Unlike Zeus, or Athene, Janus did not come to Rome from Greece but from myths about a person living early in Roman history and later deified. Janus – deity – presides over beginnings and endings, gateways and doors, invariably dual in nature.
For the Full Video – 19 Minutes
For the Shorter Version – 11 Minutes
What is dual here?
Calcium stone formers are dual. A minority arise from systemic diseases we must screen for. Each systemic disease has its own universe of causes and treatment decisions. A majority are “idiopathic”, systemic causes have been excluded.
Idiopathic calcium stone formers are dual. They have no systemic cause of calcium stones. Most form calcium oxalate stones. A minority, more women than men, form calcium phosphate (CaP) stones.
Idiopathic calcium phosphate stone formers are dual. Most have hydroxyapatite (HA, like bone mineral) as their stone calcium phosphate. Some have brushite (Br, calcium monohydrogen phosphate) in their stones. These latter have more kidney damage than HA CaP stone formers, and are a special high risk group of patients.
Both kinds of CaP stone formers need special attention. That is why I have written this article for them.
Basic Facts about Phosphate Stone Formers
Phosphate Stones Damage Kidneys
Phosphate stones, HA or Br, can grow faster and larger than calcium oxalate ones. Calcium phosphate crystals invade kidney tissue – so called tubule plugs. Tissue damage is common, as is Nephrocalcinosis from plugging – often misdiagnosed as medullary sponge kidney. Kidney tissue damage is worse with Br than HA stones. Potassium citrate, a common stone prevention, may not be appropriate as a treatment because it raises urine pH.
Alkaline Urine Causes Phosphate Stones
Stone phosphate replaces oxalate when urine is too alkaline. Kidney and GI tract physiology raise urine pH, especially in women. Diet is not the cause of higher urine pH. Diet will not reliably lower the pH, and we have no specific drugs to do it, either. So although treatment uses the same tactics as for the more common calcium oxalate patient, it must follow a different strategy.
How Stone Analysis Distinguishes CaP from CaOx Stone Formers
Only CaOx and HA Present
If the average stone mineral composition of all available stones for a given patient is above 50% calcium oxalate, the patient is considered a calcium oxalate (CaOx) stone former. If the average calcium phosphate content is above 50% the patient is considered a calcium phosphate stone former.
The average must be computed using 0 – for example, given CaOx/CaP percentages of 100/0, 0/100, 40/60, the correct classification is 140/3 vs 160/3 or 46% CaOx vs 53%, and so a CaP stone former.
Uric Acid, Struvite, Cystine Also Present
If uric acid, struvite, or cystine are present we name the patient for that constituent. A patient who forms mixed stones – for example, 60% calcium phosphate/20% struvite 20% CaOx is called a struvite stone former. The reason is that these stone types have special causes and treatments.
Any Brushite Present
Brushite is very uncommon in human kidney stones, and associates with large tubule plugs and more severe tissue damage. So when any stone contains brushite we classify the patient as a brushite stone former even though brushite is a minority of stone mineral.
Sex and Age
Single Clinic Experience
Percentages of Cases
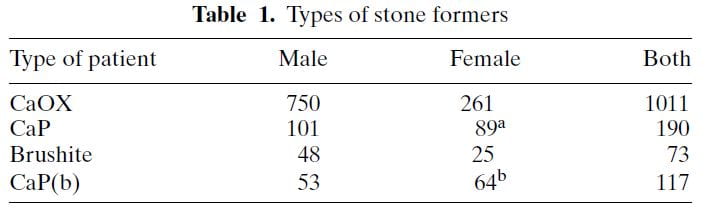 The table shows ‘CaP’ as cases where HA or brushite was the stone phosphate crystal (in early years we did not distinguish). CaP(b) are CaP stone formers with only HA, no brushite in any stone.
The table shows ‘CaP’ as cases where HA or brushite was the stone phosphate crystal (in early years we did not distinguish). CaP(b) are CaP stone formers with only HA, no brushite in any stone.
CaP predominate among females (a and b superscripts denote outsize high frequencies). Brushite does not show this difference a statistical level of significance. CaOx stone formers predominate among the total of all cases. Brushite patients are least common.
Sex vs. Percent CaP in Stones
The same study furnished this nice graph showing the sexes as the percent of stone CaP increases. The bulk of patients have very little CaP in stones (tall bars at the left of the graph). These are the common CaOx stone formers, mainly men (% female, dots, right axis about 25%). But when CaP percent is 20 – 50% in stones, women and men are nearly equal.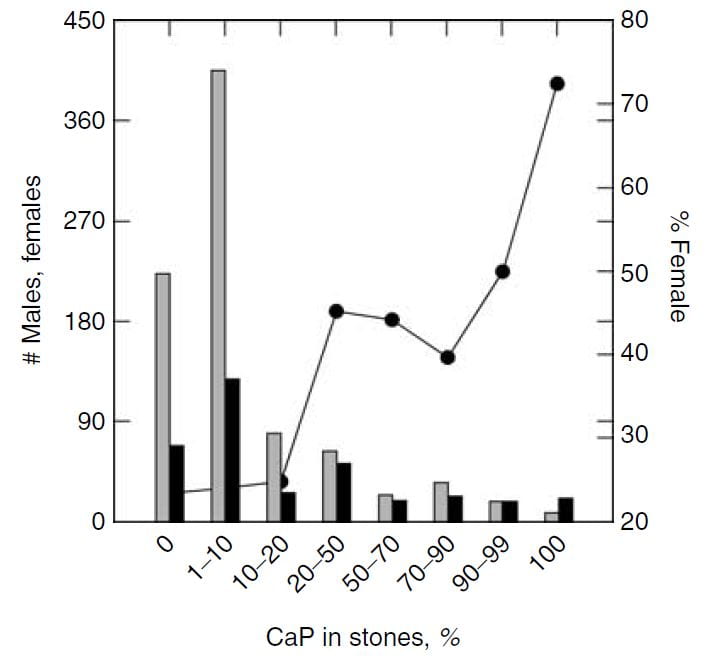
This graph blurs the sex distinction because we used stone CaP% from both brushite and hydroxyapatite. Today, I would have left the brushite to one side, which would have made the female preponderance among those with high stone CaP% more marked – because the sex ratio for brushite stone formers is closer to 1.
National Laboratory Findings
The Mayo Clinic kidney stone analysis laboratory analyzed 48,446 stones in 2010, and of these 43,545 were the first submitted to the lab for that person. From these stones, they report the distribution of stone type by sex and age. I have made a graph from Table 2 of their publication.
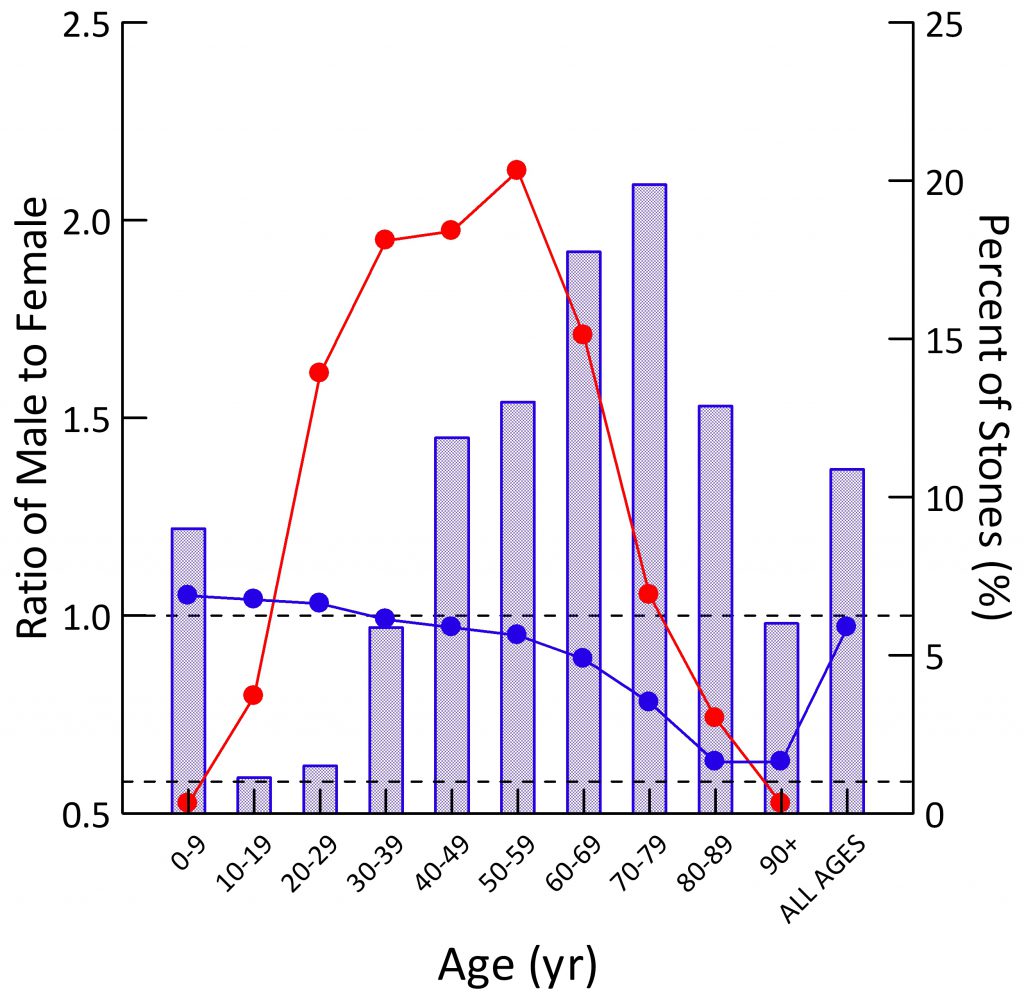 Population Sex Ratio
Population Sex Ratio
The general population contains more males than females at younger ages (blue dots). By age 30-39 the two sexes are present in equal numbers. Thereafter, as men predecease women, their blue dots slump downward.
