 Bariatric surgeries can injure kidneys by raising urine oxalate excretion. This latter causes kidney stones, and raises risk of acute and chronic oxalate nephropathy. Overall, their benefits far outweigh these risks, especially when patients and physicians take proper precautions.But risk lurks as if in shadows, and waits on accident.
Bariatric surgeries can injure kidneys by raising urine oxalate excretion. This latter causes kidney stones, and raises risk of acute and chronic oxalate nephropathy. Overall, their benefits far outweigh these risks, especially when patients and physicians take proper precautions.But risk lurks as if in shadows, and waits on accident.
The patient here inadvertently raised her risk of injury.
Like all instances this one is just that: Opportunity to inspect the details of an undesired outcome so as to reduce the chance it will happen to others. The kidneys of anyone with increased urine oxalate excretion could be injured as her’s were, so common are the causes, so seemingly innocuous.
The high resolution scan of a kidney from a child with primary hyperoxaluria shows selective crystal accumulation in a narrow stripe between the tips of the black arrowheads. Through this strip run the terminal portions of the proximal tubules, where water extraction and oxalate secretion create highest supersaturations.
What Happened
Bariatric Surgery For Weight Control
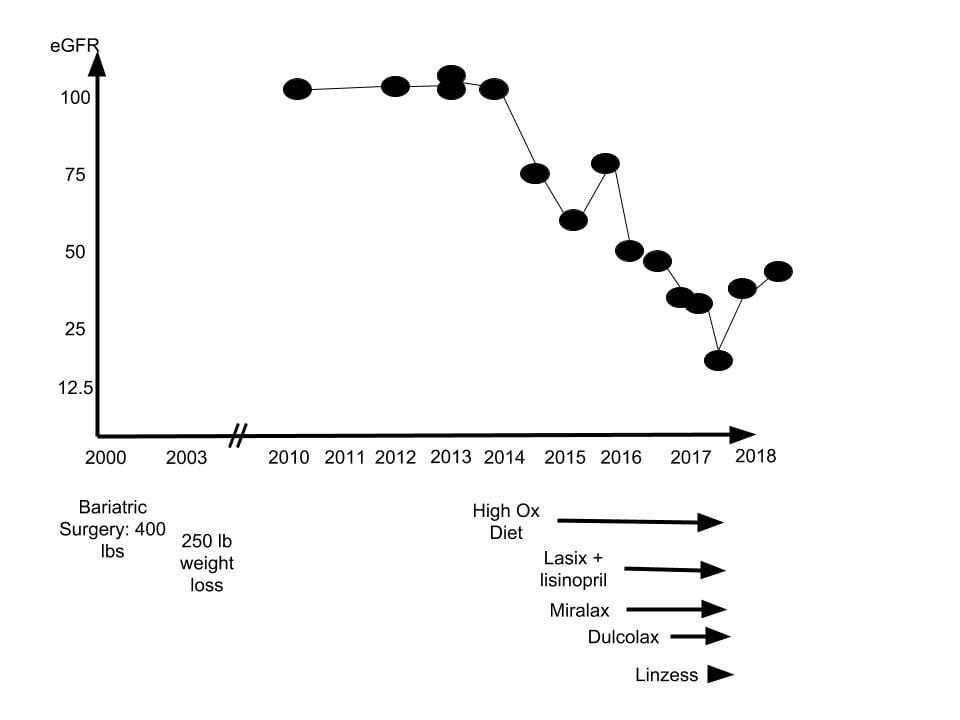
A highly educated woman, presently early middle age, had a form of Roux en Y as noted on the figure. She lost appreciable weight, and maintained that loss over 14 years.
Kidney function (eGFR) measured casually in the course of ordinary health care was stable and normal from 2010 through 2014, and had been, presumably, since 2000.
Diet Change
In 2014, aiming at optimal health, she changed her diet to mainly plant foods. Some of the foods in this new diet, such as spinach, had very high oxalate content.
For someone otherwise perfectly normal, this diet need pose no special risk. Given her bariatric surgery one might, perhaps, have wanted to measure urine oxalate excretion as a precaution. But mere diet change can seem so benign.
Fall in Kidney Function
A casual measurement at the end of 2014 revealed a fall of eGFR to about 75. Perhaps because small changes in serum creatinine easily escape notice, this did not trigger a response at the time. However ominous it may seem in retrospect, I suspect that in the course of routine care it appeared a random fluctuation. But because high urine oxalate from diet alone can cause progressive fall in renal function with cortical calcium oxalate crystal deposits, this one measurement may indeed mark the beginning of kidney damage.
A Diuretic and an Anti-hypertensive Drug
Because bothered by a sense of fluid retention, she was offered lisinopril and the diuretic lasix in 2015. Lasix is not an ideal drug because kidneys adapt to it by increasing proximal and distal tubule sodium retention, so when stopped rebound salt and water retention can continue for weeks, and swelling exceed what was there before the drug was ever taken. Even so, its use is very common, and in most people without serious risk.
Because her blood pressure was not high, the lisinopril is harder to understand.
As I shall show later on, lasix and lisinopril could have raised her risk for kidney damage from crystals, the former because of increased proximal tubule reabsorption, the latter because of reduced glomerular filtration rate.
Laxative Drugs
Also in 2015, she began Miralax, a widely used polyethyline glycol laxative. A bit later she added Dulcolax (bisacodyl) that acts differently from Miralax in directly stimulating colon motility. Linzess, a drug that increases fluid secretion by affecting ileal cell transport, was added in 2016, so all three acted through 2016 and 2017.
By depleting the body of sodium and water, these drugs would also raise proximal tubule reabsorption and lower glomerular filtration. So their risks added to those of the lasix and lisinopril.
Through the Looking Glass
Looking back, what has happened can seem inevitable. But others might have done the same as she did and escaped kidney injury depending on accidents of intake and habit. So rather than say her medications and diet caused kidney failure, say they posed potential risks that in this one instance culminated in kidney failure. Even so, this instance points out we should be wary going forward.
Acute Kidney Failure
Kidney function fell progressively throughout 2016 and 2017, so by the end of 2017 she was in kidney failure. The drugs were discontinued and a kidney biopsy performed. Subsequently, kidney function rose, as the graph shows.
