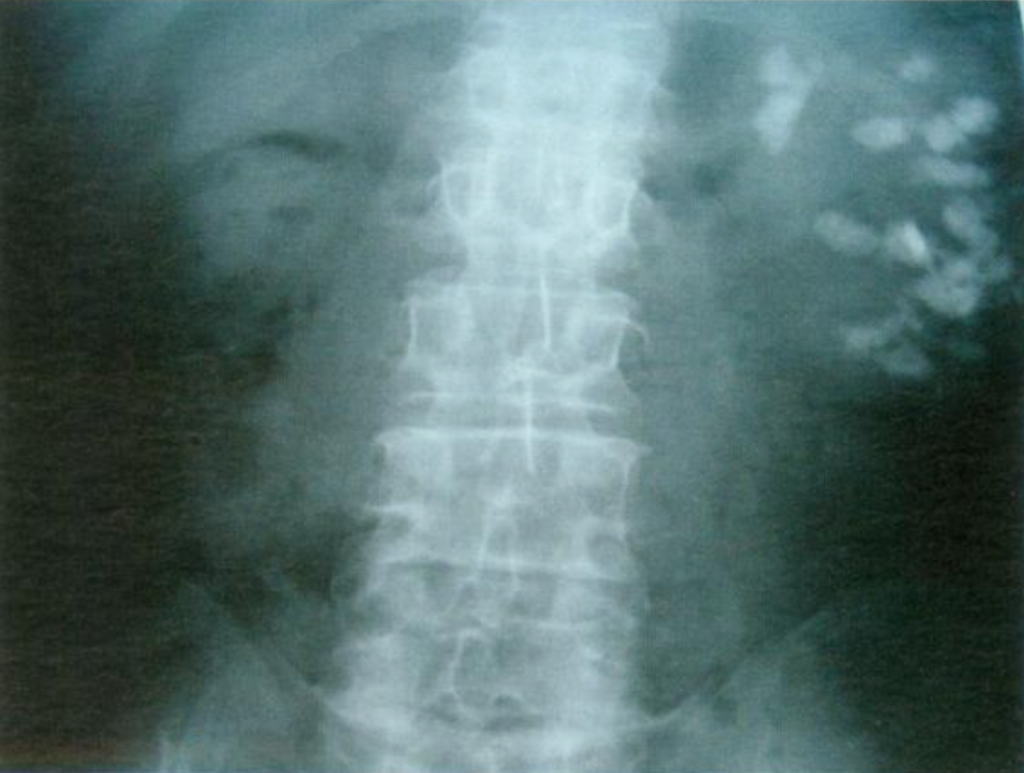
Figure 11- X-ray example of patient with MSK affecting the left kidney. Extensive nephrocalcinosis is seen.
Because radiographs cannot reliably distinguish tissue calcifications from stones adjacent to tissues, the very word ‘nephrocalcinosis’ needs to be re-defined.
In particular, the situation with respect to diagnosis of MSK has worsened as radiological techniques have changes. When IVU was the first line imaging modality for stones, contrast enhanced urographic phase imaging gave additional clues to MSK – the papillary blush illustrated in a prior section.
Nowadays since a single non-contrast CT series is all that is typically performed, the papillary blush effect cannot be seen, and diagnosis of MSK must rely more on the presence and pattern of nephrocalcinosis itself, which is not very specific to MSK. This means that the diagnosis of MSK by radiology has become unreliable.
The Power of Endoscopy
Differentiation of Nephrocalcinosis from Stones
During endoscopic procedures, stones and tissue calcifications can be directly identified and told apart. For example, in the images below, some of the calcifications were identified as stones. Others were tissue calcium deposits such as plugging or extensive plaque.
This has led to the notion that kidneys should only be labeled as having nephrocalcinosis once confirmed visually on endoscopy.