For all ages combined, the ratio of men to women is just under 1 (last blue dot at right).
Stone Former Sex Ratio
The blue bars show male to female ratios among stone formers. Remember this is counted from the sex of the person whose stone was analyzed. A survey based on symptomatic rates of stone passage, by contrast, might give different results altogether.
In childhood, men have slightly more stones than women (blue bar is above 1.0). In the teen years and up to age 39, women predominate over men (blue bars are below 1.0). After age 40 men predominate, until at age 90 and more, in this and perhaps most things, the sexes come into a near perfect alignment. Averaged over all of life, men have more stones, which appears to be because of their midlife excesses (Height of the ‘ALL AGES’ bar above 1.0).
The fraction of all stones formed (red dots; scale along the right axis) for both sexes combined is highest from age 20-69, with only a small fraction in childhood or old age.
Types of Stones
The men are on top, women on the bottom of the picture to the left.
Stones were classified using the system I have used on this site. Uric acid in any amount meant the stones were classified as uric acid stones, and likewise for any struvite or cystine.
CaOx stones preponderate among both sexes over all ages, except in women between ages 20 – 39 stones were about half CaOx and HA. With age, HA stone frequency fell in both sexes, so that most men, and most older women (over 40) have CaOx stones.
Brushite stones, in both sexes, are very uncommon. You can see them as triangles along the bottom of both graphs.
Over age 50, uric acid stones become a significant concern in both sexes.
Struvite stones, which always arise from infection with bacteria that possess urease, are more common in women than men, a fact known for ages.
The Mystery of Brushite
Brushite stones are rare but should be rarer still. I have written a whole article on brushite because it is so important and yet so evanescent. It forms first of all crystals in human urine. If pH is not too high, oxalate steals away its calcium atoms so it vanishes. If pH is high, HA does the same, and brushite vanishes.
Why, then, are there any brushite stone formers?
I do not know nor does anyone I know of. It is an open question that seems obscure but whose answer might well lead to some new understanding of how stones form.
The Importance of Brushite
Being the first crystal to form, brushite supersaturation is crucial for stone prevention, a fact not intuitive but worthy of special emphasis. Rare in stones, vanishing in most urine, yet brushite supersaturation is foremost in importance for clinicians and patients. The goal is a supersaturation below 1, so brushite cannot form. For those who want to know more about why, please look at the parent article.
Time and Shock Wave Lithotripsy
We (left hand figure below) and others have noted an increasing percent of CaP in stones over the past 30 years. In women (black dots) CaP percent is always higher than in men, but it has risen in both. For those of a quantitative bent, the time trend of stone CaP tested by ANOVA with post hoc contrasts was significant for both sexes, and women were higher than men throughout.
In the publication, we found a relationship between CaP 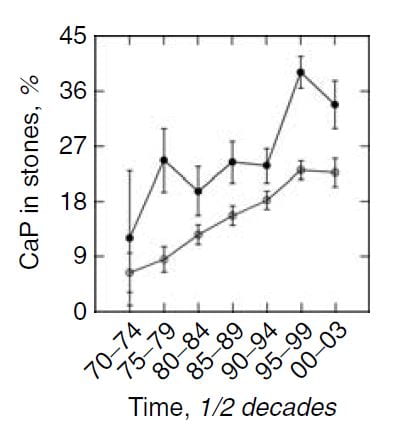 percentage and numbers of shock wave lithotripsy procedures. Use of potassium citrate, however, did not seem to increase stone CaP.
percentage and numbers of shock wave lithotripsy procedures. Use of potassium citrate, however, did not seem to increase stone CaP.
The number of shock wave procedures per patient adjusted for the number of stones and the years of stone disease rose with the percent of CaP in stones (Panel A of the figure below to the right) and the percent of CaP likewise adjusted for number of stones and duration of stones and sex rose progressively with the number of shock wave procedures (Panel B of figure to the lower right).
Not shown here, but of interest, the number of shock wave treatments was higher among BRSF than HASF suggesting a link between shock wave treatment and brushite stones.
One might infer from this set of graphs that the advent of shock wave lithotripsy caused the increase in phosphate stones, and there is nothing to contradict the idea. In fact, the very physiology of phosphate stone formation and the effects of shock waves on kidney 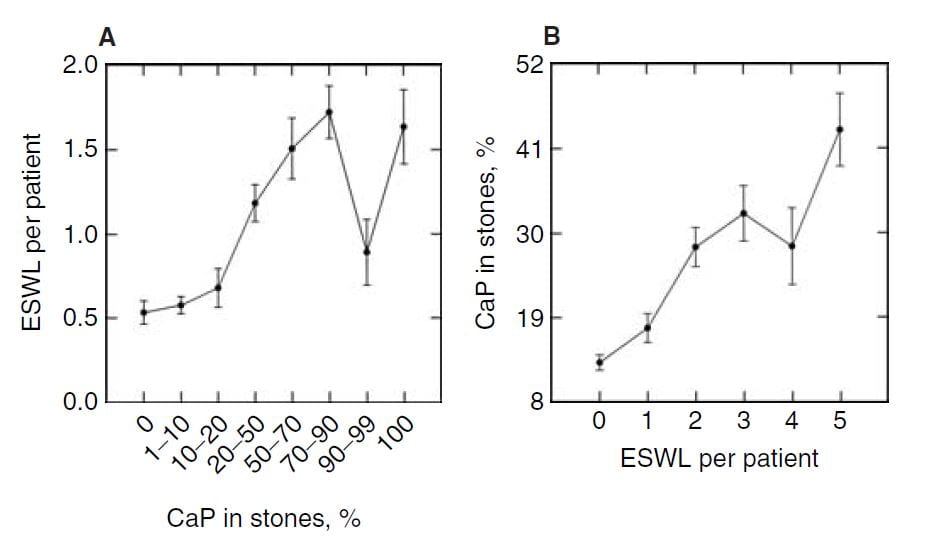 function strongly support that idea as I shall show you.
function strongly support that idea as I shall show you.
Mechanisms of Phosphate Stone Formation
High Urine pH
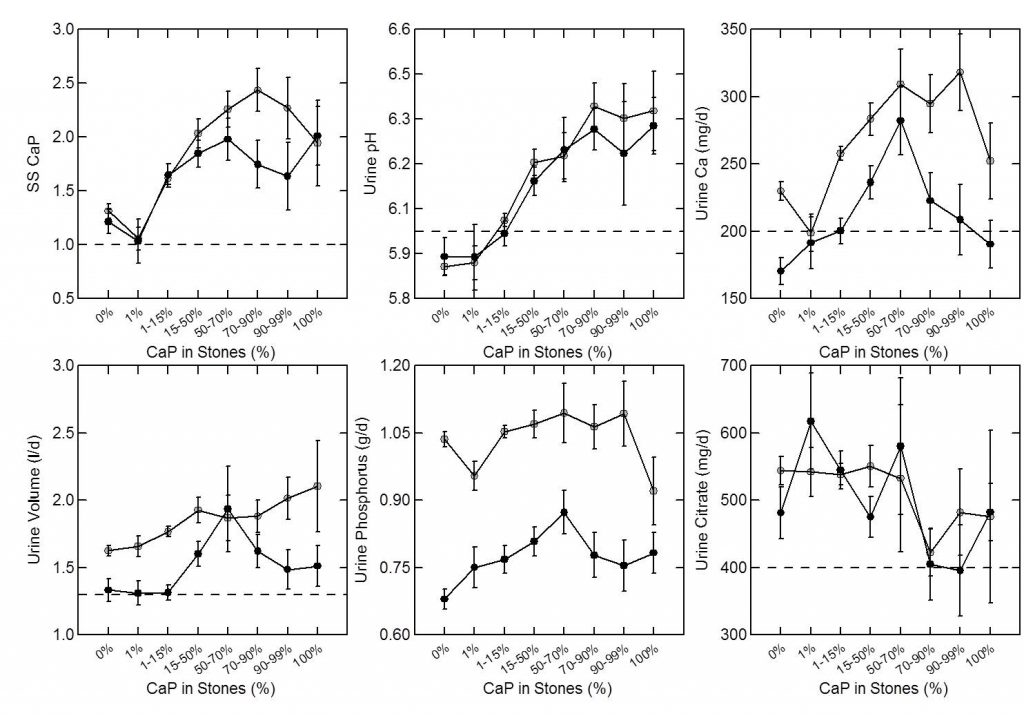
As expected, percent CaP in stones (upper left panel of the figure below) rises with CaP SS. I have shown elsewhere on this site that stone crystals parallel urine supersaturations.
