
We have known for a long time that phosphate stone formers are mainly women. We also have long known that phosphate stones form when urine is more alkaline, and that women produce a more alkaline urine than men. So the fact of phosphate stones being more in women seemed a consequence of their physiology.
But why?
Why do women have this physiology, this alkaline urine? Have it so consistently they form calcium phosphate stones as geological relics?
If asked, most would say women do this because of how they eat.
Compared to men women eat more veggies, a source of alkali, and less meats – a source of acid. More alkali in their diets, a more alkaline urine, phosphate stones, and that was that.
And that was wrong.
Not only wrong, but in being wrong diverted our gaze from something interesting, something, perhaps, evolution favored because it increased the fitness of our species.
The study, Young Spartans Exercising, by Edgar Degas, hangs in the Art Institute of Chicago. The painting itself hangs in The National Gallery, London. Degas never expressed his own reasons for creating this scene of young women and men exercising together, but the independence of Spartan women is well documented. I put it here because it illustrates one way a culture sought to enhance fitness of the species, to increase its chances for successful continuation. Although the London painting is more finished, this one is, to me, more graphic, more vital, more exciting, higher in energy.
Alkaline Urine is the Least of It
When setout to understand how women produce a more alkaline urine, our intent was, if not modest, certainly circumscribed. One possibility was prevailing opinion: higher urine pH in women was from their diet and when women and men were studied while eating the exact same foods their  urine pH values would no longer differ.
urine pH values would no longer differ.
It is Not the Food
That possibility was eliminated at the very beginning. Everyone ate in our clinical research center, so we know their diets were identical. Of course men eat more than women, but we adjust for body surface area.
Here are the urine pH values for women (F) and men (M) fasting, fed, and overnight (ON).
Fasting, and ON the sexes do not differ. The female bars seem higher but the variation is large (the thin standard error lines).
But when they ate, women far exceeded men. This is because the women not only start higher, fasting, but vigorously increase their pH when fed whereas men scarcely increase it.
So food is critical, but the food was the same food. This means that when they eat women extract from their food different proportions of things that regulate urine pH. It is biology, not what they eat, that matters.
Food Alkali
What food has that can make urine alkaline is alkali, substances that pose an alkaline load on the body. The kidneys respond to that load by removing the extra alkali, and in the process make the urine more alkaline.
So this one graph, showing the sex difference in pH from eating the same food, tells us that women extract more alkali from their food than men do. It is true that the resulting alkaline urine pH predisposes women to calcium phosphate stones. But it is equally true that the extra alkali may be highly beneficial.
But some people might argue that perhaps women extract less acid from their diets than men do, so the result is a higher urine pH for a different reason. To sort this out, we need to expand a bit on just what alkali and acids are.
What are Alkali?
Like the positive and negative electrodes of a battery, or the north and south poles of a common magnet, acid and alkali are names for opposites, names that may have historical meaning but are, in reality, verbal conventions. With apologies beforehand to my acid base colleagues, I offer a simplified image to further my article on women. If what I say is not exact, it also is not wrong.
Protons Personify Acid: pH
Protons – the nuclei of hydrogen atoms – can be viewed as the currency of acid base exchanges. Protons have a single positive charge. Because their concentration can range very widely, we use logarithms to express it, and call the logarithm pH. If [H+] is the concentration of protons in water, what we are made of, pH is the logarithm to the base 10 of 1/[H+]. So, as proton concentration falls, pH rises.
Acids and Bases Viewed from a Proton Perspective
An acid is a molecule that can donate a proton, a base – or alkali in the plural – is/are molecules that can accept protons.
Right away you can see the relativity in acid – base exchanges. Consider two acids. One is ‘stronger’, meaning it gives up its proton more readily than the other. So it is the acid in the relationship while the other of the two plays the role of base.
Water Is an Acid and a Base
Since most of what we are is water, we usually speak of being an acid or base in water.
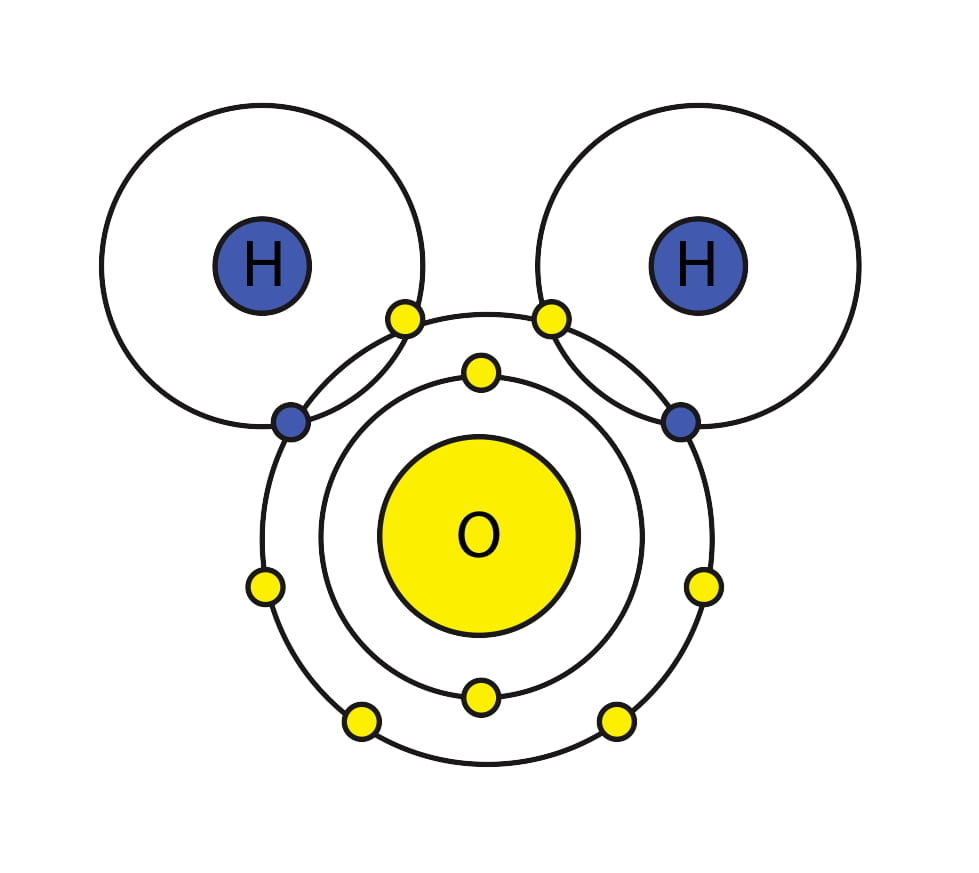 Water is an oxygen atom (Yellow) with two hydrogen atoms (H): H2O. The hydrogen atoms are bonded to the oxygen atom because they share electrons (small circles orbiting the big central nuclei). The hydrogen wants one more electron to fill out its shell, the oxygen wants two more electrons to fill its larger outer shell. They share their electrons and both are happy.