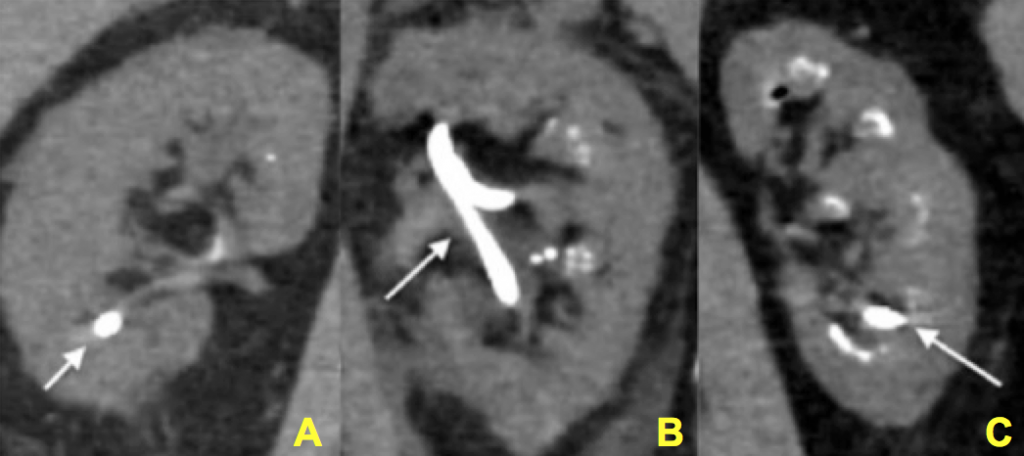
Figure 12 (A-C) – Evidence of increasing degrees of nephrocalcinosis confirmed visually at the time of percutaneous nephrolithotomy. In each image a nephrostomy tube (not calcification) is depicted by an arrow. The remainder of the images have increasing degrees of calcification (bright white) within the kidney. Ignoring the nephrostomy tube, one can clearly appreciate a minimal but very present degree of accessory brightness (calcification) in panel A, a moderate amount in panel B and a severe amount in panel C.
Diagnosis of MSK
Of course, all of the problems of nephrocalcinosis are compounded when it comes to MSK. Stones and tissue mineral are easily mistaken for one another in CT scans, and ‘MSK’ affixed as a label to patients who do not have it. Endoscopy will easily identify the remarkably abnormal papillary shapes and dilated sponges, so MSK patients are properly diagnosed.
Tips for diagnosing MSK
1) Confirmation with flexible renal endoscopy can make a definitive diagnosis in patients suspected to have MSK and can be diagnostic as well as potentially therapeutic in terms of stone removal.
2) Consider urographic phase imaging either with IVU or CTU to confirm MSK suspicion in cases where renal endoscopy is not clinically indicated.
3) MSK should not be confused with ductal plugging as these are distinct clinical entities.
4) Nephrocalcinosis is more common than previously appreciated and does not necessarily indicate systemic disease or the specific developmental disorder of MSK.
A Final Word on Treatment
MSK is a true disease and deserves more attention and research efforts to help clarify its etiology and optimize treatment strategies. Because diagnosis by CT scanning is unreliable, the condition is easily overdiagnosed, a problem which leads to many kinds of confusion, clinical and research.
Alternative diagnoses to MSK, such as severe ductal plugging, are not benign and require their own treatment in order to prevent progression. Very often patients with plugging have multiple and severe abnormalities of urine chemistry which can be treated with diet and medications. Proper classification of patients is therefore especially important as different disease states may require unique treatment strategies.
In the event that an accurate MSK diagnosis is made, the tendency to give up on treatment and surrender to the inherent challenges of the disease should be avoided. In fact, these are the patients where metabolic evaluations and attempts at stone prevention are most critical.
Moreover, just because many of these patients tend to have extensive nephrocalcinosis on imaging does not mean they can’t form symptomatic stones in the collecting system as well. In fact, for such patients the clinical history is especially important as visualizing new stones in the setting of extensive nephrocalcinosis can be quite challenging.
Oftentimes when there is a high index of suspicion based on clinical factors, the optimal approach is ureteroscopy as this can be both diagnostic and therapeutic.
That being said, realistic surgical goals should be established. Clearing all stones from such kidneys is rarely feasible, though unroofing those pockets closest to the collecting system or largest on CT imaging offers a good place to start.
MSK is a complex and poorly understood disease that can manifest uniquely from patient to patient. In that respect what works for one person may not be appropriate for another and treatment strategies should thus be organized on a patient to patient basis.



Hi! I know you are based on the USA… I need a urologist or nephrologist that has experience in MSK in Alberta Canada. No one is taking me seriously and my stones are growing at a fast rate and multiplying but they don’t pass.. just cause pain. I’m scared… the pain just gets worse and I need treatment.