Because CaP SS depends powerfully on urine pH one expects and finds (upper middle panel) that urine pH tracks very closely with stone CaP percent. Urine calcium, volume, phosphate, and citrate excretions (remaining panels) had no important relationship to stone CaP percent.
But take a look at the urine calcium excretions (Upper right panel). They are very high on average. This is because a high fraction of all calcium stone formers have genetic (idiopathic) hypercalciuria. Risk for stone forming begins at a urine calcium of 200 mg/d in both sexes.
So you can think of CaP stones as a two hit model.
Genetic hypercalciuria promotes calcium stones, and urine pH controls the fraction of phosphate in the stones. High CaP SS and CaP stones require a urine pH significantly above 6 as shown in the upper middle panel.
Kidney Tissue
Plaque and Plugs
CaOx stones can be produced as overgrowths in interstitial HA deposits, called plaque.
Idiopathic CaP stone formers, and patients with stones from bowel disease, ileostomy, renal tubular acidosis, and primary hyperparathyroidism, form stones on plaque but also on plugs of HA that fill and damage the last millimeter or so of the nephron, the inner medullary collecting ducts and ducts of Bellini.
Although we are not certain, I think it is fair to say that the plugging of CaP stone formers is because more CaP crystals form in urine and can produce plugging. In a recent article I trace out how calcium phosphate actually forms, how it is a rapid process compared to calcium oxalate, and therefore more able to make plugs during the short times it takes for urine to pass out of tubules into the renal pelvis.
Distinctions Among the Three Idiopathic Calcium Stone Formers
We have 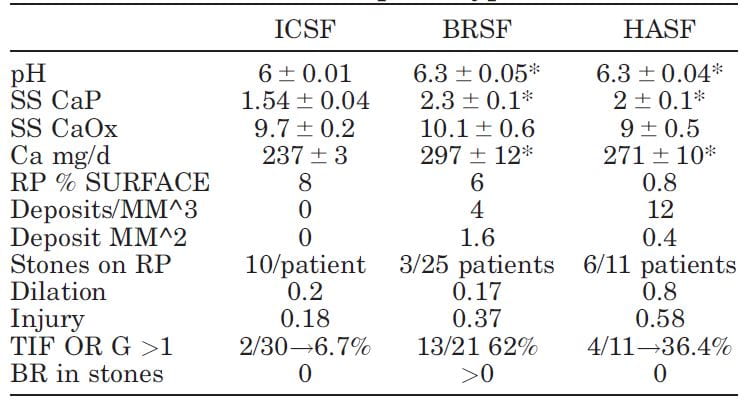 published selected laboratory and tissue findings of CaOx, brushite and HA stone formers, in an attempt to clarify differences in how stones form, and amounts of tissue injury.
published selected laboratory and tissue findings of CaOx, brushite and HA stone formers, in an attempt to clarify differences in how stones form, and amounts of tissue injury.
Numbers are small because each patient had been studied with intra-operative imaging of the renal papillae and papillary biopsy: 11 CaP (HASF), 25 BR (BRSF), and 30 CaOx (ICSF) stone formers.
As expected, urine pH was higher in the BRSF and HASF than in the ICSF, as was supersaturation (SS) for CaP. Incidentally, urine calcium (Ca) was also higher in both CaP groups than in the ICSF.
Mainly CaOx and BR stone formers formed plaque, and mainly CaOx SF form stones on it. About 8% and 6% of papillary surfaces were covered with plaque in ICSF and BRSF but only 0.8% among the HASF. CaOx stone formers had an average of 10 stones/patient attached to plaque, vs. only 3 plaque stones in 25 BRSF and 6 in 11 HASF stone patients: 10/ CaOx patient vs 0.12/brushite patient and 0.54/HA patient. These are 80 and 18 fold differences, respectively!
Plugging (‘deposits’ in the table) was absent in ICSF, but common in BRSF and HASF. Plug size averaged 1.6 mm2 in BSRF but only 0.4 mm2 in HASF – a 4 fold difference. The number of plugs was 3 times higher in HA vs. Br patients: 12 vs. 3/mm3 of tissue volume. BRSF formed fewer but much bigger tubule plugs.
Calyx dilation (a abnormal finding) estimated during surgery was higher in HASF than in the other two groups, and papillary injury (papillae are the parts of kidneys inside calyces) higher in both phosphate groups than in ICSF.
In the kidney cortex, far from where stones form, many CaP stone formers had scarring (TIF, tubular interstitial fibrosis) vs. very few CaOx patients. Brushite patients had most cortical damage.
So phosphate stone formers have injury involving the papillae and cortex, whereas CaOx stone formers have almost none.
Why is Urine pH High?
Being Female
I wrote a whole article on how women raise their urine pH. They do it by absorbing from their food a higher fraction of its alkali content. No sense copying all that here, it is better to read the article. High GI alkali absorption is not easy to treat. Those alkali are nutrient – anions that cells metabolize to get energy.
Being Young
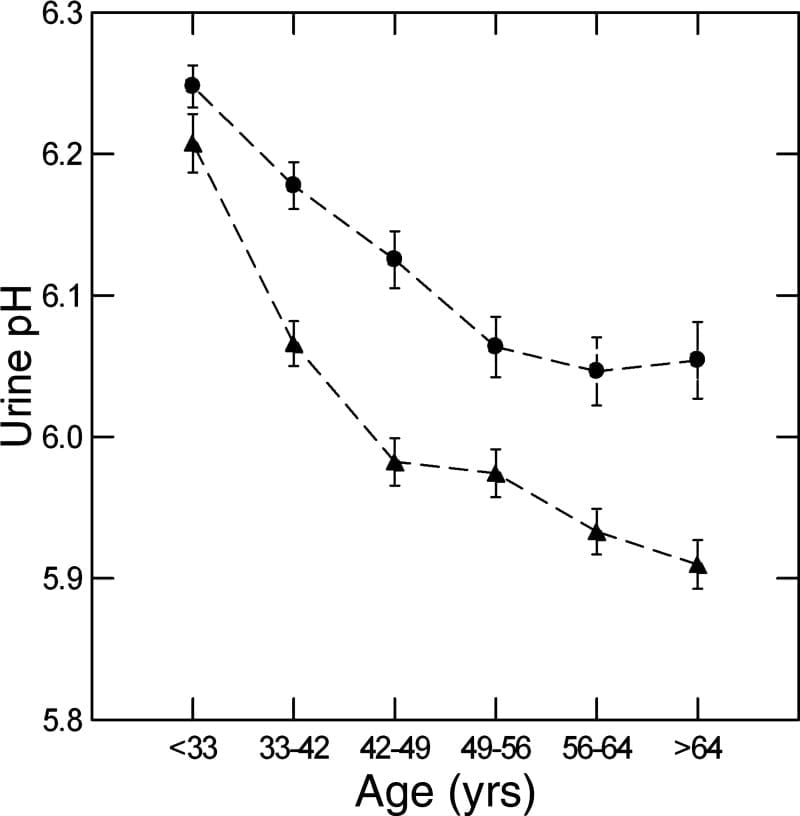
We used a massive database of kidney stone formers to ask what happened to urine pH in men and women with age. The answer is it falls, in both sexes (women are circles, men triangles).
Why is a long story. We could exclude gain in BMI, loss of kidney function, and GI alkali as reasons, but could not find the reason itself. In fact, GI anion absorption rose with age, as if to offset the falling pH.
Here the important fact is on the graph – higher pH in women and in youth are an obvious cause of more CaP stones.
Shock Wave Lithotripsy (SWL)
No practical experiments permit us to measure effects of SWL on urine pH in humans.
For these reasons we turned to an animal model: The farm pig whose kidney is much like that of a human, and likewise is similar in size.
In these animals we could shock one kidney, and then compare the treated to control side at time intervals after the treatment, the untreated side being a perfect control as both kidneys are bathed by the same blood.
SWL Raised Urine pH.
Urine pH from the treated kidneys was 0.18 pH units higher than the control, meaning SWL had increased urine pH (first line of table under ‘Basal’).
SWL Damaged Kidney Tubule Function
There was a lot more.
Urine flow, and excretion of bicarbonate, potassium, chloride, sulfate, calcium, magnesium, sodium and oxalate all were higher from the treated side (bolded). This means that shock wave treatment affects tubule handling of multiple molecules, presumably because of injury.
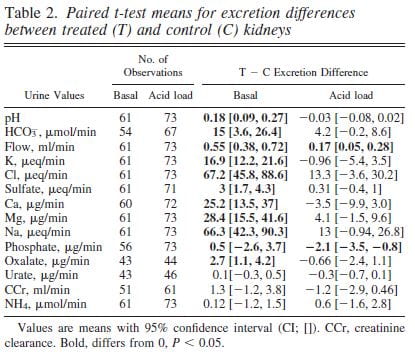 We could find these abnormalities up to 90 days after shock wave. The control kidney reduced its losses in compensation so blood remained entirely normal.
