 Nephrocalcinosis means kidneys contain many calcium deposits. You can see them on CT scans or during surgery to remove stones. This article tells about where in kidneys the deposits reside, what they mean, and what significance we need to attach to the word.
Nephrocalcinosis means kidneys contain many calcium deposits. You can see them on CT scans or during surgery to remove stones. This article tells about where in kidneys the deposits reside, what they mean, and what significance we need to attach to the word.
Fuller Albright Made Up the Word Nephrocalcinosis
The boyish face of perhaps the greatest 20th century scientist concerned with kidney stones, Fuller Albright, fills the featured picture. In 1934, Albright, then an Assistant Physician at the MGH and an instructor in medicine at Harvard Medical School, used his newly coined word, nephrocalcinosis in a remarkable paper. That paper described calcium deposits in kidneys of patients with hyperparathyroidism – a mineral disorder caused by enlargement of one or more parathyroid glands.
Who Were His Patients?
He described three types of kidney involvement in primary hyperparathyroidism. Whereas type 1 patients simply formed kidney stones, type 3 patients suffered from acute ‘parathyroid poisoning’, with kidney failure and death. Midway between these two, type 2 patients had stones and kidney tissue calcium deposits but adequate kidney function.
About these Type 2 patients he wrote this passage in which the word first appears (In text box below).
 What Limitations Did His Patients Impose on His Understanding?
What Limitations Did His Patients Impose on His Understanding?
All of his patients suffered from primary hyperparathyroidism, a disease found in only 5% or so of calcium stone formers we see today in our clinics.
Moreover, the tissues he observed came from autopsies, meaning from only his type 2 and 3 patients. His Type 1 patients, who simply formed kidney stones, rarely came to autopsy, so he did not have tissues from them.
As a result Albright coined the word nephrocalcinosis to describe the scarred, contracted kidneys of patients whose primary hyperparathyroidism had caused kidney disease. Their kidney calcifications were a mixture of those from stone formation and those that occur with kidney failure.
Who Are Our Patients?
Like Albright, some have primary hyperparathyroidism. But none have significant kidney failure. They resemble his Type l patients.
Unlike the patients for whom Albright coined nephrocalcinosis, we mainly study patients whose stones arise from no systemic disease at all. They just form stones we ascribe to excessive amounts of daily calcium or oxalate excretion, or low urine volume or citrate, or to combinations of these – so called idiopathic calcium stone formers.
So physicians today use the word nephrocalcinosis to describe very different patients than those Albright studied when he made the word up.
Who Uses the Word Nephrocalcinosis?
Radiologists
They mean many calcified – radio dense – regions overlay the outlines of the kidneys on various kinds of imaging studies: Simple flat plates, ultrasound studies, and CT scans.
But, as in the Cave of Shadows, radiographs are to the reality of tissue as shadows to real objects.
Many Others
When I looked up nephrocalcinosis in PubMed, I found 2686 entries.
Of these, most concerned diseases that calcify kidney tissues: Medullary sponge kidney, kidney transplant, distal renal tubular acidosis, primary hyperparathyroidism, inherited disorders of the kidney, hyperoxaluria, loop diuretics in neonates, vitamin D and A toxicity, FAM20A mutations – enamel renal syndrome -, claudins, hypomagnesemic states, and hypophosphatasia.
As well, I found an excellent review from which this this article takes its starting point: ‘What is Nephrocalcinosis?’ by professors Shavit, Jaeger, and Unwin.
That review begins with a definition: ‘Strictly, the term ‘nephrocalcinosis’ refers to the generalized deposition of calcium oxalate (CaOx) or calcium phosphate (CaPi) in the kidney.’
But where do they form in kidneys, and what do they signify?
Where Kidney Crystals Form
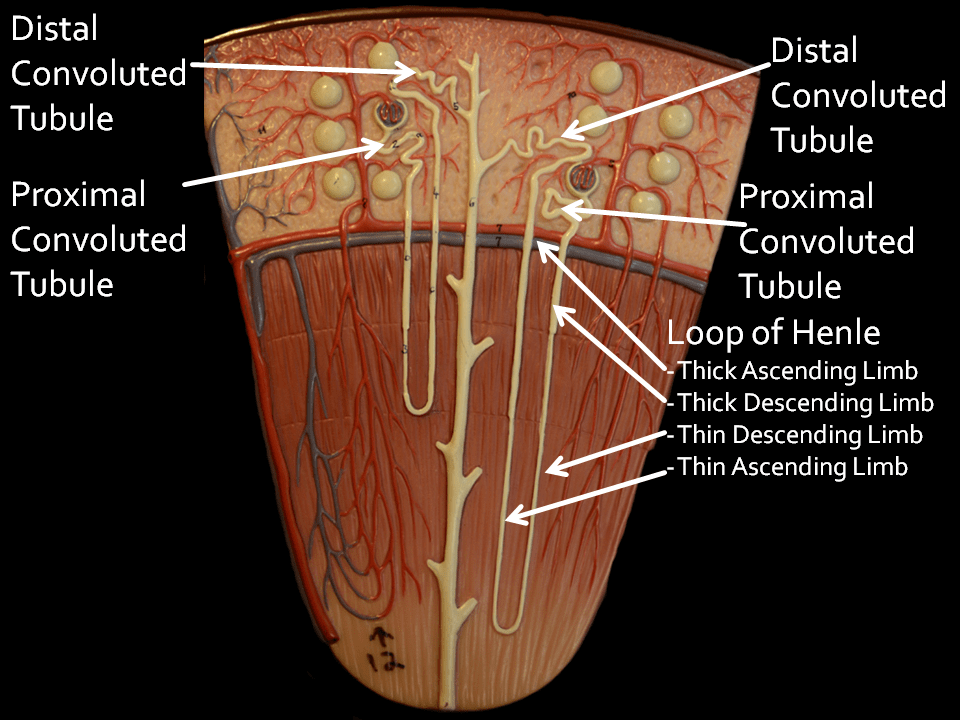
Cortex, Medulla, and Papilla
This stock web drawing depicts a slice through a kidney. The outer capsule runs along the top.