Crystal Deposits
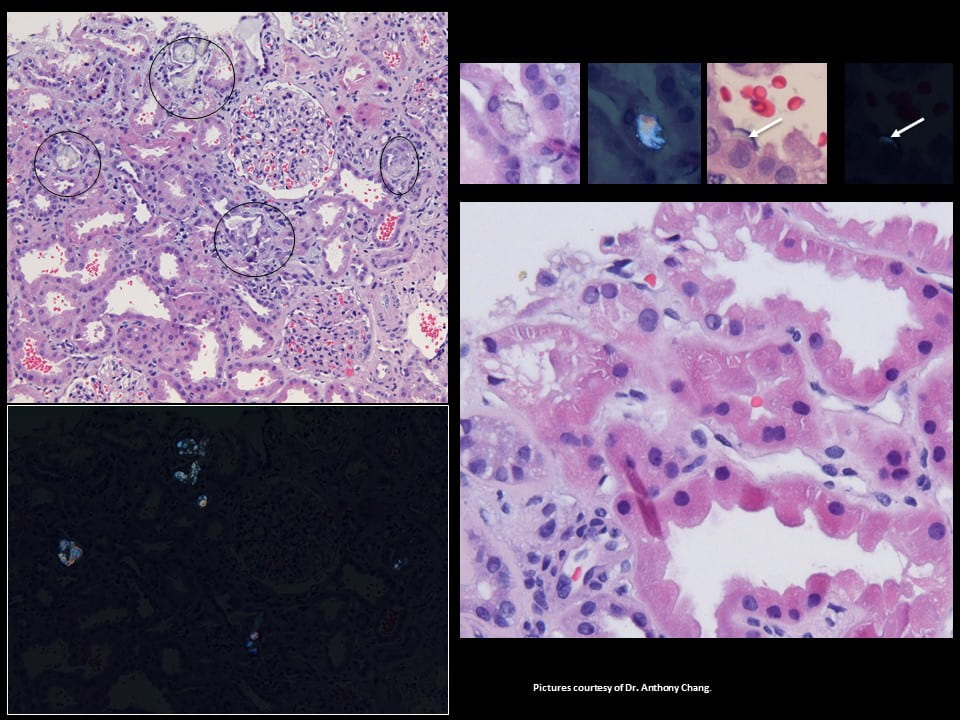
The tissue is from the cortex of the kidney, where glomerular filtration occurs. The large round balls not circled in black are glomerulae (upper left panel). Black circles highlight proximal tubules filled with crystals, clear, sharp edged, and obviously different from cells.
The black plate (lower left panel) replicates the one above it in polarized light so the crystals shine – this proves they are calcium oxalate (birefringent, for the experts here).
The first two tiny squares at the upper right show a crystal in a single proximal tubule cell and next to it a polarized view. The high power tiny square next in line shows a thin crescent of crystal in the lining of one proximal tubule cell (white arrow), facing into the tubule fluid. Last in line to the upper right is a polarized picture showing the crescent – at the white arrow (squint to see it!).
Kidney Injury
The expert renal pathologist who made these pictures for me pointed out the crystals could seem benign because the tissues show little if any scarring. But if you look close, as in the lower right large plate, the cells of the proximal tubules are irregular, their nuclei (dark inner circles) often missing. This means injury and regeneration, with risk of later scaring.
What the Crystals Do
This is not the time or place for a disquisition on crystal mediated cell injury. But the outlines are easy to state.
The crystals probably form in the cell membrane, as in the thin crescent pictures. Cells incorporate the crystals and seek to destroy them or move them out the other side, into the interstitial space. This whole process consumes energy and activates pathways in the cells that can injure them.
Cells might safely dispose of few and scattered crystals. Perhaps that happened all the years when kidney function was normal. We cannot know.
Presumably the high oxalate diet and medications increased crystal formation and forced cells to cope with more and more crystals that damaged them and caused to acute kidney failure. We can assume this as we found crystals and cell injury. As the drugs wore off, kidney function rose.
My Few Observations
Months after the biopsy I found urine oxalate was 87 mg/d – a high value. Her blood pressure 130/80. Given what we know, I began a high calcium diet with avoidance of high oxalate foods.
What Makes Calcium Oxalate Crystals in the Kidney Cortex?
Stone Crystal Formation Requires Supersaturation
Anyone who has even glanced at this site knows about supersaturation and how calcium oxalate crystals form in the final urine. It is the central paradigm of stone formation, and measurement of supersaturation a prime guide to stone prevention. I have shown over and over that the main force that produces supersaturation in urine is water conservation itself, a prime function of kidneys that protected us as a species throughout our long evolution in Africa. Thence the central importance of hydration in stone prevention.
Oxalate Secretion
Elsewhere on this site I have detailed how oxalate is secreted by kidneys from blood into the tubule fluid that kidneys process into the final urine. This occurs only in the proximal tubules, the parts of the nephrons directly following filtration at the renal glomerulae. Oxalate can also move back into the blood after it is filtered – the process of reabsorption. Kidneys of normal people filter oxalate and then excrete most, perhaps partly reabsorb some, rarely secrete any. But in people with high urine oxalate excretion, like primary hyperoxaluria (PH1) or bariatric surgery, secretion is prominent.
Beware – Difficult Material
What follows is difficult, a detailed look at secretion as we measured it in humans.
You may skip it and still get a lot out of this article, or read it to get a deeper look.
From now on I will write ‘proximal tubule’ so often, I introduce the abbreviation ‘PT’ to save letters.