We could find these abnormalities up to 90 days after shock wave. The control kidney reduced its losses in compensation so blood remained entirely normal.
Bicarbonate Losing Raised the pH
The higher urine pH could have been due to damage of final urine acidification in the collecting ducts or to high delivery of bicarbonate from higher up in the nephron so that acid secreted lower down was neutralized by a flood of bicarbonate.
To tell these apart we gave the pigs an acid load that lowered their blood bicarbonate and therefore filtration and downstream delivery (‘Acid load’ columns). Acid load brought urine pH and almost all other measurements to equality between the shocked and control kidneys (loss of bolding).
The tissues from the pigs showed widespread injury to the thick ascending limbs, and you can read the paper for details.
SWL Can Raise Urine pH by Damaging Kidney Tubules
The meaning of the work is clear.
After shock wave treatments the treated kidney may excrete excess calcium and produce a urine of higher pH than it would otherwise do. The effects are precisely those needed to produce calcium phosphate crystals. From the bladder urine, which mixes urine from both kidneys, one could never know this was happening.
It is possible that the advent of shockwave lithotripsy has contributed to the rise in CaP stones, and I hope that further science sorts out whether this hypothesis is false or true.
High Kidney Ammonia Production
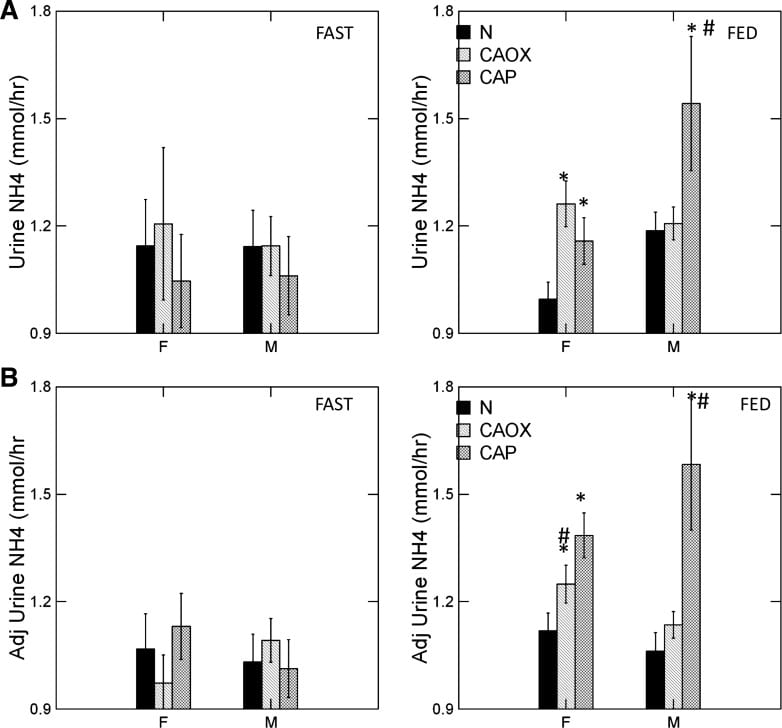 Ammonia Production Regulates Urine pH
Ammonia Production Regulates Urine pH
Kidneys excrete acid by making ammonia that can carry acid (protons) into urine without lowering urine pH. They also excrete acid by titrating urine phosphate, which does lower urine pH. If ammonia production goes down, from kidney disease, for example, urine pH has to fall so acid can be lost on phosphate.
Ammonia production relates itself to body acid load – from food and metabolism – so that the average urine pH is just around 6. But what would happen if regulation were abnormal so more ammonia than normal was made for a given bodily acid load?
Urine pH would rise.
CaP Stone Formers Make More Ammonia
The graph shows urine ammonia excretion from normals, and CaOx and CaP stone formers studied eating the exact same diet in a research center.
Fasting, all three groups are the same (left panels). Food increased urine ammonia in male CaP patients (#). Fed, the female CaP stone formers produce more ammonia than female normals (*, top right panel). So do the female CaOx stone formers. Ammonia production is governed by body acid load, which we measure as GI anion and urine sulfate – a residue of metabolic acid production. When we adjust ammonia for acid load (lower right panel) CaP stone male and female stone formers remain high compared to same sex normals.
We suspect the high urine pH that causes CaP stones arises in part from high ammonia production, perhaps an inherited trait.
Low Urine Citrate
Many articles on this site explore the powerful effects of citrate to bind calcium and inhibit calcium crystal growth. In these closely
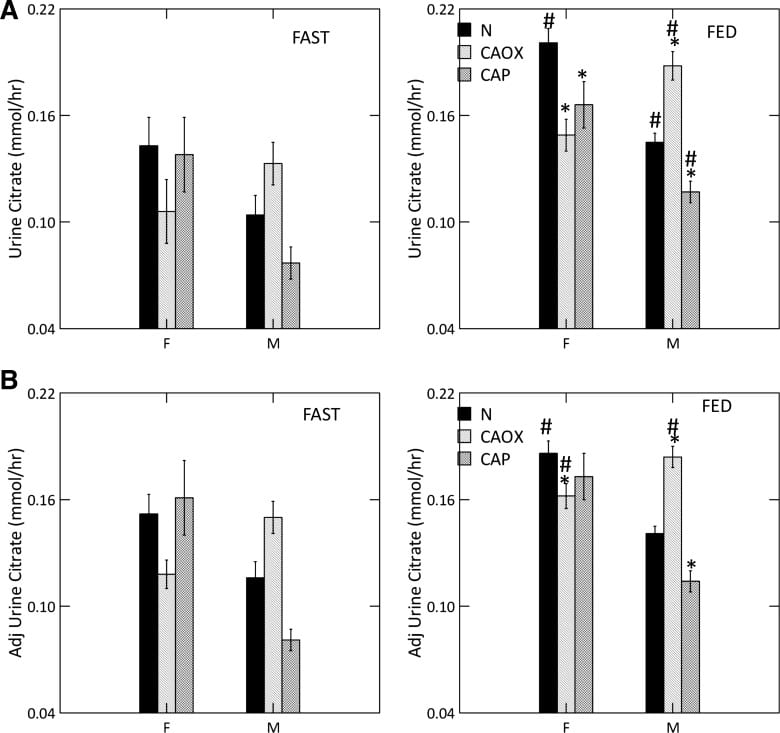
studied men and women we could document a uniquely low urine citrate of CaP stone formers vs. normal people.
Low Citrate in CaP Stone Formers
Food increased urine citrate is normal women and all three male groups (#). With food, CaP stone formers of both sexes have urine citrate excretions below their same sex normal counterparts (*, upper right panel) as did female CaOx stone formers.
As is well known, citrate is lower in normal men than women (compare black bars; we did not choose to compare the sexes statistically).
Adjusted for GI alkali and urine sulfate, (lower right panel) low citrate is concentrated among male CaP and female CaOx stone formers. Normal men remain below normal women.
Male CaOx stone formers have abnormally high urine citrate with and without adjustment for systemic acid balance.
Abnormal Kidney Cell Citrate Handling
Alkali loads, most famously potassium citrate, raise urine citrate and is an established stone prevention. Citrate also raises urine pH, because the alkali appears in urine as bicarbonate. That is why potassium citrate is not an ideal treatment against CaP stones, and why we have for decades needed a controlled trial to see if it works or makes things worse.
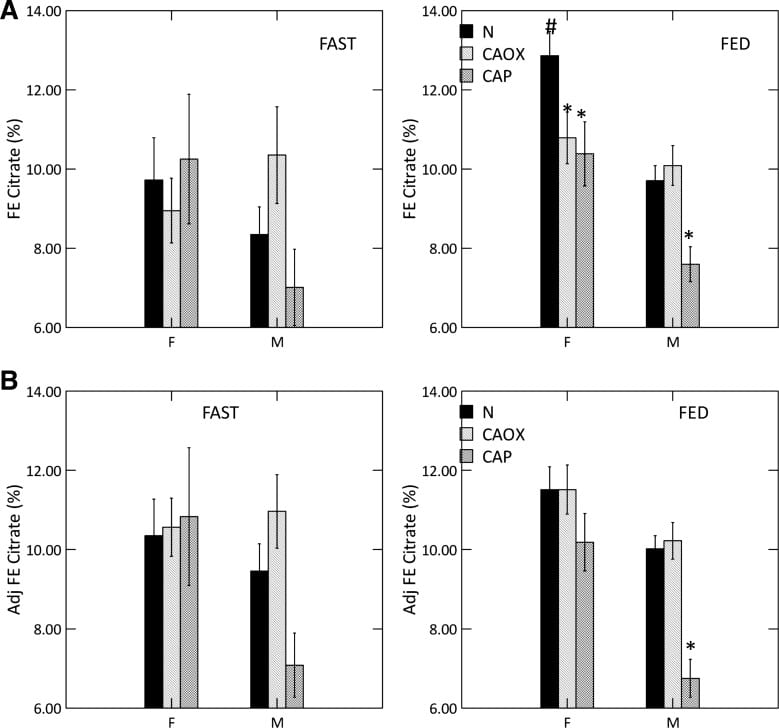
But here we have a high urine pH coupled with low urine citrate, in male CaP and female CaOx and CaP stone formers. That points to something wrong with kidney cell regulation.