The cortex occupies the upper 1/3 of the kidney slice, above the crescent of red and blue vessels. In it are the filtering units that begin the nephron, the glomerulae, shown as round balls.
Below that crescent of vessels lies the medulla.
At the very bottom of the medulla lies the rounded papillum where urine drains into the renal pelvis and thence down the ureter. Urine exits through the terminal collecting ducts of Bellini – the opening of the thick long tube that runs vertically from cortex to the bottom.
Glomerulae
Each contains a tuft of capillary held within a complex web of cells. The force of the heart filters water and salts out of the capillaries into the tubule of the nephrons. Normal human kidneys contain about one million nephron units. Common measurements of ‘kidney function’ such as serum creatinine reflect the sum total of filtration through all two million glomerulae. Obstruction from stones can reduce filtration.
Nephron Segments
Proximal Convoluted tubules
Each glomerulus drains its filtrate into a squiggly – convoluted – ‘proximal’ tubule which gives way into the remaining nephron. These tubules reabsorb much of the filtered water and salt back into the blood. They leave behind materials destined for export into the final urine.
Proximal Straight Tubules
Mislabeled thick descending limbs on the drawing, a part of the proximal tubule extends below the arc of vessels into the medulla and is called the S3 segment. I mention it only because crystals form there sometimes.
Loops of Henle
As they travels downward below the proximal convoluted tubule each nephron thins into a hairpin shaped loop. Those hairpin loops of glomerulae that lie near the outside of the kidney (look at the nephron to the left) reach down only part ways into the medulla – the portion of the kidney below the red crescent of vessels. By contrast, loops from glomerulae near the medulla – just above the crescent of vessels – reach down into the deepest parts of the medulla.
These hairpin loops permit kidneys to concentrate the urine, which means extracting water from the filtrate and putting that water back into the blood. Unlike the proximal convoluted tubules that reabsorb water and salts back into the blood the loops permit reabsorption of water without the salts, so the salts destined for export are concentrated.
Names for the Segments of the Loops
As already mentioned, the ‘Thick Descending Limb’ is actually the S3 segment of the proximal tubule.
It gives way to the descending and ascending thin limbs of the hairpin. The top of the thin ascending limb widens into the ascending thick limb. This segment reabsorbs sodium chloride back into the blood without water, leaving the ‘extra’ water – without its sodium – as a diluted fluid in its lumen. The sodium chloride collects in the medulla around these thick limbs which becomes saltier than blood.
So called Randall’s plaque, over which calcium oxalate stones may form, originates in the outer parts of the thin limbs,
Distal Convoluted Tubule
The dilute fluid drains into the ‘Distal’ convoluted tubule’. Here, the ‘extra’ water leaves, back into the blood. This segment can make the fluid more acidic, and remove calcium back into the blood.
Collecting Ducts
From there, fluid drains through the unmarked straight connecting segment and thence into the collecting ducts. Like any plumbing drains, these run from the cortex down the medulla all the way to the papilla where the final urine flows out. Along the collecting duct the ‘salty’ interstitium around the thick ascending limbs draws water – but not calcium or phosphate or oxalate back into the blood, supersaturating the fluid that remains in the tubule. Collecting duct cells make the fluid more acid, a protection against calcium phosphate crystals.
We name the portion of the collecting ducts that run alongside the medullary thick ascending limbs the outer, and the remainder the inner medullary collecting ducts.
Ducts of Bellini
These terminate the nephron and empty the final urine into the urinary collecting system through tiny holes in the lining of the papillary tip. Because they hold the final, most supersaturated urine, crystals often form in them, creating plugs over which stones form.
Where is the Interstitium?
Envision a tall building. Pipes run from the basement to the roof – water, steam, drains, electrical conduits, elevator shafts, stairwells. Now, think about the space between the elevator shafts, stairwells, and all the pipes and conduits: That is the interstitium, what stands between.