Reduced Kidney Function
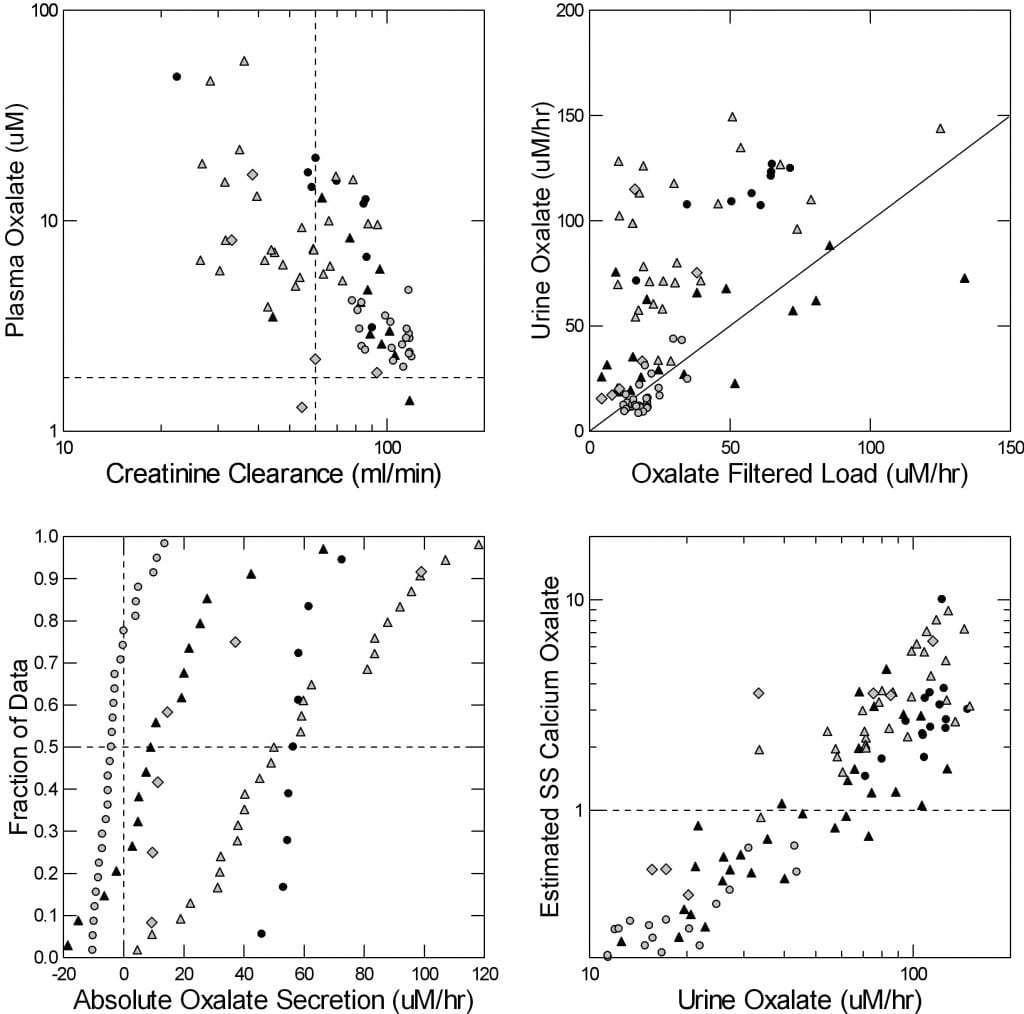
Because kidneys are the main organs to remove oxalate from the body, as kidney function fails (creatinine clearance, upper left panel) plasma oxalate concentration rises.
Circles are ordinary stone formers and normal people. The effect is small as they have good kidney function and low urine oxalate excretion. The other symbols are all from PH1 patients. They develop kidney disease so their kidney function falls, and they produce excessive amounts of oxalate in their livers. Plasma oxalate rises by 10 to 30 fold, and even the blood can become supersaturated with calcium oxalate.
Secretion Rates
As the filtered load of oxalate rises, from rising plasma oxalate, urine oxalate rises way above what is filtered. See how the points in the upper right panel are above the diagonal line of identity? All that is secretion, in the PT. Normals and common stone formers (open circles) have little or no secretion; their points line on the diagonal line.
The lower left panel shows the secretion rates directly. On the horizontal axis secretion is points above 0, reabsorption from filtrate back into blood is below 0.
Normals mostly filter oxalate and reabsorb it back (round circles). All of the PH1 patients secrete except for scattered few who have had a kidney transplant – black triangles. Those with severe kidney failure waiting for a transplant (black circles) secrete most reliably.
Total Oxalate Delivery Into Proximal Tubule (PT)
No oxalate can get into the urine except by going through the PT, and once oxalate leaves the PT it can go only into the urine. So to say urine oxalate rises is to say that the sum of filtered load and secretion rises.
If there were no oxalate secretion, kidneys would excrete exactly as much oxalate as they filtered, so as filtration rate fell plasma oxalate would rise. Secretion reduces that rise because it provides an alternate pathway to move oxalate into the proximal tubule lumen.
This matters because as plasma oxalate rises plasma itself can supersaturate with calcium oxalate and crystals form within the body. As is evident from the upper left figure in the four plot just above, plasma oxalate rises as g falls, meaning secretion cannot compensate fully and filtered load of oxalate must rise.
Proximal Tubule (PT) Supersaturation
The bottom right panel is the payoff. As urine oxalate excretion rises, calculated PT supersaturation with respect to calcium oxalate rises smoothly. The axes are in logarithms because of the large range of values. Normals and routine stone formers have no appreciable PT supersaturation; they lie in the lower left corner of the graph. But when urine oxalate rises as high as about 60 micromoles/hour (about 120 mg/d) saturation rises above 1 (the dashed horizontal line), and this means crystals can form in the proximal tubule.
In PH1 this is well known, and leads to kidney failure. In gastrointestinal diseases like bariatric surgery that raise oxalate absorption into the blood, urine oxalate can rise as high as in PH1, and PT supersaturation rise enough to create crystals, as in our patient example.
Let me be clear here: PT supersaturation behaves differently from urine supersaturation. The latter depends on urine flow rate that provided water to dilute excreted calcium oxalate salts. The former depends on glomerular filtration that provides water to dilute the oxalate coming in from filtration and secretion. Water intake does not affect glomerular filtration.
How To Calculate Proximal Tubule (PT) Supersaturation
Harder than what you just read, I wrote this for those with a quantitative bent, some algebra, and a lot of patience.
Skip down to the next section and take my word on it, or read it through and follow the logic.
Use The Fact that Oxalate Enters and Leaves Only in PT and Final Urine
Oxalate can enter and leave only in the PT (I labeled it PCT here, forgive me). I drew only secretion, but reabsorption also occurs, as I showed just above. The water to
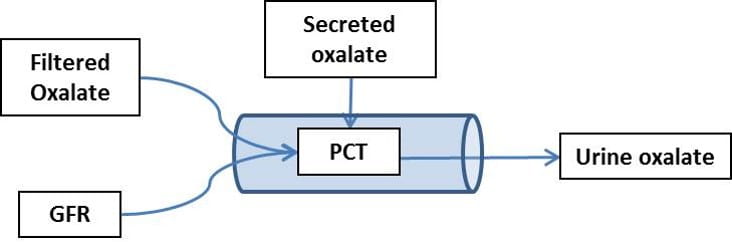
dissolve that oxalate is just the glomerular filtrate. So if oxalate enters at some rate (mg/time) there is only the filtration rate (ml/time) to dissolve the calcium oxalate salt it will make with the calcium in the filtrate (calcium is about 1.1 mmol/l).
Because it cannot enter or leave after the end of the PT, the net amount of oxalate entering is identical to the urine oxalate excretion. So the concentration of oxalate in the fluid in the [Ox]pt is simply:
Eq. 1: [Ox]pt = V*[Ox]u/g(1-r),
where V is urine flow rate, [Ox]u is urine oxalate concentration, g is glomerular filtration rate, and r is the fraction of g reabsorbed back into the blood.