We measured serum citrate and glomerular filtration so we could calculate the fraction of filtered citrate excreted (FE Citrate), shown in the upper right panel of the graph at left.
FE citrate is low in female CaOx and CaP stone formers and in males with CaP stones. This means that CaP stone formers are reabsorbing abnormal amounts of citrate back from the filtrate. It is used by kidney cells to produce metabolic energy.
Adjusting for GI alkali absorption (lower right panel) removes the female abnormalities but makes the male one even more prominent.
That male CaOx stone formers have abnormally high urine citrate excretion with normal FE citrate is because their serum citrate concentration is higher, a fact for which we had no explanation.
CaP Stone Formers Have Proximal Tubule Abnormalities
Citrate reabsorption and ammonia production are linked in the proximal tubules of the kidneys as part of overall kidney regulation of bodily acid base balance. In general alkali loads raise urine pH and urine citrate, and reduce ammonia production, whereas acid loads do the opposite.
Here we have high pH and high ammonia production coupled with low urine citrate, more marked in male CaP patients but detectable among the women as well.
It is as though the cells perceive a need to produce more acid excretion (ammonia) and conserve potential alkali (citrate is metabolized to bicarbonate), but there is no need. So urine pH rises and converts calcium stones to their phosphate forms. The cause(s) of these proximal tubule abnormalities are not known.
Incomplete Distal Renal Tubular Acidosis (dRTA)
A Questionable Disorder
Some have proposed that CaP stone formers have high urine pH and low citrate as part of “Incomplete renal tubular acidosis”. In proof, when given extra acid they may not reduce urine pH as low as normal people. In my primary article on dRTA, I present contemporary evidence that acid loading creates a continuous spectrum of urine pH responses, even among normal controls, so it is not a good basis for diagnosis. It seems better to say that CaP stone formers have abnormal proximal tubule functions, and make those the focus of new science.
Heterozygotes of Familial dRTA
With one exception, hereditary dRTA arises from gene disorders of the main proton transporters or of carbonic anhydrase itself, and these disorders are in general recessive. They are recessive because you need two defective genes to knock out a transporter whereas one good gene copy will maintain function.
Of course dRTA causes massive CaP stones and kidney disease. But heterozygotes – meaning one good and one defective gene – from families with dRTA if studied in detail, may not lower urine pH normally. These might be diagnosed as ‘incomplete dRTA, because in fact that is what they are.
CaP Stone Formers are Not Like Incomplete dRTA
Unlike our CaP stone formers, urine ammonia is low in dRTA and heterozygotes from families of dRTA, when compared to their acid load – urine sulfate. Urine ammonia is never high. I suspect that some CaP stone formers have high urine pH because they are indeed heterozygotes of dRTA. Low ammonia may be a way to separate them from the high ammonia of routine CaP stone formers.
Risk of Conversion From CaOx to CaP Stones
Some patients gradually increase their stone CaP percent, often enough to alter their classification to CaP stone former. The opposite, conversion from CaP to CaOx stones must be very uncommon, as we have no cases to report. We wanted to know how to detect risk of conversion.
Who We Studied
From 4767 patients in our program, we collected all CaOx stone formers who had two or more stone analyses and clinical follow up data (445 patients). From these we selected all who had a last stone CaP% at least 20% higher than that of the first stone (62 patients). Men and women were combined because we had so few cases.
Of these 62 cases, 26 had had three initial (pre-treatment) 24 hour urine studies before they passed the stone whose CaP percent was at least 20% higher than their first stone. We labeled these transformers with prior laboratory work – labs before they transformed – as ‘TP’.
For controls we chose 181 patients whose first stones were >90% CaOx and who increased their stone CaP percent <20% between the first and last stone.
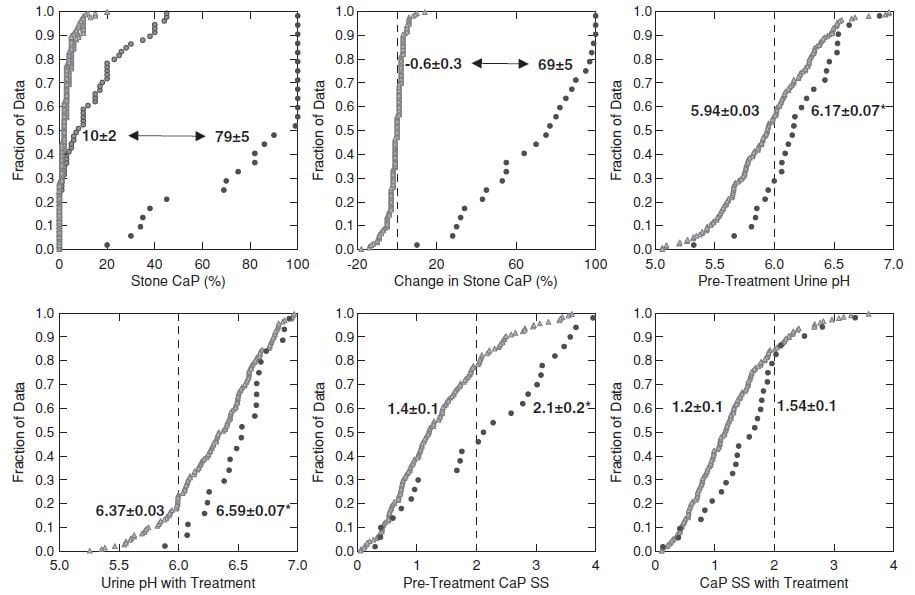
This figure shows the 26 TP cases and the 181 controls.
CaP% Was High at the beginning
Even though their initial stone CaOx percent was >50%, the 26 TP cases (black circles, upper left panel) had an average stone CaP of 10% before treatment, whereas it was much lower in the controls – who never added significant CaP.
During follow-up (upper left and middle panels) the 26 TP (black circles) increased their stone CaP markedly (average 10% to 79%, top left). The controls (gray triangles) hardly changed (-0.6% for controls, 69% change, for TP, upper middle panel).
Higher Urine pH Increased CaP SS
Urine pH and CaP SS before treatment and before conversion (upper right panel and lower left panels) and during treatment (lower middle and lower right panels) were higher in TP (black circles) than controls. CaP SS rose because we used potassium citrate as part of our treatment program.
SWL May Have Played a Role
ESWL associated with conversion: 112 of the 136 total cases with no ESWL procedures were controls, whereas only 21/41 cases with >2 ESWL were controls (X2=17, p<0.001). Furthermore, a predominance of ESWL procedures preceded the final stone (not shown here but shown in the paper), meaning ESWL could have been a causal factor.
Who is at Risk?
When stone CaP is above 10%, average 24 hour pH is as high as 6.3, or CaP supersaturation is above 2 before treatment risk of increasing stone CaP may be high. More than 2 ESWL procedures likewise. Given these risk factors in a CaOx SF perhaps one is prudent to treat as if CaP stones were already forming, so as to possibly prevent further stone CaP accumulation.
Prevention of Calcium Phosphate Stones
The objective is to lower CaP SS – reported with respect to brushite – below 1.
The main modifiable factors are urine volume, and calcium and citrate excretion. Because we cannot lower urine pH, the most crucial factor, we have to use what is left to achieve our goal. Likewise, because citrate regulation is abnormal in CaP stone formers, use of potassium citrate may not raise urine citrate so much as it raises urine pH, and therefore this otherwise valuable treatment can be ineffective.
Fluids
Relative calcium stone risk falls to 1 (no excess risk) at about 2.3 l/d of urine volume. Given the limitations of our treatments, I usually strive for 2.5 l/d spread out over the waking hours. This is an achievable goal if patients understand why it is important for their stone prevention.
Reduced Calcium Excretion
Genetic hypercalciuria is very common among calcium stone formers. If we understand that relative risk of stones rises above 1 at a urine calcium of 200 mg/d, both sexes, our goal is to reduce urine calcium to or below that point.
Reduced Diet Sodium
Multiple articles on this site detail the power of diet sodium to control urine calcium and bone calcium balance. The US diet recommendations for sodium are 100 mEq (2300 mg)/day as a tolerable upper limit, and 65 mEq (1500 mg)/day as ideal. These values concern blood pressure and bone rather than kidney stones. But if we achieve an ideal diet sodium it will lower urine calcium as well as defend blood pressure and bone mineral. So I have no reservations about promoting the ideal diet sodium, but also am prepared for compromise in this fast food dominated world.
Reduced Diet Sugar
As for diet sodium, I have written extensively about sugar as a factor that raises urine calcium, abruptly after the sugar load and with proven increase in supersaturations. Once again, US guidelines call for reducing sugar intake, and there is no benefit to anyone from eating refined sugar in any form. So I am shameless in my zeal to encourage patients to eat as little of it as possible.