In the kidney the long structures are the tubules and vessels; the interstitium is the space between them. That is where plaque is. There are cells in the interstitium – it is as though, as an example, insulation blocks were stuffed into the spaces between pipes.
Mice can live in the spaces between things, but not in the pipes. Rust can plug pipes but not the spaces between them.
The Reward for Brave Hearts
To those who have read the foregoing: My regards and admiration. Like tourists who climb the ancient, winding, broken stairs up into the towers of medieval cathedrals or the battlements of long abandoned castles, panting and worried about getting back down, you now come into the reward of so much virtue and endurance. Before you lies the architecture of the nephron.
Where the Crystals Form
From what I have told you, where would you surmise crystals might form?
Surely not, you might say, in the glomerulae or proximal tubules, or in the thick ascending limbs with their excess of water. The distal tubules, perhaps, as they extract water, but – you might think – it is only the extra water.
Ah! That vast long drain, where water extraction supersaturates urine – there would crystals form.
And, those uncanny thin limbs, so deep into the medulla.
You are right.
Who Sees Them?
When radiologists spy calcium deposits in kidneys so numerous they name them nephrocalcinosis, the deposits lie – with rare exceptions – in the medulla, the papillum. Surgeons can see them in the collecting ducts and interstitium. For pathologists they lie in the thin limbs, the collecting ducts, and the interstitium – the space between the ducts.
Crystal Deposits in the Cortex
These occur in rare stone diseases. I list them for completeness only.
Glomeruli
In humans, high blood calcium can produce glomerular crystals. Hyperparathyroidism for example, when severe.
Shall I mention intravenous dibasic phosphate in rats, Vitamin D intoxication in suckling rats, calcifications of large immune deposits? No; I demur. Not relevant to humans.
Proximal tubules
We have found calcium oxalate crystals in the proximal tubule S3 segment in primary hyperoxaluria. 2,8 dihydroxyadanine crystals due to APRT deficiency can plug proximal tubules. In transplanted kidneys we have seen scattered birefringent crystals presumably calcium oxalate.
In other words, common stone formers have no proximal tubule calcifications, only patients with rare diseases.
Distal Convoluted Tubules and Cortical Collecting ducts.
Acute phosphate nephropathy from bowel preparation is well known. Distal convoluted tubules contain calcium phosphate deposits in cystine and primary hyperparathyroid patients,and calcium oxalate in primary hyperoxaluria stone formers. In transplanted kidneys tubule and interstitial deposits are found not rarely and are said to be calcium phosphate. In primary hyperparathyroidism with stones, and ileostomy patients, deposits were found in the cortical collecting ducts.
This means, here and there, uncommonly, scattered deposits can lie in the cortex.
Cortical Interstitium
When kidneys fail and blood accumulates excesses of salts and molecules kidneys normally eliminate, crystals form in the space between tubules, the interstitium because blood itself supersaturates. The same for primary hyperoxaluria: so much oxalate is produced it can accumulate in blood and crystallize in the interstitium.
Cortical Blood Vessels?
We believe it is potentially confusing to lump vascular disease and its associated calcifications together with calcifications within the renal tubules and interstitium.
Crystal Deposits in the Medulla and Papilla – Work by Us
Our reports comprises the bulk of human kidney tissue work to date.
Thick ascending limbs
No deposits have been found in any stone formers to date.
Thin Loops of Henle
We have found rare hydroxyapatite deposits plugging thin limbs in ileostomy, cystinuria and primary hyperoxaluria patients with reduced renal function. These are best illustrated in Figure 4 of the ileostomy reference. As I have mentioned, plaque begins in the outer shells of the thin limbs.
Outer Medullary Collecting Ducts
Patients with primary hyperparathyroidism are the only stone formers who show deposits (calcium phosphate) plugging this tubular segment.
Inner Medullary Collecting Ducts
Here is the main place for crystal formation. Are you surprised? The tubule contains supersaturated fluid progressively approximating urine itself. No wonder of it: crystals from where supersaturation is.
Hydroxyapatite crystal plugs
Crystal intraluminal plugs have been found in all eleven stone forming phenotypes examined. The majority of these plugs are composed of hydroxyapatite. Note the link goes to an article on this site which lists 10 phenotypes; ICSF, the 11th phenotype, did not reveal collecting duct deposits in our work but deposits of HA were found in cases of ICSF reported by Wang et al.
Calcium oxalate crystal plugs
Obesity bypass surgery, distal renal tubular acidosis, small bowel resection, and medullary sponge kidney stone patients form calcium oxalate deposits.
Mixture of sodium acid urate and ammonium acid urate was admixed with biological apatite in ileostomy stone formers.
The Odd Microliths of MSK
Microliths, myriads of extremely small, round, non-adherent stones have been found only in the dilated IMCD of MSK patients. These differ from plugs in virtually all respects. Plugs adhere to IMCD lining cells and cause cell damage, and death, MSK microliths do not adhere nor cause any perceptible damage. Microliths are round, not cylindrical, and made up of concentric layers of crystal; plugs also have layers but much less regular.