Hi Dee, I know there is a fine stone program in Toronto at the large medical school there. Perhaps that is your best bet. I wish I could do more for you. Regards, Fred Coe
Hi, I recently got an MRI with contrast and unfortunately I totally forgot that I was diagnosed with MSK many years ago. When I search for information on if it’s safe, I‘m not finding anything specific to MSK but finding the possibility of nephrogenic systemic fibrosis (NSF) in people with impaired kidney function. Should I be concerned about NSF?
Hello Dr. Coe,
Brief history: 75 yrs. old, total stones over 22 years is approximately 30, R extra renal pelvis, MSK, multiple calyceal diverticulum with stones, narrow infundibulum (don’t know how many), 2 ureteroscopies with laser lithotripsy on R and 1 on the L over past 8 months. The surgeons notes on R (large stone load) and on both kidneys – “some calcifications appeared to be outside the collecting system either in the cortex or in calices which had strictured at the opening”. Mild narrowing of UVJ and distal ureter on both. I’ve searched and searched and can find no information regarding stones in the cortex – though ultrasound looks that way! Can they actually be in the cortex?
I found a new, excellent urologist/surgeon 10 months ago who, after 22 years, referred me to a nephrologist. I’ve done a lot of research on my own and also found Jill Harris…. MSK was acknowledged by other urologists, but that was the jest of it, along with drink plenty of waster, don’t eat spinach and almonds and watch the chocolate intake.
I have had osteopenia for 28 years and just went into osteoporosis and my PCP wants to put me on medication for it, but medications and I don’t get along – bad side effects that can linger for days to a couple of weeks following the final dose (my father was the same).
My question after this article and reading “slow filtration… stagnant flow…”:
Since medications are excreted in the urine, is that one reason why the side effects can linger? Are they not getting out of my body as fast? With so many side effects of bisphosphonates, it makes me very nervous to take any of them.
This is a wonderful and informative site.
Thank you so much for your time,
Marla
Hi Marla, MSK is a hard diagnosis to make. Many patients with calcium phosphate stones have a picture easily confused with MSK. Quite possibly you have idiopathic hypercalciuria and calcium stones, the former can cause osteoporosis. If so that will help direct stone and bone treatment. TO know the causes of both get a comprehensive evaluation – this is my best on the subject. Regards, Fred Coe
Thank you Dr. Coe, but I’m a bit confused = these findings could be incorrect?
Results of IVP With/Without Tomography from 2004 – “There is bilateral fan-like opacification of the renal tubules… Impression: Bilateral renal tubular ectasia (medullary sponge kidney) with multiple small bilateral renal calculi.” (looks to be confirmed with CT IVU w/ + w/o contrast and intraoperative retrograde pyelogram in 2022). I was also told by the surgeon that there was indeed plaque. Along with larger stones, there are multiple areas with clusters of small stones – 3 to 7 per cluster. His words, “ Your kidneys are an anatomical, probably congenital, mess.” (Thanks mom and dad = lol.)
I don’t have calcium phosphate stones. My stones analysis vary somewhat:
1.) Calcium Oxalate Monohydrate (Whewellite) 97% and Calcium phosphate (Apatite) 3%
2 & 3.) Calcium Oxalate Dihydrate (Weddellite) 20% and Calcium Oxalate Monohydrate (Whewellite) 80%
Comprehensive renal panels and 24-hour urines have been done (due again in 1 month) – primary results reported…. hypercalciuria and what ‘was’ urine pH of 5.4, even though report said dietary protein did not seem to be the cause (now, after dietary changes – very little meat, it’s 6.5). ??? I’ll see if it is sustained in next 24-hour urine.
It just seems to make sense to me that if MSK has “slow filtration and stagnant flow” that medications would stay in the system for a longer length of time. No doubt I could be incorrect!
Bottom line: I can’t change any of the anatomical characteristics of my kidneys, so just keep keeping on with the Kidney Stone Diet.
Thank you,
Marla
Hi Marla, Given the findings MSK seems probable. Calcium oxalate stones are the rule. The urine pH is not important for calcium oxalate. Glomerular filtration rate can be normal in MSK. The problem is that the sponge regions are dilated and flow though them is slow for their volume (like a narrow river widens and becomes a sluggish marsh. Crystals seem to form because urine dwells in this ‘marshy’ area, and there is time for the almost universal calcium oxalate supersaturation to dissipate in crystallization. High urine flow is use, and if urine calcium loss is elevated lowering it would also be predicted to help. Medications are cleared from the blood normally. The kidney stone diet included low diet sodium that will lower urine calcium, but if you are forming new stones thiazide is a good idea – chlorthalidone or indapamide and possibilities your physicians might want to consider. Regards, Fred Coe
Hello Dr I wonder if you can give any input regarding my situation. All I can give you is my latest CT scan results. I’ve had a lot of fluid retention and been in a lot of pain. Further investigation here (Australia) costs a lot of money. Any advice would be great.