Thiazide
Drugs of this class lower urine calcium about 80 to 100 mg/d below the level predicted by sodium intake. They act in part to increase proximal tubule calcium reabsorption. They are trial proven agents to reduce calcium stone recurrence. We have shown thiazide drugs lower urine pH, a possible benefit.
I have often argued to use diet as much as possible before adding thiazide to avoid drug side effects. But phosphate stones are not easy to prevent, so far as I have observed, and they damage kidney tissue. Moreover, we have no trials – none. These patients may have been in trials but are doomed to perpetual minority status unless specifically a focus.
So I am not shy about adding thiazide after perhaps only one to two efforts at diet control, should CaP supersaturation remain above 1.
Why NIH has yet to fund a calcium phosphate stone prevention trial escapes me. I cannot imagine how this has not been a priority.
Potassium Citrate
This drug will lower urine calcium below the level predicted by diet sodium intake. It may raise urine citrate excretion. But It may also raise urine pH.
Being as it is therefore able to raise or lower CaP supersaturation, I do not so much avoid using it as view it with a cold eye.
If thiazide is not attractive to a given patient I will try citrate and watch the effect on CaP supersaturation. CaP supersaturation is the final resultant of whatever changes it induces in urine calcium, pH, and citrate. If it indeed lowers CaP supersaturation, I am prone to use it but with appropriate 24 hour urine followup and an inextinguishable skepticism.
Reduced Diet Oxalate
I am aware that calcium oxalate in stones matters, and that even high phosphate stones often contain that crystal. If urine oxalate is high enough to confer risk – above 25 mg/d in both sexes – I make appropriate diet recommendations.
But patients cannot do everything all at once, so I generally put most emphasis on the calcium phosphate side. The exception is when urine oxalate is quite high – above 40 mg/d, for me – whereupon I do what I can with diet.
Monitoring Treatment
The objective is to lower CaP supersaturation below 1 in the 24 hour urine, and that is what I aim to achieve.
If fluids are enough, so be it. If not I add more treatments more or less as in the paragraphs above. Lacking trials, this is the best we can do. I watch supersaturation for calcium oxalate as a secondary endpoint, and if it is high enough to promote risk – above 3 – I attempt to lower it by reducing diet oxalate.
Monitoring is crucial. What we try to do may not be done because patients cannot or will not do it, so we have to know when to try another approach.
Put another way, for stone prevention, especially calcium phosphate stones, deliberation is reality.
I wish to thank Dr John Asplin for his careful reading of this article and suggestions for improvement.



Hi Dr. Coe. What is your thought on betaine for lowering urine pH? My doctors seem to be happy with the improvement of my 24h urine results after CTD and KCl administration and don’t seem to want to do more despite brushite SS still being so high. They don’t want to raise the CTD as they’re worried about gout risk. I’m on 37.5mg CTD, 100mEq KCl (potassium kept dropping too low). I adhere strictly to your recommended KSD and drink 13-15C of water per day, even before sleep and have to urinate multiple times thru the night. Nephrologist thinks I have incomplete distal RTA (said “walks like a duck”…) as I have other autoimmune conditions, but there’s nothing that can be done. My last 24h urine results (have had 4 24h urine tests since starting meds):
Calcium 218
Oxalate 10
Uric Acid 762H
Citrate 604
pH 7.2
volume 3.27L
Sodium 37
Phosphate 1046
Magnesium 120
Potassium 154H
Creatinine 981
Sulfate 8
Sodium Urate SS 0.26
CaOx SS 0.28
Brushite SS 2.78
Uric Acid SS 0.08
The Ca came down from 496, brushite SS was 2.29 (w/o medication and on KSD for a month). Brushite SS on the last 4 24h urine tests on medication: 2.78, 2.11, 3.18. 2.96. My doc has been great and has helped me over the last 9mo with the CTD (I couldn’t tolerate Indapamide), but is content with where I am. I do wonder if I should try to find another practitioner who can help me further, but perhaps he’s right and nothing more can be done? Thanks for your thoughts, Jen.
Hi Jen, indeed you have a high urine pH of 7.2 but unlike in renal tubular acidosis your urine citrate is very high. Of great interest your urine sulfate is very low indeed (8) suggesting a low protein diet or a diet in which protein is arising from veggies (or you put in the wrong number). The high pH and high citrate fit well with a very alkaline diet, incidentally. You do not give an ammonia, urea nitrogen or protein catabolic rate or urine chloride so I cannot interpret your values more exactly. But if I am right then diet is the ‘problem’ as it were. Best, Fred Coe
Thanks for your response Dr. Coe. Unfortunately, Quest Urorisk test doesn’t include Nh4,Un, Pcr, or UCl. The sulfate number is correct. Other 24h urine tests had sulfate numbers of 9,10,15, 22 (22 was probably high due to going out to eat 1 or 2 days before the test, a rare occurrence). I’m 110lb and get about 35g meat protein / day (not including eggs), with a total daily protein of 75g from all foods. 25g fiber / day and actually eat 1/3 less vegetables than when my urine pH was normal. The high pH started around menopause. That being said, I wouldn’t say my diet is very acidic since I don’t eat a lot of grain foods (skin issues) and follow the KSD diet to a tee. If there are other foods or supplements I can eat to acidify my system, I’m open to suggestions.
I asked my nephrologist if it would be useful to bring in other specialists like endocrinology or do some genetic testing. He didn’t think they would be helpful for stone disease. He’s now offering to raise CTD but must check serum uric acid first since urine uric acid was elevated in the 24h urine for the 1st time. That will like require even more KCl (at 100mEq – 2 pills with breakfast, 3 with dinner). I think he said too much CTD could make me prone to gout, or other side effects. Dietician said what I ate the day of the collection was fine for purines.
Do you think a 2nd opinion and other avenues are worthwhile or just accept it and keep flushing my kidneys out with water around the clock? How much would I even need to drink to keep stone formation at bay? I currently drink 3L and go to the bathroom 2x in the middle of the night, as there are “flakes” forming at the bottom of my container when I check for volume of daily output. =(
Thank you so much for your thoughts and all the wonderful information on your website!
Hi Jen, I would change my vendor to Litholink – their panel is better in being more comprehensive. As for genetic testing, I use it when urine calcium is high, treatment is weighty, and serum phosphate is below normal and 1,25 vitamin D high. Sometimes I encounter defects with simpler treatments like phosphate.The flakes you notice need testing. I presume they are calcium phosphate crystals and that might influence your treatment. If treatment is too much your physicians might wish to consider obtaining a second opinion, perhaps from a geographically convenient stone program at a university. Best, Fred Coe
I keep getting calcium phosphate stones, no matter what I do. My doctor refuses to give me a thiazide and only recommends drinking more water, which I try to get 2L. My 24 urine :Hydroxyapatite crystal: 6.09 Brushite .57 PH : 6.7 Calcium 240mg
I know I need to lower my sodium and sugar. But my stones keep coming, the last 1 was 6mm I wouldn’t let them do surgery or lithrotripsy. The stone before that less than 9 months prior was big all around 7x6x5 mm. Please can you help me? I can send my Mayo Clinic report of the 24 hour urine but have had a stone evaluation since 2015 & my diet has changed & stone composition would be different, never caught any stones since then. Please give an email address so I can attach the documents.
Hi Tina, I do not privately read reports as that is to practice medicine. If you post the results I can say what I see. Also, telehealth is available. My clinic can arrange things – 773 702 6134. Best. Fred Coe
Here are my results:
Calcium oxalate crystal: 0.80
Brushite crystal: 0.57
Hydroxyapatite crystal: 6.09 H
Uric acid crystal: -4.34
Volume: 2000 ml
Sodium: 110 mmol /24h
Potassium: 50 mmol/24h
Calcium: 240 mg/24h H
Magnesium: 140 mg/24h
Chloride: 94 mmol/24h
Phosphorus: 500 mg/24h
Sulfate: 12 mmol/24h
Citrate excretion: 1078 mg/24h
Oxalate: 0.14 mmol/24h
Oxalate: 12.3 mg/24h
Ph: 6.7
Uric acid: 400 mg/24h
Creatinine: 1240 mg/24
Osmolality: 333 mOsm/kg
Ammonium: 16 mmol/24h
Urea nitrogen: 6.9 g/24h
Protein catabolic rate: 68 g/24h
Hi Tina, Your urine calcium is too high (over 200 mg) and diet sodium also 110 mEq/d. If you lower the latter the urine calcium will come down and lower stone risk. I do not trust the 24 hour urine lab because an oxalate of 12 mg/24 hours does not occur. The measurement is no good. I would recommend Litholink, a service of LabCorp for the next study. Best, Fred Coe
Hi Dr. Coe, this article is extremely helpful. I am a 22 year old male and had my first kidney stone about a year ago, which was a 6×4 in the upper pole of my left kidney:
(NOTE)
20% Calcium oxalate monohydrate (Whewellite)
50% Calcium oxalate dihydrate (Weddellite)
30% Carbonate apatite (Dahllite)
I got it removed via ureteroscopy, but within 7 months I had another 6mm stone. I then had an ESWL done, but it failed, sending me to the ER where they found a 7×4 stone in my ureter, and several smaller stones in the lower pole of my left kidney (4mm or less), only a week after thr inital scan showing a single 6mm stone. I had a stent for a month, and then a second ureteroscopy and a new stone compositon upon analysis, with this stone in almost thr exact same spot of the upper left pole of the left kidney:
20% Calcium oxalate monohydrate (Whewellite)
10% Carbonate apatite (Dahllite)
70% Calcium hydrogen phosphate dihydrate (Brushite)
My stent removal was two weeks ago, and my urologist said it was already encrusted after a week, and now 3 weeks after the ureteroacopy I already have a new 3.5mm stone (though this one is according to an ultrasound) in the middle pole of my left kidney. While the scans show that the stone is non-obstructing, my side has been hurting every single day in the same way as my precious, obstructing stones (but lesser so).