Water is an oxygen atom (Yellow) with two hydrogen atoms (H): H2O. The hydrogen atoms are bonded to the oxygen atom because they share electrons (small circles orbiting the big central nuclei). The hydrogen wants one more electron to fill out its shell, the oxygen wants two more electrons to fill its larger outer shell. They share their electrons and both are happy.
A hydrogen atom without its electrons is the proton I have already spoken about. Being so powerfully charged and small, protons hardly exist alone but are almost always found attached to other molecules via charge attraction.
When water gives up a proton we have, instead of H2O, the hydroxyl ion: OH–. The minus sign connotes the loss of a positively charged proton. When water takes up a proton we have H3O+, the hydronium ion, + denoting the extra proton. So, in water, pH really connotes the concentration of the hydronium ion.
A molecule that will take a proton from water can be called a base, or basic or alkali molecule – compared to water, and one that donates a proton to water can be called an acid, or an acidic molecule compared to water. Comparison to water is only one way to define acids and bases, and hardly the most general way. But for this article it is a good way, one that will get across the main points of what we have found out about women and men.
pH of Water
When pure, water has a pH of 7. Since water is the medium our cells live in, and also the medium of their interiors, we tend to speak of acidic and basic – or alkaline – solutions as below or above pH 7. Our blood pH is 7.4, in health, or slightly alkaline. Urine pH varies from 4.5 to nearly 8, because kidneys need to excrete protons or alkali in order to maintain the blood pH at 7.4 despite what we eat and drink, and what our cells choose to let into or take up from the blood.
What Alkali Do We Extract from Food?
Products Food Made When Alive
Women and men extract molecules their body cells can utilize as fuel, can oxidize to carbon dioxide and water thereby releasing energy that sustains life.
Some of these fuels are taken up into cells and oxidized with a proton, because they are actually acids. They gave up their proton when part of a living thing. We inherit the molecule without its proton and give it one back so it can be used by our cells to produce energy. Because it takes up a proton, it is, in the process of its metabolism, a base.
Think about potassium citrate, a molecule well known on this site.
Citrate as An Example
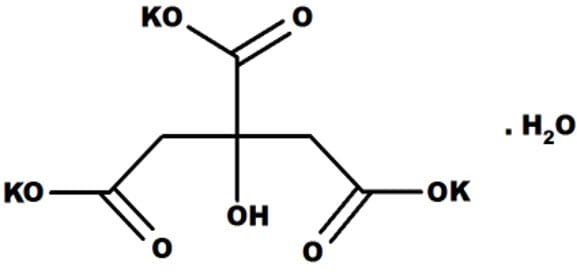 Citric acid is a proton donor of considerable force. It is also critical to one of the most important metabolic energy producing cycles we own, called, incidentally, the citric acid cycle.
Citric acid is a proton donor of considerable force. It is also critical to one of the most important metabolic energy producing cycles we own, called, incidentally, the citric acid cycle.
What we eat can have in it considerable citrate, the acid molecule that has given up some of its protons. Being highly charged negative where the proton would bind, it must be accompanied by positively charged atoms or molecules – usually potassium, K, as shown here. Nature never allows highly charged molecules to swim in water unaccompanied by some opposite charged mates.
Note that citric acid has three negative sites to hold a proton, presently occupied by potassium atoms (‘K’ in the figure). So it can take up and give out three protons. When all three are in residence we call it citric acid; when even one is missing we call it citrate.
Plants, especially, have a lot of citrate, but even meats have it because the cells were once alive and processing this molecule. We absorb it with its potassium, or sodium, or calcium, or magnesium (all positively charged atoms). Some will be metabolized, but to enter into the citric acid cycle it must take up all of its protons, and the only place to get the protons from is the blood.
So citrate acts like a base in the body. The linked article reviews all of the forgoing material in a lot of detail.
Citrate is Just an Example
Citrate is part of an army of like molecules, perhaps hundreds of them.