Cystine Plugs
Cystine plugs also differ from all other plugs in not adhering to tubule cells. They move freely and do not appear to damage the cells.
Ducts of Bellini
We have found Bellini duct crystal plugs in all stone forming phenotypes.
This is expected as these ducts contain the final urine.
Bellini duct plugs may attract overgrowths at their distal ends that protrude through a dilated opening of the duct into the flowing final urine. These proto stones may well grow to clinically significant size. Growth on plugs is one pathway for stone production.
Interstitium
To date, all interstitial deposits found in human kidneys have been hydroxyapatite ‘Randall’s’ plaque. Growth of stones over plaque is another pathway for stone production.
What About Blood Vessels?
No evidence exists showing calcium deposits within the vasa recta within the medulla or papilla. Deposits of hydroxyapatite can be found within and involving capillaries, but this is not evidence of a primary calcification. Theoretical papers proposing vascular injury and calcification as causes of plaque have failed to advance direct evidence in support of the theory.
Crystal Deposits in the Medulla and Papilla – Work by Others
Idiopathic calcium oxalate stone formers
Idiopathic calcium oxalate stone formers display variable amounts of interstitial plaque. Those with hypercalciuria had the heavier plaque deposits, much as we have found. Unlike our work, many patients had crystal plugs in Bellini ducts. Wang et al described much the same.
In biopsy tissue from 15 patients with ‘idiopathic calcium stones’ not otherwise characterized, Khan et al found Large areas of interstitial plaque. Crystals were HA. No plugging was found. Stones were calcium oxalate. The main finding was intimate association of plaque with collagen, as we have also described.
Mixtures of stone formers
Linnes et al studied ICSF, ICSF with malabsorption, phosphate stone formers which included struvite stones, and, also, uric acid stone formers. In 99% of patients they found interstitial plaque with an average low abundance. It was only when they separated out hypercalciuric ICSF that they found high plaque abundances as we have. They found plugging in all stone phenotypes. The patients were mostly female, and hypercalciuria was not impressive. They did not analyse the crystals in the plugs.
Single case report
Report of a single case describes interstitial plaque deposits identical to those we have described. The patient had large calcium oxalate staghorn stones. By EDX analysis the interstitial deposits were calcium phosphate. Tubule plugs were found in the medullary collecting ducts and these were calcium oxalate. We suspect this patient had primary hyperoxaluria.
Putting it All Together
Overall, these and our papers more or less agree. Stone former kidneys contain interstitial calcium phosphate deposits and plugs in Bellini duct and inner medullary collecting ducts. Only we and Khan have described the crystals in plugs to date. So, when we speak of multiple crystal deposits in the kidneys of stone formers, or nephrocalcinosis, we all seem to mean plaque and plugs. MSK differs altogether, because dilated ducts contain myriads of calcium oxalate microliths.
Virtually all Stone Formers Manifest Nephrocalcinosis
Virtually all Stone Formers Form Kidney Crystal Deposits
Shavit, Jaeger, and Unwin concur with Albright: ‘Strictly, the term ‘nephrocalcinosis’ refers to the generalized deposition of calcium oxalate (CaOx) or calcium phosphate (CaPi) in the kidney.’ Since virtually all stone formers deposit crystals in their kidney tissues, virtually all have nephrocalcinosis.
The Kinds of Deposits Number Three
One kind is plaque – calcium phosphate as hydroxyapatite – in the interstitium.
The second kind is plugging of the lumens of the various tubule segments, mainly the medullary and papillary collecting ducts. These plugs are usually calcium phosphate but can be calcium oxalate, cystine, or uric acid salts.
Of the three, the microliths of MSK, unique to this one disease, make up the third.
The Word Means Plaque and Plugs, or MSK
In any one patient nephrocalcinosis means interstitial calcium phosphate crystals, tubule crystal plugs of diverse kinds, or both, and microliths in the dilated tubules of MSK.
Because of this specificity, I propose we restrict the word to this exact meaning: Calcifications within kidney tissues as demonstrated directly in the tissues themselves.
Radiographic Nephrocalcinosis
Because tissue calcifications and stones both brighten the confining shadows of the radiologist with similar points of light but stones do, also. Therefore, we propose the term ‘radiographic nephrocalcinosis’ define what radiologists report. I say this because radiological means cannot always distinguish masses of tissue plugs or of microliths in MSK from stones.
Surgery
Because they visualize stones, plaque, and plugging, and also the odd contours of MSK, surgeons can not only specify nephrocalcinosis but the type of calcium deposit. Therefore, they mean by nephrocalcinosis what pathologists mean. The only difference between them is in resolution. One has a microscope, the other simply an external view of the kidneys at modest magnification.
Meaning of Nephrocalcinosis
Since all stone forming patients deposit calcium in their kidney tissues, it signifies a quantitative vs. a qualitative distinction. Unlike other patients, those with nephrocalcinosis have more tissue calcium deposits and therefore, perhaps, what one might call more disease burden.