CT
FINDINGS
The nonenhanced scan shows increased density along the renal pyramids on both sides with early calcific formation in the left kidney mid zone. The appearances are suspicious for medullary sponge kidney with early calcinosis. In the nephrographic phase there is symmetrical nephrogram. The medullary enhancement is more intense. There are multiple cysts of varying sizes centred in the medullary region the largest cyst in the inferior pole of right kidney measures 17mm and the largest cyst in the superior pole left kidney measures 14mm. There is no hydronephrosis and no perinephric fat stranding.
On the delayed phase there is good pacification of the renal collecting system. There is no obstructing calculus in the ureters on either side.
The urinary bladder is well distended and normal.
Hi Jesse, I gather you have kidney stones. Fluid retention is not generally part of stone disease, however, so I do not understand it in your case. The report hints as calcium deposits in the kidney but good function of the kidneys and normal drainage. Pain would not be expected from this CT scan – no obstruction. But sometimes people who form calcifications pass crystals that are missed on CT but cause pain and urinary bleeding. Physicians can see them in the urine using a microscope. You need medical care if you have pain and for stone prevention. Here is a common kind of evaluation. MSK is not diagnosed well by CT, so I am not at all convinced as yet. Best, Fred Coe
Thank you for writing back to me. I didn’t expect an answer so quickly. That’s some really good information I’ll be checking out right away. Thanks again.
I know that it has been several years since “The Diagnostic Dilemma of MSK” was published, however my daughter has true blue MSK. Her rock collection started about 10 years ago when she was in her mid 30’s. Her condition has progressed to the point of exponentially declining quality of life. About every 2 months she ends up in the hospital, uroseptic with either an occluding stone or her stent is occluded and she is in excruciating pain. The CT always shows the same thing “numerous stones, too many to count measuring up to 26mm”. Every time this happens, again approx. ever 2 months, she gets her stents replaced with a hospital stay of 5-7 days for Abx and usually a bicarb gtt. On her last hospital stay her Co2 level was 9!! I don’t know if this is related to the MSK or not, but her baseline creatinine is around 2.5.
Anyway moving along, the reason I am reaching out to you is to inquire about a possible trial or study that she may be able to participate in? I mean at this point she is willing to try ANYTHING that may help because, as you know, this is never going to end.
Any info you could give us would be so very appreciated. Thank you for your time.
Hi Jackie, a serum bicarbonate of 9 is either due to sepsis or renal tubular acidosis. MSK alone does not do this. She has serious decline in renal function. I know nothing more about her but advise her physicians might want to refer her to a university kidney program – it she is not already at one. If she has MSK, as you say, and is having recurring sepsis with low CO2 levels, that is not a workable outcome. If it is renal tubular acidosis, the same. Given her very serious loss of overall kidney function she needs whatever special skills she can get. Sorry to have so little specific but I do not know enough about her case to do more. Best, Fred Coe
Dr. Coe. I am a MSK patient and member of the Facebook MSK research group, since 2013.
I just want to sincerely thank you for all the work you do, in understanding this disease, as well as the help that you offer so many by replying to questions in posts like this.
Thank you, again!
Hi Heather, Lovely of you to write this. Best, Fred
I read your article and comments twice. It was very well written. I was diagnosed 15 years ago with msk. I pass stones regularly and am always pretending I’m not in pain. I’m tired of being told I shouldn’t be in pain because my stones are small. I live in “the kidney stone belt” and am told I will always suffer from stones. I recently read another article stating that laser lithotripsy can remove smaller stones and provide up to two years of relief from pain. What, if any, information do you have regarding laser lithotripsy? I hate narcotics for a million reasons and ketororolac ( not sure of the spelling) which works well is restricted because I’ve been told it’s bad for kidneys.
Hi Andrea, MSK produces calcium oxalate stones, you do not say what yours are. Have you been evaluated for causes? What are your 24 hour urine calcium, oxalate, pH, citate etc?? Are there things that can be treated?? Perhaps a lot can be done, but I do not have enough to go on right now. Best, Fred Coe