Cells traffic in acids that are made into each other, transformed in the process of producing energy and things cells need to make. We eat the acids mostly without their protons, which have been left behind in the blood of the animal or sap of the plant before it was taken for us to eat, and for the most part – in animals at least, removed by the kidneys. A name for these protonless acids is ‘food anions’, negatively charged molecules that take up protons when metabolized.
So, although I have used citrate as a familiar example, when you think about women taking up more alkali from food than men do it is not citrate alone they take up but a vast variety of anions like citrate: Acids without their protons, that can take up protons in order to enter our cells and be useful to them as nutrients.
How Can We Measure The Alkali We Eat?
As Something Missing
Because food anions come into our blood with their positive charged mates, sodium, potassium, calcium, and magnesium, they will show up in urine as a gap, a space between the sum of their positively charged mates and their main negatively charged counterparts, chloride and phosphate. The anion nutrients are there, in the urine, but we detect them only by the charge gap between materials we cannot metabolize: positively charged sodium, potassium, calcium and magnesium, and negatively charged potassium and phosphate.
In principle, with modern high speed systems, we could measure all of the food anions in urine and determine each one by name. But for the present purposes that would add nothing of importance.
How Do We Do It?
We measure the concentrations of all six charged atoms, multiply each by its number of charges, and calculate the gap.
The concentrations are in moles, weights scaled to the sizes of the atoms.
The number of charges on an atom are known: sodium, potassium, chloride are 1; calcium and magnesium are 2. Phosphate differs from the other charged molecules, because it has a proton that can stay on it or leave depending on the pH of the solution it is in. In blood, pH 7.4, its charge is 1.8 because on average that is the fraction of sites unoccupied by protons.
So the gap is (sodium + potassium + 2 calcium + 2 magnesium) – (chloride + 1.8 phosphate).
We can call what is measured by this gap absorbed food anion.
But the common term is ‘GI anion’.
Do Women Absorb More GI Anion?
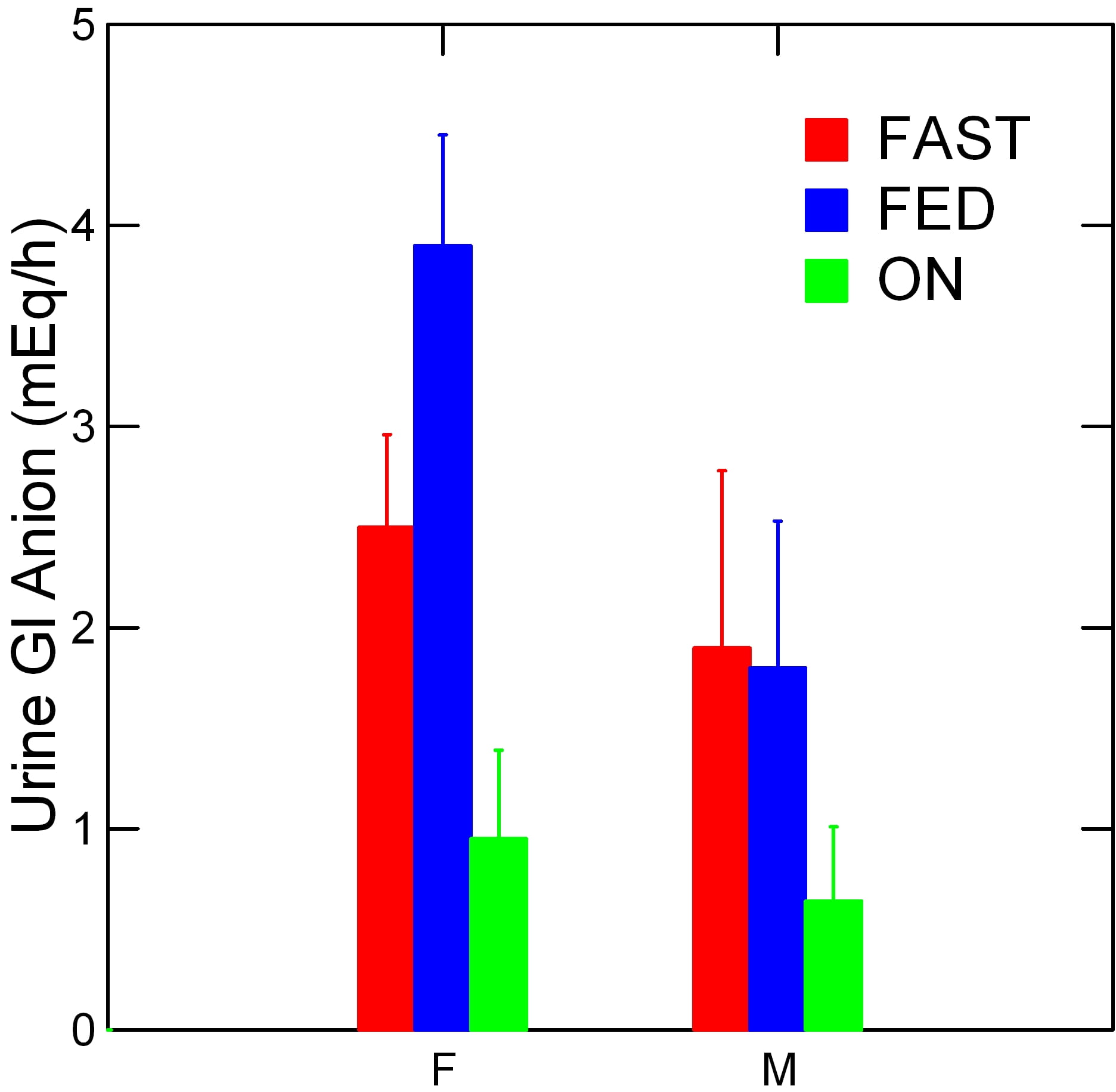 Of course. Why else would I lead you into this labyrinth?