By disease burden I mean the tissues carry more crystals in them, and therefore a greater hazard from whatever evil it is that crystals might do.
One such evil: lodgment for new stones to form on. Because stones form on plaque and the ends of plugs, more plaque and more plugs implies a greater stone production potential. Although evidence for such potential must come, eventually, from prospective observations greater tissue mineral burden seems a proper spur to greater treatment effort even now. Such greater treatment efforts mean perhaps more emphasis to patients about diet and fluid change, and earlier use of medications.
Another is tissue damage. For example, crystal plugs cause obvious tubule cell loss and inflammation in the surrounding interstitium. Although papillary, plugging may affect the cortex. Compared to patients without plugging, those with plugging have more cortical interstitial scarring that treatment might benefit. Such treatment, as opposed to stone prevention alone, would specially emphasize reduction of calcium phosphate supersaturation.
Like many stones, nephrocalcinosis quantifies stone diseases. But in a new dimension, one that complements those already in use. Because complementary, the word adds specific value, provided we use it carefully.


Where can I find more information regarding nephrocalinosis in a transplanted kidney? Specifically if a transplanted kidney in the left abdomen would still be the source of flank pain on the left side?
Hi Christine, I arranged an email exchange as noted below to get you more information. Regards, Fred Coe
Hi Dr. Coe,
I am 23 yrs old (F) and have had four stones, the first of which occurred at 17, and the last of which occurred at 21. I’ve always had residual pain, which flared last summer, and I saw a new doctor in the fall. I received an X-ray and he informed me I had no stones, but did have nephrocalcinosis and that was the probable cause of my pain. I had actually been diagnosed with nephrocalcinosis via CT scan at the time of my first stone back in 2014, where the report said I had “some nephrocalcinosis” in both kidneys. He suggested that I needed to be on something to manage it, but I was unable to follow up with him post urine testing as I moved. We did two, 24hr urine tests. I previously had one in 2015. I had bloodwork done by my primary care and he said it was normal, so I’m assuming GFR/related elements were fine.
In 2015, notably, my urine volume was low (.5), my SS CaOx was very high (10.38), Urine Citrate was ~236, SS CaP was 4.20. All dietary factors seemed to be okay.
In the first most recent test, my volume was 1.17, my SS CaOx was 5.55, citrate was 215, SS CaP was 3.26, and urine pH was 7.101. Dietary factors were Na 24 at 262 (it was 60 back in 2015!!), P 24 at 0.943 and Cl 24 at 232.
The next test on the next day was a little better because I drank more water. Vol 24 at 2.22, SSCaOx was 3.95, citrate still super low at 129 so not great, SS CaP at 1.30, and pH at 6.413. Dietary factors were Na 24 at 203, P 24 at 0.794 and Cl 24 at 214.
I guess I’m just wondering how serious NC is and should I be concerned (especially since they were able to tell on both CT and X-Ray)/try to get a new doctor to prescribe me something or do I have some time to handle this? Do you have any dietary recommendations? I was recommended a high salt diet for separate cardio issues but I am trying to resolve/get away from that. I don’t want my kidneys to get seriously messed up from the NC. I seem to be okay on the kidney stone part now but from my understanding NC cannot be cured, only managed.
Hi Madeline, At 23 with stones since 17 you certainly require expert care. I guess your stones are calcium phosphate, but the low citrate is not of obvious cause. The goal is to stop new stone formation and growth of more stones on the calcifications that are in your kidneys – what radiologists are calling ‘nephrocalcinosis’. Your blood is said to be normal, which is critical here. The ideal experts are university based and perhaps there is one in a school near you. Given COVID has permitted telemedicine to open up, you can also use consultants almost anywhere. I would advise this route. Regards, Fred Coe
My mother had a 59 year struggle with 1st kidney stones and the cancer.
I am 42 and have struggled with my kidneys, my entire life.
1. UTIs as a child. These were found when I was five and still wetting the bed.
These UTIs lasted until I was 19 and my urologist for an absest sore in my right kidney.
I had 105. Fever with no infection in my urine and was finally diagnosed with an ultrasound.
2. Kidney failure (both left and right) at 20, while I was pregnant with my 1st son.
3. Ureter reflux at 20, after my son was born.
My urologist put a dye in my kidney and found that both my kidney had reflux.
This is when I took control and watched everything I drank and forced myself to drink (a lot) of water.
4. I constantly past kidney stones for years. My first was at 18 years of age.
I can’t tell you the amount of stones I have past, maybe 1000s but luckily I pased them.
5. I start having chest pains and no one knows why. All of the doctors say it is because of my job (I drive for UPS and left a lot of weight). 6 years with crazy chest pains and finally my liver fails and this was because of gallstones completely blocking my liver. March of 2020, my Gallbladder was removed.
Now, I am having troubles with my kidneys again. Stones in both, and I am not urinating as much as I should.