Remove the Effects of Urine Volume
Because urine volume flow, V, is part of both the numerator and g, it cancels out leaving:
Eq. 2: [Ox]pt = [Ox]u * 1/[U/P]cr * 1/(1-r),
where [U/P]cr is just the urine to plasma ratio for creatinine, the marker used here to calculate g.
Calculate the PT Calcium*Oxalate Concentration Product
It is fundamental chemistry that crystals in equilibrium with a solution (at solubility) all have a unique concentration product of their constituent salts – calcium and oxalate in this case. This is often called the empirical solubility product, and for human plasma filtrate (as one finds in PT) the value is 40 x 10-9 M2. The square denotes that one concentration is multiplied by another. M means the units are in molarity. If you divide the concentration product of a solution by the empirical solubility product the result is supersaturation.
The concentration of calcium is known to be reasonably constant along the length of the PT because calcium and water are taken back into the blood at more or less equal rates. So the concentration of calcium in PT fluid is the ultra-filterable calcium concentration, [Ca]uf. One can measure that.
This gives us the PT SS,
Eq. 3: SSpt = [Ox]pt * [Ca]uf/40,
where [Ox]pt is in micromoles/liter and [Ca]uf in millimole/liter (usual value is 1.1 mmol/l).
Measurements in a Research Setting
The lower right panel of the large four plot just above shows PT SS calculated from Eq. 3. But these calculations were limited because we had only estimates of r, the fraction of filtrate reabsorbed by the end of the PT. From published estimates using lithium clearance, we assumed 75% when g was above 60 ml/min and 70% when it was below 60 ml/min. But you can see from Eq. 3 that as r rises near to 1 [Ox]pt rockets upwards, and the same as g falls toward 0. Even so, the estimates make clear that PT supersaturation is a reality when urine oxalate rises.
A Fall in Filtration Can Produce a Vicious Cycle
Welcome back. This part is a lot easier and you will enjoy reading it.
PT CaOx SS Rises as Filtration Rate Falls
 Here, I have calculated PT CaOx SS vs eGFR for urine oxalate excretions (mg/d) of 30, 50, 100, 150, and 200 (curving lines of increasing height). I assumed values for r, the conservation of water in the PT, at 70 to 75%..
Here, I have calculated PT CaOx SS vs eGFR for urine oxalate excretions (mg/d) of 30, 50, 100, 150, and 200 (curving lines of increasing height). I assumed values for r, the conservation of water in the PT, at 70 to 75%..
When eGFR falls below 25 ml/min, PT SS rises above 1 for even the lowest oxalate excretions, meaning PT crystals can form as they did in our case. At an eGFR of 60, and an oxalate excretion of 100 mg/d, SS would be present. If diuretics or laxatives raised r, SS could be very much higher.
So a fall in eGFR could cause PT crystals that damaged the kidney and lowered eGFR more. I suspect that happened in our case.
Put It All Together
It would seem that because oxalate is filtered and secreted into the PT and cannot get out again, the fluid in the tubules can supersaturate and create crystals that damage the cells and even plug the entire structure.
The main driver is how much oxalate kidneys need to remove. The secondary drivers of supersaturation are eGFR and r. Lower eGFR means less water to dissolve the calcium oxalate salts. Higher r, water leaves PT more rapidly and completely.
So anyone with high urine oxalate, from bowel disease, primary hyperoxaluria, bariatric surgery, or even very high oxalate diet has a potential risk of a downhill cycle given serious and sustained dehydration, drugs that would lower eGFR or raise PT water resorption, or underlying kidney disease. Obviously as these factors begin to overlay – someone with reduced filtration who becomes dehydrated for a significant period or takes diuretics – potential risk rises.
That is a pretty good summary of how I envision things. Be clear; risk is risk, not reality. People with increased risk may do fine, but wiser ones will avoid risk whenever possible.
Can We Prove This Vision is Correct?
What follows looks abstract and difficult but is really not. You will enjoy reading how what we only imagined – PT supersaturation from how the PT works – gives rise to predictions in the real world we could actually look for.
A Moment to Contemplate How Science Works
A Seemingly Closed Book
Here is a perfect case to illustrate all I have written about science.
Given the lack of sites elsewhere along the nephron through which oxalate can enter or leave the nephron, that one fact, one gets Equation 3 from simple conservation of matter – oxalate molecules in this case (For special readers: The ‘fact’ includes kidneys neither produce nor degrade oxalate molecules).
Equation 3 summarizes the quantitative implications of oxalate conservation and predicts PT supersaturation given conditions of urine oxalate, eGFR and r we can readily measure directly. This prediction is necessary if conservation is true. From the evident crystals and physical chemistry of crystallization we also know supersaturation is present.
So, one would seem to be done with the problem.
The Power of Theory in Science
Not so.
We already knew about the crystals and sought to explain them. We did, with equation 3. That is nice, but circular. Useful theory predicts what we have never seen because we never looked – novel predictions.
So, what does our theory predict beyond that water extraction produces PT CaOx supersaturation?
Spatial selectivity.
Because oxalate is secreted through the cells of the PT, supersaturation should be especially high in cell membranes. Because the PT absorbs water continuously along its length, crystallization should rise with length, as well.
These two spatial selectivities provided us with our first two novel predictions.
Tiny Cell Crystals
In tissues from our patient here, I showed several PT cells with tiny crystals in them. These are expected if oxalate secretion is what drives PT crystal formation. The very transporters that secrete the oxalate are on the cell membranes that face into the tubule fluid. Crystals are far more apt to form on a surface – like a cell membrane – than in the flowing bulk fluid within the tubule, especially if oxalate is moving at high concentrations through its transporters in that same membrane.
They Are Not Secondary to Tubule Plugs
In our patient, multiple tubules were plugged with crystals, so the tiny crystals on single cells could have formed as a secondary phenomenon to plugging. But in our publication, we demonstrated tiny crystals in the membranes of PT cells despite absence of any tubule plugs.
They Are In the Membranes
The white arrowheads point to tiny bright dots that are crystals viewed under polarized light. Not shown here, they all stained as well with Yasue stain that is particular to calcium.
That double staining marks them as crystals as opposed to other white dots scattered about.
The combination of Yasue stain and birefringence in polarized light assures us they are calcium oxalate crystals. Moreover they are in the borders of the tubules (you can see the path of a tubule running downward from left to right under the white arrowheads).
This view is at 200 power magnification, the same as that used for the small crescent in the biopsy of our patient’s kidney tissue. But here, these crystals are all alone. No tubules have any plugs in them, and kidney function is reasonably well preserved (creatinine clearance = 78 ml/min).