Of course. Why else would I lead you into this labyrinth?
Fasting and overnight women and men look much alike. But look at the blue bars. Women tower over the men.
Remember, this was exactly where their urine was more alkaline – higher pH.
Also, remember what GI Anion is: Just the difference in net charge between the positive and negative atoms.
And, the men and women ate the very same food. Men ate more than women, being larger. But that will not much matter. We are measuring a difference of charges, not actual amounts.
The difference in GI anion between women and men, fed, is 2.1 mEq/hr and our fed mean is over 12 hours meaning an additional flowthrough of 25.5 mEq over the fed period.
But, This is In Urine
I know.
GI anion, measured like a ghost, as a space missing between two solid masses, is what never got used in the body, It came in as something cells could eat but did not, and left the way it came. So these ghostly molecules did not take up their protons and become food. They escaped altogether and were lost.
Why then did the urine pH rise?
Some Was Metabolized
How can we know that?
Well, if it were, we should seen signs. For example, urine might have more alkali. Blood, too.
Urine must have had more alkali. Its pH went up, meaning fewer hydronium ions.
But this is too indirect, too conversational. Charming, perhaps, but not what one hopes for.
If I am to fully narrate what happened, I need to enlarge the circle of our mutual acquaintances.
I need to introduce another player, one possessing an overwhelming power and versatility in the kingdom of acids and bases.
We need to make familiar to us the prince of alkali, bicarbonate: Magister Ludi.
Bicarbonate and the Great Cycle
You can buy a five pound box of sodium bicarbonate on Amazon for $3.41. But howsoever inexpensive, bicarbonate is a magical material.
The Players
This nifty figure is from an equally nifty presentation of the true arithmetic for calculating things in this very complex molecular universe. It is complex because the bicarbonate system lives in air – as CO2 gas, and in water – as dissolved CO2 gas, carbonic acid, bicarbonate, and carbonate. The total of all four is called total CO2 or TCO2 .
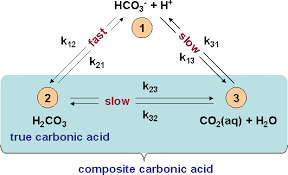
At the top is bicarbonate, a proton acceptor next to its proton. It can slowly decompose into CO2 gas and water, and the dissolved CO2 gas can leave into thin air. Bicarbonate can be made very rapidly when carbonic acid (lower left) simply gives up its proton. Carbonic acid, like bicarbonate, can slowly decompose into CO2 and water.
But these reactions go both ways. CO2 and water can slowly make carbonic acid or bicarbonate. Thence the double arrows.
In the human body, CO2 is made by cells as they metabolize their nutrients to make energy for their work, and removed through the lungs. And that removal is regulated, so the dissolved CO2 gas is very constant. Furthermore, when needed, an enzyme, carbonic anhydrase, can speed up the processing of carbonic acid into CO2 and the reverse.
The Play
If protons enter the blood, bicarbonate can take them up becoming carbonic acid – the fast reaction. Carbonic acid decomposes to dissolved CO2, and dissolved CO2 can leave the body via the lungs, in expired air. So, a steady stream of protons can push down the concentration of bicarbonate, whereas a stream of alkali can raise that concentration – by pulling on carbonic acid and dissolved CO2 to make more bicarbonate. Remember, these are equilibria, the ratios of the players will maintain themselves.
Carbonate itself is not on the figure because it releases its proton only at pH values far above blood or urine, so it is a very minor component. Even so, TCO2 includes it.
You Need Not be a Body
Put water in a bottle. Get a tank of CO2 gas. Figure out a way to put a high pressure of CO2 gas into the bottle. Carbonic acid will be made and convert to bicarbonate and one proton, making the water more acid. You have made carbonated water – acid tasting, bubbly.
Now, spoon in some proton acceptor – like phosphate. It will begin to soak up protons. If you keep the CO2 gas pressure high more carbonic acid will be made and give up its protons to make bicarbonate. You are making a form of Pepsi or Coke, but without the ingredients to make it taste nice. You are making bicarbonate out of thin air – CO2 gas.
Bicarbonate: Buffer
Our fastidious and highly evolved cells tolerate changes in blood pH poorly, very poorly.
As protons come in or go out, bicarbonate takes them up or gives them out to keep pH constant. As it takes protons up, bicarbonate becomes carbonic acid which vaporizes into dissolved CO2 that leaves the blood in our expired air. As it gives protons out, carbonic acid becomes bicarbonate, and continuously restores itself from dissolved CO2 gas which our cells are always manufacturing in their processes of metabolism.
So the bicarbonate system – if I may use this term – ‘buffers’ blood pH, keeps it relatively constant despite the comings and goings of protons. But buffering leaves a mark, or signal of its actions. As it takes up protons bicarbonate vanishes into carbonic acid and CO2; as carbonic acid gives protons out it becomes bicarbonate which appears in blood as if from thin air – which is in fact where it came from.
So the comings and goings of protons are signalled by parallel falling and rising of blood bicarbonate.
Did Our Women Make More Bicarbonate?
If they actually used some of the GI anion, did not let it go into the urine but took it up into cells with a proton, that would be to make new bicarbonate. It is a removal of protons from blood, and the bicarbonate system must respond in the only way it can.