My urologist passed away, and the doctors now do not understand my history.
I go to the urologist this Friday for another ultrasound (that I had to beg for).
I am worried that this urologist will not pursue the issues like my old doctor.
My right kidney is starting to hurt constantly and my left kidney isn’t feeling so good.
With the information that I have given you, could you suggest why I constantly have issues or is there a surgery of medical fix to my kidney issues?
Thank you.
I know this is a long shot, but I really don’t want to die at a young age like my mother.
Hi Kimberly, Your situation seems very complex. If you say where you live I an try to find a convenient center where you might get the care you seem to need. Regards, Fred Coe
I had a kidney stone a couple of years ago and it was determined at that point i had/have idiopathic hypercalcuria. i have had a lot of soft tissue sports related injuries over the years, typically slow to heal. I recently hurt my hip, glutes, SI doing weights and the imaging showed calcification (ossicle) on the rectus femois, glute medius and minimus tendons. Is there any correlation between hypercalcuria and calcification on soft tissue areas in the body that are not the kidney?
Thank-you!
Hi Barbara, Assuming your blood calcium is normal – part of the definition of idiopathic hypercalciuria, soft tissue calcifications are not part of the condition. Be sure your serum calcium is indeed normal. Regards, Fred Coe
i had a large kidney stone a couple of years ago and have hypercalcuria. My Dr put me on a low level diuretic which worked well for about 8 months (lowered urine calcium levels) but my recent 24hr urine test showed my urine calcium was very high again. He’s not sure why. I’ve been taking Vit D3 ( 3-5k) for years. My Dr doesn’t believe in Vit D and wants me to go off it to see if it lowers my urine calcium. What are your thoughts on Vit D as it relates to hypercalcuria and any thoughts on what would cause my urine calcium to spike again? All other levels are good. Thanks
Hi Barbara, The usual reason is that diet sodium went up. Check the 24 hour urine sodium. Another reason is increase of diet protein, check the PCR see if it above 1. Third reason would be a big increase in diet calcium, especially supplements. Regards, Fred Coe
Hi,
I am having medullary nephrocalcinosis problem in my both kidneys. Are these calcium deposits lead to chronic kidney failure . My age is 29 now
Hi B surendra, It all depends on the cause. Your physicians need to uncover why (and if) your medullary areas are calcified. Many causes, many outcomes. I cannot help from so far away in discerning the cause. Fred
I am 47 years’ old and have had symptoms from my MSK and nephrocalcinosis. I have been on chlorthalidone since I seen Dr. Coe 20 some years’ ago. I also barely put out urine in my first 3 back-to-back 24-hr urines and I was told, I had to drink MORE water. I haven’t left my home without my water bottle in the last 20 some years. The next time I did a 24-hr urine for Dr. Coe, I used 1.75 containers. He was much happier with me. I pee all day, everyday but it has kept me at 100% function so far. I do get pain from the crystals constantly passing out the kidneys but I’d rather that everyday than kidney failure. Make an appt at the University of Chicago to see either Dr. Coe or someone on his team. He and his team are amazing. They were the only ones who found a way to help me. I still to this day, praise Dr. Coe and the University of Chicago.
Hello,
My 17 year old daughter suddenly developed chronic flank pain and hematuria for over a year. Intense Renal Colic spasms several times per week, especially when drinking a lot of water. For over a year, sent from doctor to doctor. Nothing shows up on ultrasound or CT. No actual stones, yet she seems like she is passing stones. Our local Urologists & Nephrologists say her pain in “not real.”
Two ureterscopies with stents & kidney biopsy just done. Now…finally doctor at Mayo Clinic believes her. He found Wide-spread Randall Plaque in both kidneys. No stones, but crystals. My daughter says her kidneys feel like glass or vice grip squeezed. She doesn’t function or live a teens life with this pain.
What can be done about Randall plaque & pain? How can she be cured?
Hi Brenda, I imagine your daughter has idiopathic hypercalciuria, which is easily treated. It causes plaque. Plaque does not cause pain but does promote stones – stones grow on it. Her pain is almost certainly from crystal attacks, and these are prevented by lowering urine calcium and calcium supersaturations. In children IH is the only obvious cause of stones. Regards, Fred Coe
Hi Dr. Cole,
It has been forever since I have been to the University of Chicago. Are you still there? My uncle is Eli Jack Coffman. I am Tami Coffman. I have MSK and Nephrocalcinosis. I am 47 years’ old and back at school for Ultrasound and everyone always wants me as their test subject for kidneys because they light up. I know the last time I was there, I tested with calcium oxalate stones. I had a bad UTI at one time that wasn’t taken care of so when I found my current Urologist, he did a cystoscopy and found that I had an infection in the urethra that had been brewing for years. He had to burn it all out. I also get cystitis really bad a couple times a year. I currently have pelvocaliectasis and my stones have increased throughout the last 20 some years. My function is still at 100%. I would love to come in to see if there is anything I can do to get this accumulation to slow down.