Crystals Rise with Length
The PT begins at the glomerulus, and proceeds through the cortex. At its end, it becomes straight and plunges into the outermost stripe of the medulla – the so called straight, or S3, segment.
Most Supersaturated
This straight segment, being at the end, will have had the most fluid removed back into the blood, and if oxalate is being secreted it will also have the most oxalate compared to the earlier segments. Even more, it passes through the medulla, where the thick ascending limbs are reabsorbing sodium chloride back into the blood without water. Because the salt concentrations outside the S3 segments are higher than outside the earlier segments, water extraction will be even more marked by comparison. In other words, the S3 segment must be the most supersaturated segment PT segment.
Our Theory Predicts Crystals Will Concentrate There
Given that crystals form because of supersaturation, we should be able to find – if we look carefully enough – that crystals accumulate in the S3 segments more than anywhere else in the PT. To look we chose kidneys from children with kidney failure from PH1 whose kidneys were removed prior to transplantation. They will have formed enough crystals to destroy kidney function, but the kidneys will not have been in their bodies for years of kidney failure that allow crystals to simply fill up of their tubules.
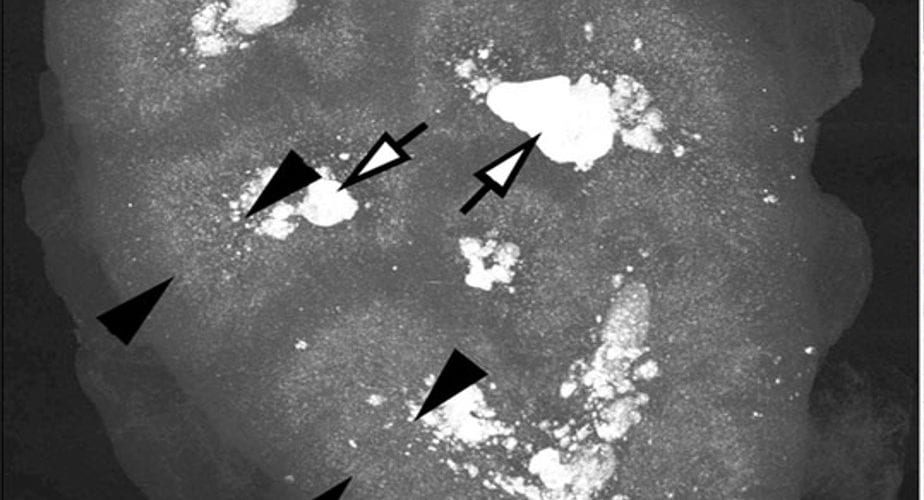
We Found Crystals Where We Predicted
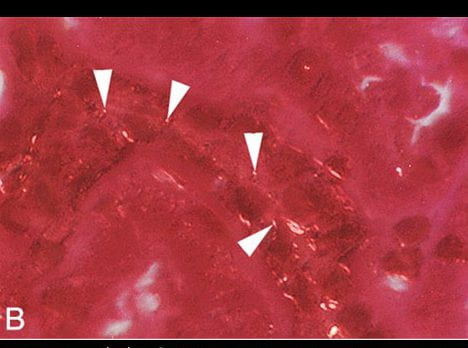
What is the picture of?
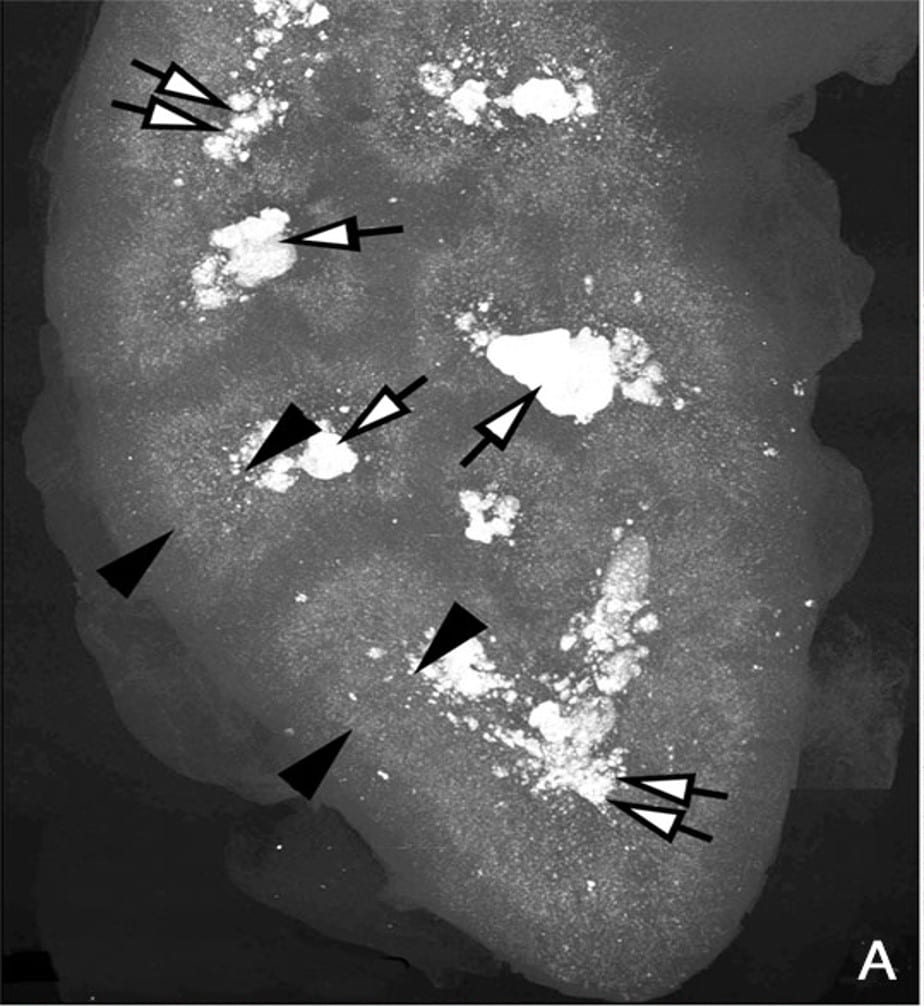
This picture is a high resolution CT image of one such kidney. The black arrowheads point to the outer and inner limits of the outer stripe of the outer medulla. That is the precise region the S3 segments pass through.
Stones Fill the papillae
The white arrows point to large deposits and stones filling the papilla and the calyces – where the final urine leaves the kidneys to enter the urinary system.
The Cortex is Relatively Clear
But the most important place to look is not between the arrowheads, or at the tips of the white arrows. It is along the wedges of the left hand black arrowheads, for they lie along the entire width of the renal cortex, where the glomerulae are, and the winding convoluted proximal tubules wend their meandering ways.