We can look in urine and ask if more is being lost, but that could mislead us. It might be lost from blood. We can look in blood, but that can mislead us – it might be retained by kidneys. We can look at both, and see what we can see.
Urine Total CO2
Urine total CO2 rose.
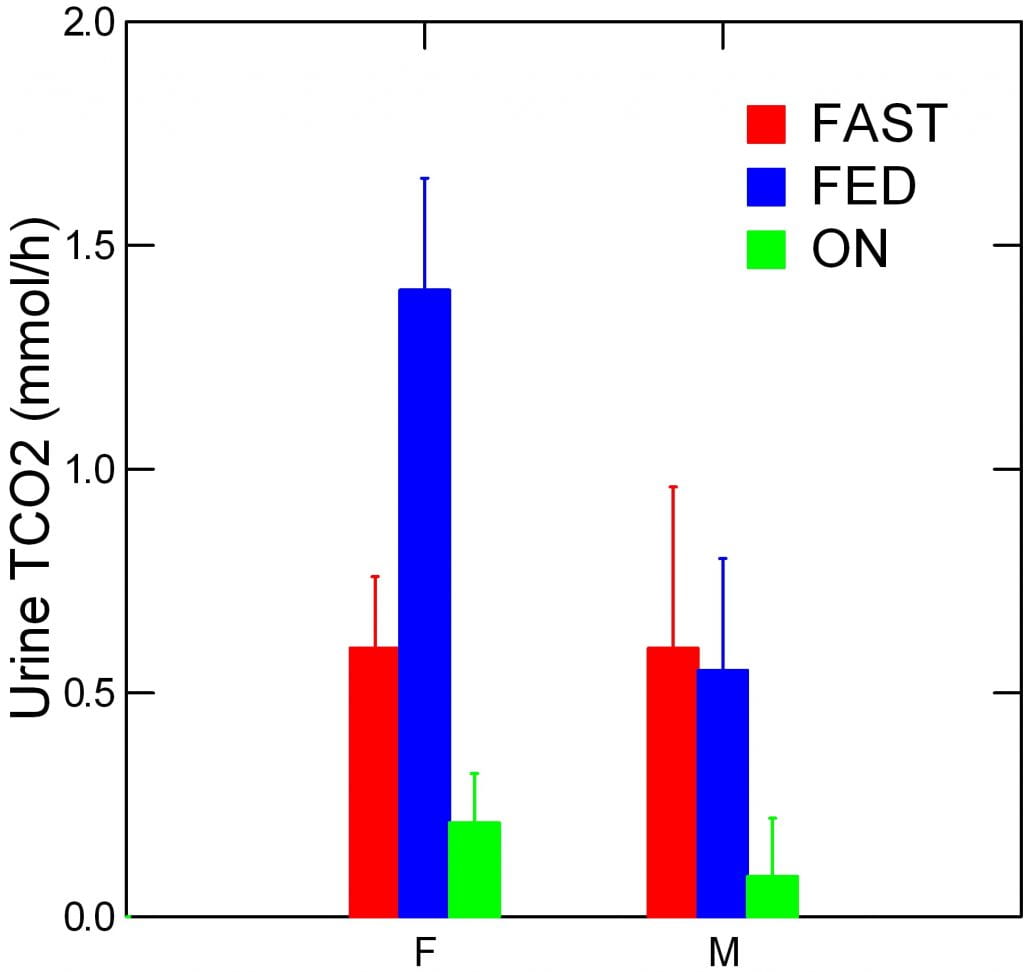
You knew it would. Would I have pestered you if it did not?
Think about this. The GI Anion we measure is anion that was absorbed from their food. It was lost in the urine. Lost from the body and lost as a source of new alkali for that body.
Instead of taking up a proton and serving as food for cells, it left, without ceremony, in the urine. Essentially it left as it came in, without effect.
But at the same time, with food, urine total TCO2 rose, a lot.
The sex difference in the amount of TCO2 excreted over the 12 hours of the fed period was 0.85 mmol/hr x 12 hours or 10.2 mmol in total. This is about 1/2 of the sex difference in GI anion I calculated just above as 25.5 mEq/12 hrs of the fed period.
That must mean women made new bicarbonate, or that the kidneys decided to lose bicarbonate from the blood into the urine.
How can we tell the one from the other?
That is not hard. We can measure TCO2 in blood.
Be clear. I have already said that almost all of the CO2 species in blood – and urine, too – is bicarbonate.
Serum Total CO2
It rose, too, at the same time.
It need not have to prove my case. If it just stayed constant and yet urine bicarbonate rose, it would have been proof enough that new bicarbonate was made. Only a fall in serum bicarbonate would have shredded my case.