I don’t know how my stones can form this fast, and my appointments with a urologist/nephrologist are months out, so I’m wondering what steps I should take to prevent more stones. I’ve been on a strict low/no oxalate diet, low sodium (my serum sodium has always been low to begin with), and barely any animal protein a day, yet a stone has still formed extremely quickly. I was told to take Moonstone Stone Stopper (Alkali Citrate), but I’m wondering why, considering Alkali is supposedly bad for brushite stones unless I misunderstood that. Here’s my 24h Urine Test results after my first 6×4 mm stone, before my ESWL:
Calcium, urine: 349 mg/vol
Creatine, urine: 1.8 gm/vol
Magnesium, Ur: 111.14 mg/vol
Sodium, urine: 260 mmvol/vol
Uric Acid, urine: 779.6 mg/vol
Citrate, Urine 24 Hours: 287 mg/day
24 hr Urine Oxalate: 42mg/day
Total volume: 2,475 (I now drink at least a gallon of water a day)
If you know any next steps I should take based on this I’d really appreciate it. I’m not sure what to do right now because it seems like not much is known about brushite stones and they’re extemely aggressive, but you’re definitely the most knowledgable source I’ve seen about them so far. Thank you!
Hi Dillon, brushite stones behave this way, and unfortunately you have converted to it. Your high urine calcium is a main cause, as is your low citrate. I would not hesitate to use thiazide to lower urine calcium and head off recurrence. Be sure and check your serum phosphate and calcium, fasting. Low phosphate may point to a common gene defect, high calcium to hyperparathyroidism. Regards, Fred Coe
Hello Dr. Coe,
I’m a 23 year old female who had a 19mm calcium phosphate stone this summer and had to get a sound wave and laser lithotripsy done to dissolve the stone. Since then, I have changed my diet to a low oxalate diet and eat one serving of meat protein a day. My doctors haven’t really helped me on how to get rid of these stones, and I’m worried these stones are going to damage my kidneys. Here are my results from my 24H Urine Test:
Urine Calcium: 117 mg/day
Urine Oxalate: 17 mg/day
Urine Citrate:779 mg/day
SS CaP: 1.31
Urine pH: 6.785
Creatinine: 1166 mg/24hr
I feel lost on what to do and do not want to end up with kidney failure at a young age. Any advice I would greatly appreciate !
Hi Mackenzie, The urine you show is not the one that caused the stone. Something has changed – for the better. The high pH is common in younger women and the high citrate likewise. Right now you have no stone risk, but if you look back a few years things might have been instructively different. Best. Fred Coe
Hi Dr. Coe, this article is extremely helpful. I am a 22-year-old male and had my first kidney stone about a year ago, which was a 6×4 in the upper pole of my left kidney:
20% Calcium oxalate monohydrate (Whewellite)
50% Calcium oxalate dihydrate (Weddellite)
30% Carbonate apatite (Dahllite)
I got it removed via ureteroscopy, but within 7 months I had another 6mm stone. I then had an ESWL done, but it failed, sending me to the ER where they found a 7×4 stone in my ureter, and several smaller stones in the lower pole of my left kidney (4mm or less), only a week after the initial scan showing a single 6mm stone. I had a stent for a month, and then a second ureteroscopy and a new stone composition upon analysis, with this stone in almost the exact same spot of the upper left pole of the left kidney:
20% Calcium oxalate monohydrate (Whewellite)
10% Carbonate apatite (Dahllite)
70% Calcium hydrogen phosphate dihydrate (Brushite)
My stent removal was two weeks ago, and my urologist said it was already encrusted after a week, and now 3 weeks after the ureteroscopy I already have a new 3.5mm stone (though this one is according to an ultrasound) in the middle pole of my left kidney. While the scans show that the stone is non-obstructing, my side has been hurting every single day in the same way as my precious, obstructing stones (but lesser so).
I don’t know how my stones can form this fast, and my appointments with a urologist/nephrologist are months out, so I’m wondering what steps I should take to prevent more stones. I’ve been on a strict low/no oxalate diet, low sodium (my serum sodium has always been low to begin with), and barely any animal protein a day, yet a stone has still formed extremely quickly.
I was told to take Moonstone Stone Stopper (Alkali Citrate), but I’m wondering why, considering Alkali is supposedly bad for brushite stones unless I misunderstood that. Here’s my 24h Urine Test results after my first 6×4 mm stone, before my ESWL:
Calcium, urine: 349 mg/vol
Creatine, urine: 1.8 gm/vol
Magnesium, Ur: 111.14 mg/vol
Sodium, urine: 260 mmvol/vol
Uric Acid, urine: 779.6 mg/vol
Citrate, Urine 24 Hours: 287 mg/day
24 hr Urine Oxalate: 42mg/day
Total volume: 2,475 (I now drink at least a gallon of water a day)
If you know any next steps I should take based on this, I’d really appreciate it. I’m not sure what to do right now because it seems like not much is known about brushite stones and they’re extremely aggressive, but you’re definitely the most knowledgeable source I’ve seen about them so far. Thank you!
Hi Dillon, Brushite stones grow rapidly and treatment is very complex. Your high urine calcium, low citrate are common causes, but the crystal is itself a mystery. Your care should be at a university stone program given your age and this specific kind of stone. Best, Fred Coe
I keep getting calcium phosphate stones, no matter what I do. My doctor refuses to give me a thiazide and only recommends drinking more water, which I try to get 2L. My 24 urine :Hydroxyapatite crystal: 6.09 Brushite .57 PH : 6.7 Calcium 240mg
I know I need to lower my sodium and sugar. But my stones keep coming, the last 1 was 6mm I wouldn’t let them do surgery or lithrotripsy. The stone before that less than 9 months prior was big all around 7x6x5 mm. Please can you help me? I can send my Mayo Clinic report of the 24 hour urine but have had a stone evaluation since 2015 & my diet has changed & stone composition would be different, never caught any stones since then. Please give an email address so I can attach the documents.
Hi Tina, I think I answered this already. Fred
Hi Dr,
I am a 32 year old female recently diagnosed with kidney stones. My stone analysis consisted of Carbonate Apatite (Dahllite) 100%. My 24 hour urine collection revealed citric acid 180 and brushite 2.56. The suspected problem on the report said Hypocitraturic Nephrolithiasis and brushite supersaturation. My urologist is recommending I start potassium citrate 3 times a day. Do you agree that this is the best way to proceed?
Hi Shelby, You do not mention urine calcium which is frequently high in patients like you. K Citrate will raise urine pH and perhaps urine citrate. The effect on brushite SS is hard to predict. I would suggest you follow your physician’s advice and see how much pH and citrate rose, and what happened to brushite SS. In some, citrate rises quite a bit vs pH and in some the opposite. If urine calcium is high, one would want to lower it with diet and perhaps meds. Regards, Fred Coe
Are you aware of any research looking at a phosphate binder like sevelamer for dRTA patients who produce CaPhos stones? If not, do you have any thoughts about that potential approach?
Best,
Bill
Hi Bill, I would not be in favor – bones will be endangered. Fred
Thank you for the very informative information. My 17 yo daughter is struggling with repeat kidney stones. Her last one required ureteroscopy. The pathology on the stone shows 30% calcium oxalate dihydrate and 70% calcium phosphate carbonate-apatite. She just starting down the road of additional testing and has some scheduled in the near future. My question – My daughter also has a rare genetic disease (Fabry Disease, FD) that can cause KD, but this usually doesn’t manifest in females until later in life. Since most doctor’s (including the urologist we are working with) have no knowledge or FD, should we consider seeing a nephrologist familiar with FD? Or could her kidney stone issues be unrelated?
Hi Jessica, Fabry is not a cause of stones but of kidney disease. Enzyme replacement is available and important so she should be cared for by experts in this rare condition. Her stones will have another cause, and should be evaluated in the usual way. Regards, Fred Coe
Hi Dr. Coe,
I’m a new stone former as of Thanksgiving 2024, discovered while on vacation in Mexico. I know I am making mixed stones, can you help me narrow down how they should be classified?