Our patient had crystals in those cortical convoluted tubules, you saw them. No doubt this kidney has them there, too. But look at the far greater density of crystals between the ends of the black arrowheads than under the bodies of the ones on the left.
Do We Have More Predictions?
We do, but have not tested them. Right after the S3 segment come the thin limbs of the Loop of Henle – critical to concentration of urine. Water is extracted rapidly as fluid descends down the limbs into the medulla and oxalate cannot leave. So we expect severe crystallization will destroy them.
We should look specially for this, and did not.
Because these thin limbs are essential for concentrating the urine, we should expect an early loss of renal concentrating power. Such a loss occurs in all kinds of kidney disease, but should be more apparent in states of high urine oxalate, present even when kidney function is normal.
We should expect crystal plugs in the distal convoluted tubules. We did not report them, but were not specially looking for them, either.
Is Our Theory Right?
No theory is ever right, merely not yet proven wrong.
Filtration and secretion with secondary crystal formation in PT has intuitive appeal and has withstood two critical tests, as I have just shown you. One might say it will therefore do as a basis for proceeding further. But if you are an acolyte of Popper and Kuhn, as I am, you know our theory will not survive. Those who come will surely overturn it, as I have overturned those that came before me.
But for now it has enough in it to guide what we say to patients, and to guide what they can do to protect themselves against kidney injury.
The Full Theory
A theory must not only predict but also explain what we found, in this case acceleration of kidney loss with high oxalate diet and use of specific drugs. Such explanation links the new theory with the whole of the relevant knowledge in the field. That is not a test, as no sane scientist would theorize against known facts. It is simply to use theory as explanation in new terms such as factors that affect PT supersaturation.
At the top of the figure just below, something raises urine oxalate excretion. In this case it was bariatric surgery, and then addition of a high oxalate diet.
To the Left Hand
To the left we have conventional stone disease with tubule plugging.
Conventional Stone Disease
Higher urine oxalate causes kidney stones, and certainly can do so after bariatric surgery. Crystals typically plug medullary and papillary collecting ducts. One might think the plugs will be pure calcium oxalate, but only in PH1 does this assumption hold. Among patients with small bowel disease and resultant hyperoxaluria, plugs are mainly calcium phosphate as hydroxyapatite, with only scattered traces of calcium oxalate. The same in those with old style ileal diversion. It produces extreme hyperoxaluria but plugs are mixed hydroxyapatite and calcium oxalate.
We do not know why the plug crystals are that way.
Plugs can obstruct those nephrons they drain leading to damage in the cortex – internal hydronephrosis. One can see such cortical damage in kidneys from animals. In humans with plugging, not from bariatric surgery but from other causes, we found scarring in the renal cortex.
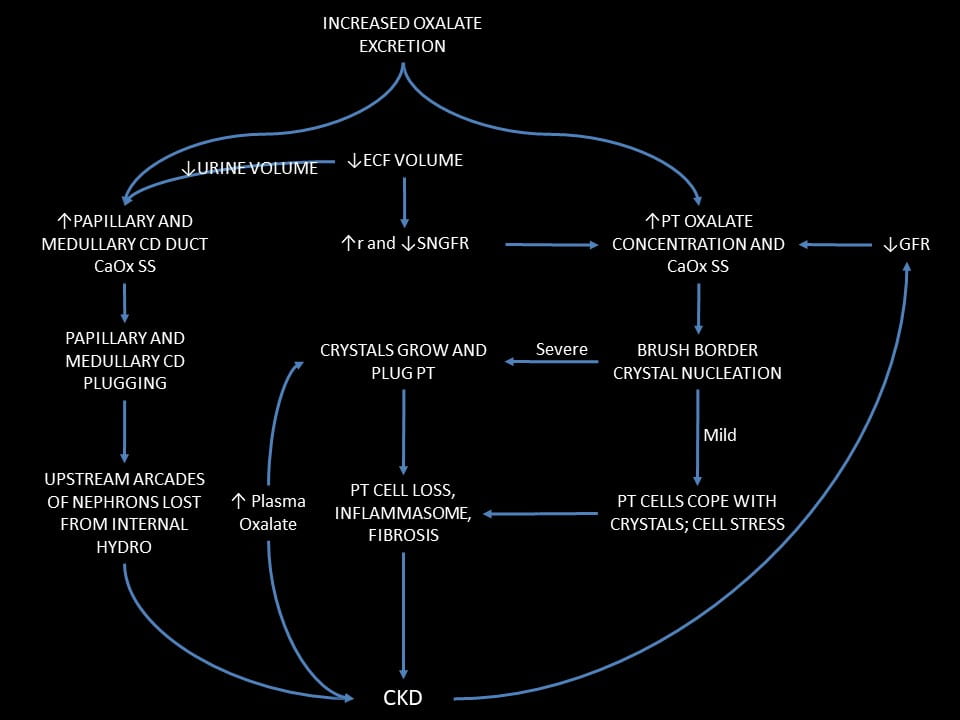
Worsened by Dehydration
A fall in water intake will lower urine volume via the hormone vasopressin. This concentrates calcium oxalate salts in the medullary and papillary tubule fluid and promotes more stones and plugging.
Chronic kidney disease (CKD)
Since plugging can cause cortical scarring, we propose it can cause chronic kidney disease (CKD). CKD and even kidney failure are more common in stone formers than other people.
To the Right Hand
PT CaOx SS
We have theory and evidence that by increasing urine oxalate excretion bariatric surgery will raise PT oxalate secretion and therefore can cause PT CaOx SS and crystallization. In our case we have the crystals to prove it. A fall in ECF volume that reduces g or increases r will raise that SS by reducing the amount of filtrate available to dissolve CaOx salts in the PT.
Mild
When SS is not very high new crystal formation can be slow and cells adapt by internalizing the crystals and clearing them. I think this may have been the case during the long years of stable kidney function in our patient after her surgery. Even so, one can imagine some cell stress and perhaps cell loss, but likely not enough to cause external evidence of injury.
Severe
Raise the rate of crystal formation and cells cannot cope. They internalize crystals and become injured. Crystals spread to involve the entire tubule lumen with the resulting crystal plugs our patient illustrated. The outcome is cell loss, inflammation, and possible fibrosis.
CKD
Any fall in eGFR will lower the amount of filtrate available to dissolve PT CaOx salts. The reason is that secretion will maintain urine oxalate excretion constant. If it does not, plasma oxalate concentration must rise, and that will raise filtrate oxalate concentration so as to increase SS just as secretion would have done.