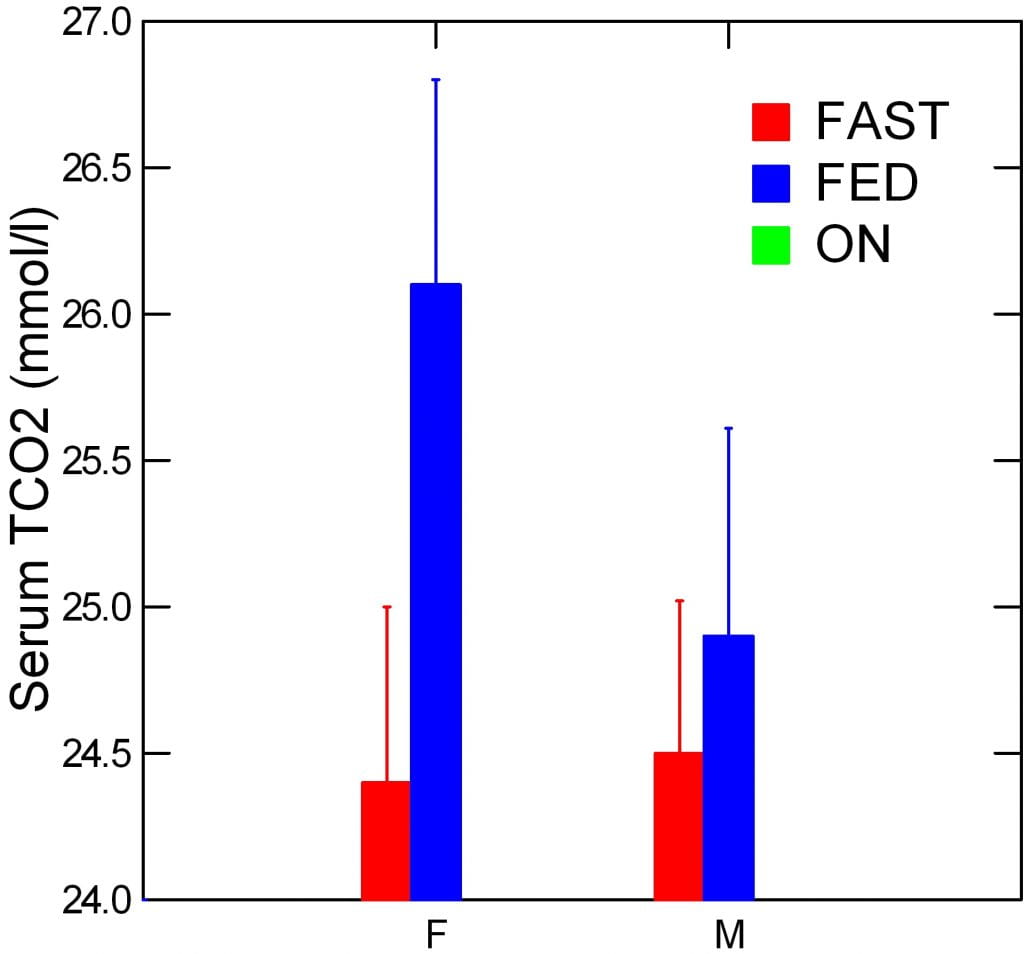
In other words, women must have absorbed more GI anion than men, and converted some of it into bicarbonate, leaving the rest to go into urine.
Extracellular Fluid Bicarbonate
We measured TCO2 in serum, but this small molecule spreads out beyond blood into the much larger pool of fluids that bathe the body cells.
If we assume the total volume of fluid outside the cells (includes serum) is about 14 liters, and the total CO2 is dissolved throughout it – very approximate estimates indeed! – the difference between men and women amounts to 1.2 mmol/l x 14 or 16.8 mmol produced. If we add this to the difference in amounts in the urine, 16.8 + 10.2 we get 27 mmol of new bicarbonate difference.
Men do less. They increased their blood total TCO2 by 0.4 mmol/l whereas women increased by 1.7 mmol/l. The mean increases of 1.7 and 0.4 for women and men, respectively, applies to the entire fed period of about 12 hours. So one can say that the particular food used in this research gave women an increased concentration x time benefit of (1.7 – 0.4) x 12 or 15.6 mmol hr.
What About More Alkali or Less Acid?
I wrote all this presumably to answer this issue – is it that women extract more alkali or less acid from their food. Clearly, it is the balance that differs. GI Anion, our estimate of food alkali is actually a difference between positive and negative charges, and when that difference is itself positive it means that the negative nutrients in the food – the anions – were more efficiently absorbed. The anions are metabolized to bicarbonate.
The other way around, a negative GI Anion, would mean that food cations predominated. When we use them for energy they release a proton.
The Value of Alkali
Biology and Desire
I have shown you a real biological difference between women and men, one that favors women with more daily alkali from what they eat. But we all know that on top of this biology women choose more alkaline diets than men do, diets richer in fruits and veggies, and less weighted by meats. Perhaps this, too, is biology. After all, many of our choices seem that way. In the real world, biology and desire add together, making women a more alkaline interior. No doubt diet and biology lead to their higher urine pH, and its unfortunate consequence of calcium phosphate stones.
Health Implications
I have no way at present of quantifying what this might mean for health except to say that contemporary science discloses negative health effects from the chronic low grade acid load our current diet imposes. While we need a lot more study of the matter the regular alkaline bath to which women treat themselves daily may well improve their overall health in comparison to men.
Men are less advantaged but certainly can make up for it. If they are less efficient in absorbing alkali from their diet, they can eat foods high in alkali such as fruits and veggies. Women presently eat more of those foods than men do, but the reverse would make more sense.
Let us say it would seem as if both sexes can benefit from fruits and veggies, but men can benefit most.
Why The Sex Difference?
But this leaves to one side the obvious question, why evolution – for what else could produce so profound a difference in behavior – has favored a greater female efficiency in diet alkali absorption.
I have no data, but I do have a rather obvious speculation. Women produce children and milk. Both impose extreme demands on their skeletal mineral stores. Alkali loads could promote bone mineral uptake between pregnancies so as to compensate for losses during pregnancy and from milk production.
The speculation is modest enough I demur to marshall published evidence. But if I did, much of it would support the idea that alkali can increase bone mineral in adults.