Hi Tami, seeing me is easy right now because of Zoom – like many physicians I practice remotely, still. My secretary’s phone is 773 702 1475. I will copy this note to her so she knows, Regards, Fred
Hello doctor I have a question about what a CT scan of my kidneys said. I’m a 39 year old woman with type two diabetes. The doctor stated that I had hyper dense medullary pyramids and that could be from dehydration or early medullary nephrocalcinosis. #1 what does this mean and two as a diabetic should I be concerned? Thank you
I should also mention I drink a LOT of water. And the CT scan was due to pain in my back and flank they thought I may have a stone, but ended up having just an infection. I am suffering from nausea and cramps in my muscles daily.
Hi Nikole, I am surprised that you drink so much and have dense papillae – perhaps the extra fluid is something new. As for the muscle symptoms, if recent they can occur with an infection and then go away after it is cured. Regards, Fred Coe
Hi Nikole, Your physician is a scholar. I have written about papillary density because it interests me. STONE FORMERS HAVE A KIDNEY ABNORMALITY FLUIDS MAY FIX Dense papillae can signal risk for subsequent stones, and it can be reversed by higher fluid intake. I guess I would favor higher fluids from what you now drink, just in case. Regards, Fred Coe
Is a CT scan or ultrasound more accurate in identifying medullary Nephrocalcinosis?
Hi Terry, A CT. Regards, Fred Coe
Doctor Coe: I have been diagnosed with Randall’s Placque back in 2017. I have suffered with kidney stones both very small and larger ones that I can not pass due to small urethers. I moved to Brunswick, MD about 1 hour from Baltimore. I am writing out of desperation of guidance in what type of doctor I should have to help me with this disease. I am on my second urologist and I think I may need a different type of doctor to see me. Do you know of any doctors in Maryland or Washington DC area that knows and understands Randall’s Placque? I would really appreciate some help to direct me to the right type of doctor. Thank you very much, Barbara Strassner
Dear Barbara, You are a fortunate person. At Hopkins Brian Matlaga is head of the stone program. He trained at Indiana University with Jim Lingeman and I had occasion to work with him. He is outstanding, and can take care of you perfectly. Just go to him. You can say I said so. Regards, Fred Coe
After contracting Covid 19 in Dec 2022, the results of my recent abdominal ultrasound indicated I have Cortical Calcification of the Right Kidney. I came across several articles indicating this is a rare disease. I am troubled about this. I live just outside Baltimore MD. Is there a specialist you could refer me to? Thank you so, so much.
Hi Irene, There is an excellent kidney stone center at Hopkins, Brian Matlaga is head of it and outstanding. I would use his expertise. Regards, Fred Coe
My 17yo was just dx with bilateral medullary nephrocalcinosis of unknown etiology. It was an incidental finding after blood work was drawn due to what I thought was a Lyme flare. His BUN 47, creat 2.0, uric acid 12. Childrens in Boston is taking forever and while my head understands the rationale for needing more data (ie labwork trend after starting allopurinol and Mg) my heart is having a hard time not doing more while his GFR is 39. Any suggestions are beyond appreciated!!
Hi Erica, The institution is justly famous and certainly will come to know what is causing his kidney function and calcification problem. The high uric acid and I gather low serum magnesium point toward genetic disease, but I am guessing. What I know is that he will be challenging even at that superb institution and everyone will have to be patient for a while. Because the diagnosis is not as yet established and the problem is complex I would not hazard any comments. Best wishes, Fred
I greatly appreciate your response!! So far, PH1 has been ruled out, but now waiting on paperwork for genetic testing which can take 1-2 months. In the meantime, no diet changes. This coupled with the fact that my husband has MM is heartbreaking!
We have found the cause through Natera testing. He has mutations to Claudin 16 which caused FHHNC. Is it possible NC causes lower back pain?
Hi Erica, I have your much longer email and plan to answer it. Nephrocalcinosis can include tissue calcium deposits and stones, which latter cause pain. Infection is also possible. As for the underlying condition I will answer as above. Regards, Fred Coe
Hello Dr. Coe. Thank you so much for your invaluable website, which i just discovered. Could you please recommend a medical team /center in Los Angeles that practices stone prevention at your level? I’m 52 and was just diagnosed with nephrocalcinosis after never having any (known) kidney issues in my life, so I want to work with a great medical team on understanding it and working to prevent it as much as possible. I so appreciate your advice… – Warren.
Hi Warren, Dr Dunn at UCLA classifies himself as particularly well trained in kidney stone surgery. Under prevention I found this, but they do not name particular physicians. The institution is well known, and generally accepted as excellent. Regards, Fred Coe
Thank you very much Dr. Coe! I’ve made an appointment with Dr. Dunn, and will also be seeing a nephrologist at UCLA who works with him. I so appreciate the help you’ve provided me and others. My best, Warren
I’ve had many kidney stones removed they are all calcium oxalate stones. My 24 hr urine says I have too much calcium in my urine. Is there ANY place that could help me dietary, as I don’t want to take medication. I’m in Peoria IL.