What Our Patient Did in Functional Terms
We know what preceded kidney failure in our illustrative case, but need to restate it in terms of our ruling equation and the scheme of injury I just detailed for you.
Effects on Urine Oxalate
Bariatric Surgery
Of course it all began with the bariatric surgery that raised urine oxalate excretion. I know it did so as her urine oxalate was still 87 mg/day when I first made a measurement. The blue arrow attaches the surgery to that component of the equation as a means of raising PT supersaturation.
Kidney function remained stable and normal for 14 years after the surgery. I presume that if PT crystals even formed, the cells coped with them, leading to no disease or one so indolent we found no trace of it.
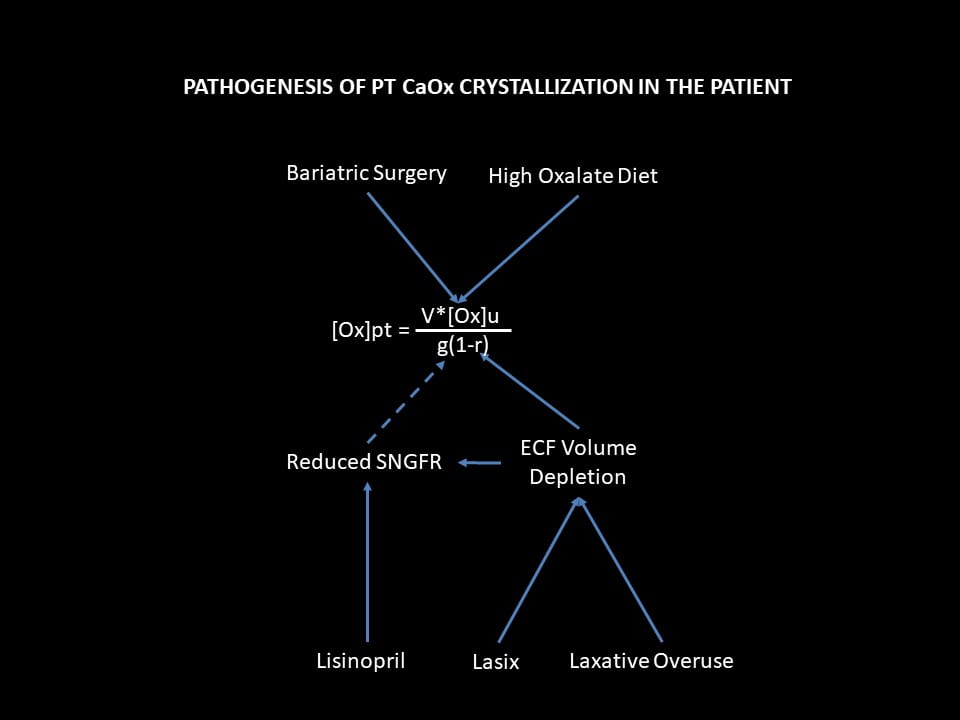
High Oxalate Diet
Being only an exaggeration of the surgery itself, the diet would simply have increased PT SS and rates of crystal formation. But we did find, looking back, a fall in eGFR from only the diet – if the history is so exact one can tell such fine points.
Effects on g
Linsinopril
Lisinopril, like all drugs that reduce angiotensin 2 production or action, lowers single nephron GFR (SNGFR) by dilating the efferent glomerular arteriole. If you have not read the parent article the link goes to, this might be a good time to do so. Variation of SNGFR is usually harmless, even beneficial in some cases, but here not when urine oxalate excretion is high. The fall in g must raise PT CaOx SS.
Lasix and Laxatives
ECF volume depletion from lasix and laxatives, the same. Such a fall will reduce renal blood flow and therefore eGFR. Because the usual compensation for such a fall is efferent arteriolar constriction via angiotensin 2, the combination of ECF depletion and lisinopril would have a magnified effect to reduce rGFR, and therefore to raise PT SS and crystallization.
Effects on r
These same two drug classes, lasix and laxatives lower ECF volume. To save us from death by dehydration, any fall in ECF volume triggers multiple pathways that actuate renal salt and water conservation. Prime among these pathways, PT reabsorption rises because this segment controls the largest flows of salts and water.
What Do I Have to Offer?
To Patients
If you have any condition that might raise urine oxalate – bariatric surgery, small bowel disease, vegetarian diet, very low calcium diet – be sure your physicians have measured 24 hour urine oxalate excretion. Every calcium oxalate stone former needs such a screening measurement at least once to exclude the rare but vicious genetic primary hyperoxalurias. When present, high urine oxalate poses not only a risk for stones but for kidney damage and disease.
Your physicians are responsible for your care, for detecting high urine oxalate, and for treating the condition. But you need to know the precautions you must take if urine oxalate is high. Your physician will tell them to you, so I am just repeating here what you should expect to hear directly.
Avoid ECF volume depletion: diuretics, laxatives, very low salt diets – these are not for you unless your physician has made special treatment initiatives to lower urine oxalate. The ACE/ARB drugs may well be used, but with precautions to avoid a marked fall in eGFR. If eGFR falls, one needs to somehow control urine oxalate specially well. Acute GI diseases are usually brief, so keep up some salt and fluid intake. If your urine oxalate is very high and not controlled your physician may prescribe brief at home IV fluids to tide you over. NSAID OTC drugs – like Motrin – can lower eGFR, so be sure your physician has explained to you how you can safely use them.
Obstruction from a stone lowers eGFR and raises r, a lot. Your urologist will consider acute obstruction an far more urgent matter in you vs. common stone formers.
X ray contrast agents all reduce eGFR, and even though brief the episode might trigger acute kidney injury. Physicians are here to assure you are protected. But they must know who you are and what you have. Records may not always be clear or even present; you are, and can warn them.
To Physicians
You do not need me to tell you how to control enteric hyperoxaluria, from bariatric surgery or any other cause. You already know how. Low oxalate high calcium diet, low diet fat intake, oxalate binders if needed, pyridoxine in PH1 patients who respond to it – these things are part of your routine practice. You know to measure urine oxalate in any stone former, and certainly in a patients with any bariatric surgery. You know all about drugs, contrast agents, inter-current illnesses that can lower SNGFR and raise PT reabsorption.
So what is there for me to say?
Simply this.
The patient who kindly let me use her as an example has physicians, good ones, yet things got by.
The oxalate diseases remind me of a policeman’s life – mostly nothing happens, but when it does it comes all of a sudden, with stunning violence.
Homage
“Opportunity to inspect the details of an undesired outcome so as to reduce the risk it will happen to others.”
I said that at the beginning, and say it at the end.