80% calcium oxalate monohydrate,
10% calcium oxalate dihydrate, and
10% calcium phosphate (hydroxy- and carbonate- apatite)
Hi Caroline, these are common stones. You need the common evaluation for cause and treatments for prevention. Best, Fred Coe
Dr Coe, could these findings have any bearing on the formation of Calcium phosphate stones in the prostate? My husband had prostate reduction surgery and the prostate was full of Ca Phosphate stones. He has passed 8-9 since then and has one stuck at present. Is there anything he can do to prevent these from forming?
Hi Annette, Prostate stones are calcium phosphate and are tissue calcifications, not urinary stones. Is his physician sure he does not have both? If they are from the prostate gland why are they still passing? Regards, Fred Coe
Hi Dr. Coe. I have been reading others’ comments with interest. I’m a 56 year old male with a history of large calcium phosphate kidney stones. I’ve had several procedures including 2 PCNLs in the past couple of years removing some but not all stones from my right kidney. About a year after the first surgery, my right kidney was filled up again with stones, some well over 1cm. I’ve been to three urologists and a nephrologist and they can’t tell me why I form such large stones so rapidly. The most recent urologist, a university expert, asked me to take Potassium Citrate 15 mEq twice daily but I hesitate because I know that will raise my urine pH, which is already high. The most recent stone I had analyzed was composed of 10% calcium oxalate, 60% brushite, and 30% hydroxy- and carbonate- apatite. Could you take a look at my numbers and see if you have any advice?
Numbers from 24 hour urine test, * means the lab flagged it as outside the normal range:
Total Volume: 2.299L
pH: 6.86
Sodium: 94 mEq/TV
Potassium: 45 mEq/TV
Uric Acid: 788 mg/TV*
Creatinine: 1405 mg/TV
Calcium: 193 mg/TV
Magnesium: 81 mg/TV
Chloride: 63 mEq/TV
Ammonia: 31 mEq/TV
Citric Acid: 296 mg/TV*
Phosphorus: 735 mg/TV
Oxalate: 35/TV
Sulfate: 21 mmol/TV
Urea Nitrogen: 8.54 g/TV
Relative Supersaturation
of Calcium Oxalate: 1.62
of Brushite: 2.64*
of Sodium Urate: 1.28
of Struvite: 4.1
of Uric Acid: 0.25
Comprehensive metabolic panel:
Glucose: 108 mg/dL*
BUN: 18 mg/dL
Creatinine: 0.93 mg/dL
eGFR: 97 mL/min/1.73
BUN/Creatinine Ratio: 19
Sodium: 141 mmol/L
Potassium: 3.4 mmol/L*
Chloride: 103 mmol/L
CO2: 24 mmol/L
Calcium: 9.3 mg/dL
Protein Total: 6.7 g/dL
Albumin: 4.3 g/dL
Globulin: 2.4 g/dL
Bilirubin, Total: 0.7 mg/dL
Alkaline Phosphatase: 71 IU/L
AST: 28 IU/L
ALT: 31 IU/L
Thank you.
Hi Jordi, Low urine citrate high pH high CaP SS ammonia (acid excretion) higher than sulfate (acid load) brushite. Brushite is a major problem stone and not like any other. I think the brushite stones are all coming from the right kidney and the left one makes more common HA or CaOx stones. The final urine is from both kidneys so one cannot tell what is happening in the one most affected. I notice a low serum potassium, and that will raise urine ammonia and therefore urine pH. Possibly replacement with KCl will help lower urine ammonia and urine pH. All the usual measures, thiazide, low diet sodium high fluids together can often bring SS CaP below 1 and reduce stones, but I fear for the right kidney. This is an area where we need more research. Best, Fred Coe
Hi,
I had staghorn stones last year and 5 PCNL procedures to have them removed (1 on right, 4 on left). 7 months after last surgery, they came back. I was in the ER 8mm stone blockage, urine sepsis, January 7. I have stones in both kidneys again… stone analysis always 100% calcium phosphate. Drinking 100 ounces of water, no meat, low sodium. They started 50mg hydrochlorothiazide, potassium citrate ER 10 MEQ.
Litholink was done week before blockage on January 1
Urine volume- 3420
Calcium oxalate saturation 3.52
Ph 6.2
Calcium urine 185
Oxalate urine 35
Citrate urine 974
Calcium phosphate saturation 0.58
Uric acid saturation 0.21
Uric acid urine 615
Sodium urine 140
Potassium urine 53
Magnesium 64
Phosphorus 875
Ammonium 36
Chloride 135
Sulfate 24
Urea nitrogen 9.22
Protein catabolic rate 1.0
Creatine urine 1340
Creatine body weight 19.1
Calcium body weight 2.6
Calcium creatine ratio 138
I also found out I have hundreds of gallstones but not inflamed.
Any recommendations would be greatly appreciated.
Rose
Hi Rose, Your present urine is not high risk for more stones. But often there are fragments that can grow. I do not know the details, but right now things look good if they stay that way new stones should not be likely. That is the best I can say with just the numbers. Best, Fred Coe
Hi Dr. Coe
I am a renal physician, and I find your article very informative.
However, I have question that is not covered here. We have a 52-year-old woman with a long history of kidney stones.
She is a former of mainly apatite and brushite stones. Her urine pH is high. She also has hypercalciuria and hypocitraturia.
Her urine has also been colonized for years with E. coli. On rare occasions she had symptomatic UTI’s. She received multiple treatment attempts with antibiotics, even when asymptomatic with the idea that she has infection stones.
I am not entirely sure about the role of the E. coli in terms of her kidney stone formation though. She never had struvite components in her stones, and as far as I know E. coli do not produce urease.
However, do you think we should continue to aggressively treat the bacteria?
Hi Dr Gerber, I apologize for the late reply. This pattern of high pH low citrate and high calcium is indeed the common one in HA and BR SF. Tubule plugging with HA is the rule, plaque can be part of the stone formation cycle. Brushite often forms in one kidney the other HA, and the Br forming kidney often is infected or has urological or structural abnormalities. Mechanisms for low citrate are not clear but it arises from increased renal citrate reabsorption. Often, renal ammonia production exceeds sulfate pointing to some disorder ot PT acid base sensing. Potassium chloride loading does not shut off the ammonia nor raise urine citrate – we tried it. As for e coli, the ‘correct’ remark is that it plays no role but – and I should write about this – e coli and other non urease bacteria can express cell surface phosphatases that release inorganic phosphate the leads to crystals forming over the bacteria. As well, neutrophils responding to inflammation – perhaps from infection – seem to play a role in brushite stones. My only recourse is to lower urine calcium as much as I can with low sodium diet and chlorthalidone and raise urine volume – this often is enough even though it does not get to the primary problem. Best, Fred Coe
Would this information all pertain to calcium phosphate bladder stones as well?
Hi AMB, I am not sure it does. Bladder stones arise mainly because of outflow obstruction and residual urine. Best, Fred Coe
Cystine, Urine, Qualitative 01 Neg Urine Volume (Preserved) 01 4500 High mL/24 hr 500-4000
Calcium Oxalate Saturation 01 1.08 Low Negative
6.00-10.00
Calcium, Urine 01 80 mg/24 hr 550
Calcium Phosphate
Saturation 01 0.14 Low 0.50-2.00
pH, 24 hr, Urine 01 6.264 High 5.800-6.200
Uric Acid Saturation 01 0.12 <1.00
Uric Acid, Urine 01 406 mg/24 hr <750
Sodium, Urine 01 61 mmol/24 hr 50-150
Potassium, Urine 01 40 mmol/24 hr 20-100
Magnesium, Urine 01 78 mg/24 hr 30-120
Phosphorus, Urine 01 556 Low mg/24 hr 600-1200
Ammonium, Urine 01 37 mmol/24 hr 15-60
Chloride, Urine 01 75 mmol/24 hr 70-250
Sulfate, Urine 01 <14 Low meq/24 hr 20-80
Urea Nitrogen, Urine 01 5.56 Low g/24 hr 6.00-14.00
Protein Catabolic Rate 01 0.7 Low g/kg/24 hr 0.8-1.4
Creatinine, Urine 01 821 mg/24 hr Not Applic.
Creatinine/Kg Body Weight 01 12.0 mg/24 hr/kg 8.7-20.3
Calcium/Kg Body Weight 01 1.2 mg/24 hr/kg <4.0
Calcium/Creatinine Ratio 01 97 mg/g creat 51-262
Hi Coleen, The 24 hour urine results who almost no stone risk of any kind. I cannot imagine you making any new stones under these conditions. Regards, Fred Coe
What would you recommend to increase blood potassium when on Indapamide…(for high calcium in urine) my blood potassium was at 2.9. I have had 10 surgeries (over 100 stones) since 2018. Started with calcium oxalate stones and have progressed to Calcium phosphate/brushite stones. I have been on potassium citrate at various levels for years 1-3 tablets a day and feel like this may be making my problem worse by focusing on citrate rather than ph. I just purchased ph strips… what is the ideal ph to prevent phosphate/brushite stones in women and what other potassium supplement would you suggest?
Hi Kathy, Amiloride 5 mg tabs 1 to 2 daily can reduce kidney potassium loss. Potassium chloride is preferable for you in that it will not raise urine pH and increase risk for growth of calcium phosphate stones. Best, Fred Coe