Hi Janice, Jill Harris would be ideal. I work with her. Regards, Fred Coe
Is there any way to have a televisit with you?
Of course. My secretary is at 773 702 1475. SHe can make all of your arrangements. Regards, Fred Coe
Hi there,
Thank you so much for this comprehensive article. I’m writing to ask about what pain typically looks like. I am 5 1/2 months pregnant and in the last few weeks have been having rather intense right sided pain. After my doctor suggested a possible kidney stone I was sent for a kidney ultrasound and told I have nephrocalcinosis. I have a history of endometriosis and most of my pain would have been on the right side previously, so that’s where my brain originally went in regards to the pain. The pain for which at first only struck when I’d wake up in the middle of the night to pee, but has now spilled over into the day hitting me at random times. Unable to lift my right leg when it comes on.
Any information you could provide on if this is a normal presentation for pain would be much appreciated. I was told the deposits are roughly 5mm
Thank you so much,
Vanessa
Hi Vanessa, During pregnancy nothing should be done unless a stone passes and requires urological care. Surely your condition has a cause and treatment but 24 hour testing is highly affected by pregnancy and testing therefore not helpful. Try to complete the pregnancy successfully, and breast feeding if desired. Thereafter your physicians can look into the causes of this renal calcification and fashion a treatment program. Regards, Fred Coe
Hi there,
Thank you so much for this comprehensive article. I’m wondering if you may be able to provide some insight on what to expect in regards to pain levels. I am 5 and 1/2 months pregnant and have been having some very intense pain on my right side. It started when i’d get up to pee in the middle of the night, but has since spilled over into the day, hitting me sporadically. I originally thought it may be related to my endometriosis, but after a kidney ultrasound I was told I had nephrocalcinosis. I have yet to receive any real information from my doctor on what to expect in regards to pain, and if indeed the pain I am feeling is fully related to this. When the pain hits, I am unable to lift my right leg. Any information you may be able to provide would be so greatly appreciated.
Thank you so much,
Vanessa
I believe I answered to this already, Fred
I have Renal Tubular Acidosis, diagnosed 18 years ago. Kidney stones began 22 years ago. I now have been diagnosed with dense bilateral medullary nephrocalcinosis with stone formation. Over the last several years I have passed on average 25-30 stones. Many up to 10mm, with only flomax and OTC Tylenol. I have been in more pain in the last 4 weeks, having passed around 12, leading to the CT scan diagnosis. My RTA is managed, though I am in state 3 CKD. The stones are not. What are my treatment options since there is so much calcification?
Hi Michelle, I apologize for the extreme lateness of my reply which is because the site has been under reconstruction. A lot depends on what kind of RTA you have. Do you have the gene type?? Likewise, the details of the 24 hour urine chemistries matter. If you offer more information perhaps I can make some suggestions for your physicians to consider. Regards, Fred Coe
Hi,
Need support for the nephrocalcinosis treatment.
Thank you,
Hi RK, can you be a bit more specific about the problem and your requirements. Perhaps I missed this in the post itself. Bests, Fred Coe
Hello,
I am 6 months postpartum and breastfeeding. I had not passed a stone that I was aware of prior to the c-section. I have had numerous UTI’s since his birth as well. CT scan shows innumerable calyceal stones throughout both kidneys that may reflect medullary nephrocalcinosis. 24 hour urine collections have shown low citrate and urine ph of 6.6 as my risk factors. My 24 hour urine calcium has all been below 200. My stones are calcium phosphate. I pass multiple stones a month and am desperate to feel better. I am in the Houston, TX area if you have any recommendations for experts.
Hi Angela, Your low citrate and high urine pH are typical for calcium phosphate stone forming and may reflect genetic distal renal tubular acidosis.The medical school at Housten faculty in urology to not list kidney stones as special areas of interest. The UT SW medical school in Dallas has a world famous program and is perhaps your ideal. Dr Pearle is famous for stone surgery and prevention. Regards, Fred Coe
My daughter, about 49 years old, has just been diagnosed with nephrocalcinosis. Has had kidney stones in the past, maybe 3 that manifested. Now her kidneys show dense calcium cysts. No diet or treatment has been ordered. She is seeing a nephrologist but would like to know if you could recommend a nephrologist in the Murfreesboro/Nashville, TN area?
Hi Sheree, You are fortunate in that Vanderbilt has an excellent program with two experts I know: Drs Hsi and Miller. The institution is superb. See if you can get your care there. Regards, Fred Coe
Hi.I recently had a whole abdomen ultrasound and the result said “tiny cortical calcification considered in the right, otherwise unremarkable kidneys”. Is this a cause for concern? Also, right kidney length is 9.3 cm, left kidney length is 9.4 cm. Is this normal?
Hi Oli, cortical calcifications are not the rule in stone disease. It would point to some other kind of problem – or trivia – about which I am not expert. Regards, Fred Coe