More broadly, to stare at so clear a sex difference is to wonder at it. And, if my science is too dim and weak, others can step forward with better, and make some progress as to how the difference arises, and for what vast and presently mysterious purposes it was made to be.

Considering all of the above, why have all my stones been calcium oxalate do you think?
Hi Christine, You are a very shrewd person. The paper we have just finished answers your question. Women who produce calcium oxalate stones seem to have lost this remarkable alkali physiology. But don’t tell anybody as the work is not even submitted for publication yet. Why they lose it, or how, remains for another day. Fred
How you are able to marry your deeply pleasing writing style with this complex science is a wonder. It keeps me reading to the end with your humor and deft summations. I understand little, but manage to grasp the salient points and derive great pleasure in the process. 2 stones in 20 years and hope to never encounter another. Thank you for your work
Hi Margaret, Thank you for the compliment. The marriage began as a convenience and gradually became a marriage of love. Nature is wonderful wherever you encounter her, and medicine is perhaps one of the best places of all. As for you, a stone every 10 years is still too many. Be sure and figure out what causes them. Warm regards, Fred
The stones that I collected – more like sand were 95% Calcium Oxalate (dihydrate 40% monohydrate 55%) and 5% Calcium Phosphate. (I also have a large bladder stone 20×23 mm. Yikes!) There were translucent stones, again sand size, that I was unable to collect because they dissolved in the attempt. I don’t know what type those would have been but assume that the percentages may not reflect what is actually in my body. Is it common to have both types? The four in my right kidney (the largest being 12mm had completely dissolved with minimal discomfort by the time I had a CT scan aprox 2-1/2 weeks later. The 2 in the left had reduced in size 50%.. The bladder stone had reduced to 15x20mm. Is it common to have so many stones? The only thing I can think of that I did differently was to take an Ayurvedic herb called Parnavadi guggalu. I had a few stones 31 years ago. I had been prescribed over 500 milligrams of sustained release Vitamin C daily to see if it would address a symptom… which it did… but it may also have created kidney stones. My urologist attributed the stones to the high vitamin C intake and the fact that it was sustained release. I have thought the current stones may have developed due to the way I am have been eating to try to gain weight (I needed to gain 10 pounds) I loaded up on avocado, olive, nuts (especially almonds), 3 eggs for breakfast with chard or collard greens every day, 3 meals a day plus a protein drink… and very little dairy except for occasional goat cheese and yogurt…. did I create 7 monsters?
Hi Donna, I am confused in that calcium oxalate stones do not dissolve. Was the initial imaging ultra sound and the second image CT? If so, I suspect the ultrasound was faulty as it is very poor compared to CT. Are you sure the bladder stone was calcium oxalate – uric acid stones do dissolve, readily. Frankly, I do not understand what has happened but I suggest you use ultra low dose CT to follow up and see if stones are really changing – minimal radiation dose. Likewise, I would hope you have had blood and 24 hour urine testing to see what is wrong with your urine chemistries and causing crystal formation. Regards, Fred Coe
There is a common suggestion to drink diluted apple cider vinegar as a preventative for kidney stones, especially calcium oxalate. This would seem contrary to your conclusion that alkali extraction from food is beneficial. Am I missing something?
Hi David,
We are not fans of drinking ACV. There is no science discussing its value.
Best, Jill
Hello Dr. Coe, My eyebrows raised after reading your comment “Only Uric acid stones can dissolve” where do I sign up? I am 54 years old & have uric acid stones of the plenty – ct scan on 7/14/19 in ER suggests 3 to 4 stones 3.5 cm located in my left kidney.Since then I have had 2 laser lithotripsys with stent placement. First procedure doctor said he removed 15 stones from the ureter. After my first stent was removed I started getting severe stone pain again and within 5 days I was back in for my 2nd procedure as ct scan showed 3 stones in ureter. With that stent in place I passed alot of tiny stones or red gravel. After 2nd stent removal doctor stated stones were already forming on stent which was in place 1 month. Pain started again the next day 30 hours later passed many stones largest being 9mm x 5.1 mm, 7mm x 5mm and many smaller stones. 3 days later passed similar sizes but larger quantity. Now 12 days later awaiting ct scan results due to ongoing stone pain but nothing passing on its own. Likely another lipotripsy/stent placement I’m afraid but how many of these procedures can I safely go thru in such a short period of time? Can not take Vicodin or like meds as makes me very ill so INSAIDS has been getting me thru but doctor says I need to back off of these since for the last 6 weeks I have been taking 800 mg. 1 to 3 times daily. Tried tylonal at doctors request but it does not subside my discomfort. The ibprophen isn’t helping my gerd situation either. I take 20 mg of Prilosec daily otherwise no other health issues. I have failed to drink adequate water throughout my life but now drink 65 to 75 ounces daily without fail. What are your thoughts besides I am long winded. Thanks – Sheila
Hi SHeila, I answered your email directly but copy more or less the same here so others can share. Indeed uric acid stones will cease when urine pH is raised above 6, and also such stones can dissolve. I would advise you ask your physicians about this and I am sure they will want to treat you in such a way as to achieve this higher pH. I assume the stones are uric acid, perhaps your physicians found otherwise, so that is very important. If they are uric acid prevention is virtually certain. Regards, Fred Coe
Dr. Coe, Thank you for sending a direct email however for some reason I did not receive it. Just an update since my original note a couple weeks ago I did have a third lithotripsy with stent. Each time the stent is removed numberous stones will pass but within days to a week they stop passing and block the ureter. Just had stent removed 2 days ago and my pain has returned but the stones stopped passing already so I know my fourth lithotripsy will likely be next week. My stones are uric acid – in regards to taking medications to prevent or dissolve stones my Urologist said we’ll get into that once we get thru this. Thank you so much for providing all this knowlege and for answering everyone’s questions! It can be very overwhelming scouring articles online but finding your website has been so helpful! Thank you, Sheila
Hi Sheila, I tried to answer you directly but somehow the mail did not get through. I think it is because of the mailer used by my site engine, WordPress. You are making uric acid stones, and they stop instantly when urine pH is raised to above 6 with potassium citrate. You should be on that agent immediately. Repeated lithotripsy for uric acid stones is not an ideal approach when medical treatment can dissolve the crystals. I would bring this suggestion to your physician and ask her/him if this might not be helpful. Regards, Fred Coe
I have RTA distal type 1 & MSK since birth. Dx’ed at 12. Came from my mother’s maternal side, my grandmother died in her 40’s, my mother died at 62.
My mother had renal transplant, RTA, MSK, also dx’ed w/ polycystic kidneys?? CABG x 3, brain aneurysm, HTN, hyperlipidemia, restless leg syndrome.
Both my younger brothers had dialysis, renal transplants & CABG. We were told that I had the worst kidney disease; I am the oldest, 64 yrs old;
The last 2 years I slipped into stage 3 kidney disease. I have 2 children, my daughter has no kidney disease, my son has RTA distal type proximal 1
& MSK. He is 37 & already in stage 3. Naturally I am very concerned for him.
My 24 hour urine
pH is 7.866
Ox 24 43
Vol 4.25
ss CaOx 0.82
Ca 48
Ox 43
Cit 686
ss CaP 0.16
ss UA 0.00
UA 0.396
Bood work
CR 1.25
GFR 42
Ca 10.1
T chol 142
LDL 136
HDL 86
Tri 62
Everyting else CBC, other chemisties, PTHI.
My Dr just reduced my K Citrate to 45 meq/day from 60 meq/day. Also told me to decrease my Vit D3 to 1000 IU’s.
I was taking 3 gm’s of NaHCo3/day on my own, which I was advised to stop completely.
I take Mg Citrate, lisinopril 5mg Turmeric, Cranberry extract 2x week. I take Zetia & Crestor intermittenly,
since I am not a fan of statins & I already have muscular pain. I am going to start on D-Mannose. I drink
2.5 to 3.5 L/day of water. Eat animal protien 2,3 x’s wk. Lots of salads, with no dressing or balsamic dressing.
No soda, processed foods. I am 20 lbs over weight, but exersice 4 to 5 x’s a wk, 1 hr on the treamill, weights,
stretching.
Other HX
CAD
Hyperlipidemia
lots of ortho problems (because I’m a nurse haha)
How can I get my urine pH more aciditic? The foods that are acidotic have high oxalate, ie walnuts, cashews, potatoes
are also high oxalate.
Is it ok to eat more meat? I wory because that is hard on the kidneys.
Thank you both, Dr.Coe & Jill RN!!
Hi Laura, You seem to have a familial kidney disease, but I am not so sure about the cause. IN your urine you have normal citrate, something very hard to accomplish with alkali in distal RTA. Your blood bicarbonate and chloride and potassium are reported as normal – I recognize you are taking some potassium citrate. Do your prior labs show the expected low serum potassium and bicarbonate + high chloride? Presently, with so low a urine calcium you have little potential to form crystals. In fact your SS values are all below 1. I presume you have massive renal calcifications and stones have been calcium phosphate. In terms of treatment, stone prevention seems perfect right now, and lowering urine pH not of importance. Presumably your physicians have enough detail to know precisely what is wrong in your family, and I do no, so this is the best I can add. Regards, Fred Coe
Hello Dr. Coe. Since my email changed 7 months ago I do not get your papers. Its ok because my sister celia sends them to me but I’m nevertheless wanting to find your signature as by email a few days ago you mentioned that is where I csn sign up again. I’m still looking lol
Hi Laura, I did put your new email into the system so this should be fixed. Best, Fred
Hi Dr. Coe — I just had a urine test at my request because of frequent nightly urination. I am 65 so considered this to be normal. Everything in the urine test was fine except for a PH of 8.5 I am a vegetarian for many years. I am reading that this might be a sign of a UTI but I am asymptomatic. Do I need to make any changes to my habits? I do take vitamin C and a probiotic among some other vitamins. No other meds.
Hi Debbie, Look at the urine ammonia – if it is not high, it is not infection. Your diet can produce a very alkaline urine. Since you do not form stones the ammonia is not an unreasonable criteria. It should be rather low. Regards, Fred Coe
Dr. Coe, like the article states I’m one of those women that eats more fruits and veggies a day than men and my urine PH is high and have phosphate stones. Are there certain ones other than lemons that should be very limited so PH doesn’t rise even higher? Is it okay to eat a daily orange/clementine and fresh tomatoes? I appreciate all your hard work!
Hi Carol Lynne, The alkali absorption women perform does raise urine pH and probably is a reason women are more prone to calcium phosphate stones. I favor controlling the other risk factors, urine calcium excretion and urine volume – it is very hard to reduce pH presently. As for veggies and fruits, the best bet is to follow a balanced diet, and use low sodium and perhaps thiazide to lower urine calcium, and also maintain high fluid intake. Here is a good article about the matter. Regards, Fred Coe