The ‘opportunity’ consists in the experiences of a person who has consented to appear here – albeit disguised – as a proof absolute of what can happen, and as a warning to us all.
I have concealed her so no one save for the rare few can know who she is. But to see rehearsed on a public screen the events of your own life can be no pleasure.
That she has given of herself in this regard is a mark of grace.
It is a gift to us for the which I offer – so far as I can so presume – our collective gratitude.

Dr. Coe,
I have taken Jill’s class about a year ago, and while I have not had any new kidney stones form, I did have bariatric surgery in 2012 to remove a GIST tumor from the antrum of my stomach. Do you have a way where I could discuss my latest LabCorp results with you, get advice for my urologist? I had a urologist who recently left town, and I have a fairly experienced urologist now, but I would like to be able to communicate my Roux-En-Y plus resection to him. I take 12.5mg of Chlorthalidone every other day, as we were testing it against my Sulfa allergy, and I have had no adverse side effects, however, my recent results from LabCorp show my calcium at 700 mg/24 HR, volume at 5,000 ml/24 HR, Phosphorus at 2355 mg/24HR, Uric Acid at 860 mg/24HR, Oxalytes at 50 mg/24HR, Sulfates at 70 mEq/24HR, and Creatinine at 3,100 mg/24HR. My urine citrates are at 925 mg/24HR. I really want to try and avoid forming further kidney stones, but I continue to be discouraged by the fact that nobody seems to know the impact my surgery has had on my various levels. I could do a better job at reducing my sodium, but I do try and stick to 1,500 mg/day. At my last CT Scan, I did not have new stones forming (about 8 months ago) from the previous year, and my former urologist said to just keep going as long as they weren’t forming. I don’t feel that was sound advice with my supersaturations above. Other items were in the normal range. My email is johnoneill2003@hotmail.com. I’m more than willing to pay whatever I need to in order to get the advice I need to try and get ahead of this. I’m only 40 years old, and I want to be as proactive as possible. Please, I beg you to help! Please! (979)220-1261 (cell)
Hi John, Obviously I cannot practice medicine via the web – too early in history, my institution does not permit it. I will email you privately to help you find someone. Regards, Fred Coe
Dear Dr Coe,
Thanks very much for these informative essays. A continually updated website such as this, with comments and responses, is an extraordinarily valuable resource. All the better with art, philosophy of science and delicate and insightful statistical analysis. I think https://scienceofparkinsons.com performs a similar role for Parkinson’s Disease – though nothing matches the mathematical sophistication of this site!
In addition to the likely causative mechanisms for nephrolithiasis listed in the above-linked “Medical Management of the Postoperative Bariatric Surgery Patient”, here are some other suggestions regarding RYGB.
Perhaps the lack of stomach acid affects oxalate binding to calcium and its absorption in the colon. For instance, if the patient takes calcium carbonate supplements rather than the recommended calcium citrate, then perhaps (my guess) the lack of stomach acid might render that calcium less available for absorption and binding to oxalate.
Likewise perhaps this alters the rate at which vitamin C degrades to oxalate in the lumen. RYGB patients are advised to take supplemental iron with vitamin C 500 to 1000mg, so perhaps some of this converts to oxalate. This ingestion is supposed to be at times different from meals and calcium supplement ingestion, so there’s a good chance that any such oxalate would not be bound to calcium, and so be more likely to be absorbed in the colon.
Other micro-nutrient absorption problems common, to some degree, to all RYGB patients might also affect these oxalate generation, binding and absorption processes, despite the efforts to compensate for them with supplements.
Food and supplements pass through the digestive tract much faster than before the operation, due to both the shorter path and the absence of the stomach sphincter. Moreover, there is no stomach in which food and supplements spend any time, so there would be less time for oxalates to bind to calcium.
The lack of stomach storage and mixing probably results in the intestinal contents of a meal being less thoroughly mixed than before surgery. Before surgery, alternating, minute-by-minute, swallowing of, for instance, cheese and spinach, might be fine, since the oxalates and calcium mix well in the stomach. Likewise with an after-dinner cheese platter. After the operation, more discrete meal components may travel through the GI tract with less mixing, which would reduce the ability for oxalates to bind to the calcium ingested at times differing by one or more minutes. Substantial fluid intake during the meal, and taking a long time to eat, would exacerbate such effects.
Post RYGB, the above-mentioned study showed little correlation between oxalate intake and urinary oxalate levels. Perhaps this was due to some experimental anomaly, though I have not read the article. Surely it would make sense to continue to try to minimize oxalate intake, though this would be challenging given the restrictions on diet imposed by the surgery.
The post operative impact of fat ingestion is clear from your analysis. However, reducing it may be tricky, since the desire (really, in the long-term, need) for satiety would drive the person to eat more carbohydrates instead. This might have two undesirable effects beyond the likely consequent weight gain.
Firstly, the person would be at greater risk of Post Gastric Bypass Hypoglycemia (previously, Late Dumping). Even without an actual hypoglycemic episode, perhaps the greater glucose ingestion and higher insulin levels would upset other processes and cause immediate or long-term harm.
Secondly, while some carbohydrate foods are a good source of potassium, many of them contain oxalates – certainly more oxalates than the oily or fatty foods which would otherwise have been enjoyed.
A final suggestion is that the increased scatter of urinary oxalate levels may cause more crystal growth and stones than before, even though the average (per population of subjects, or within the levels of any one subject over months and years) appears about the same. This scatter is both per meal average, and at different time periods during which the food is being processed.
I guess this would be the case because initial crystal formation would occur at the peaks of saturation, and there would be more peaks above some above-average crystal formation threshold with the larger scatter than with the pre-operative lesser scatter. In principle those crystals might dissolve rapidly once the peak has passed, but they might do damage before dissolving and/or may remain as nuclei for later episodes of crystal growth. Overall, the RYGB patient’s GI tract is in for a much wilder ride than before – even though total quantities of food are reduced. (It is not my place to seriously criticise this accepted medical practice, but the whole approach seems reckless and destructive to me. Obesity problems are profound, but RYGB seems analogous to ripping out some pistons and con-rods when the actual problem is a sticky accelerator cable and weak throttle return spring.)
I am not a healthcare professional or RYGB patient. These are the speculations of an electronic technician and computer programmer who is keen to understand long-term dangers of kidney damage due to oxalates and diet in general.
Robin Whittle Daylesford, Victoria, Australia.
Hi Robin, You are a very good reader and critic. RYGB is gradually giving way to gastric sleeve that has fewer complications. So perhaps these complex issues will become less relevant. No doubt the surgery is a major event but the diabetes and other effects of obesity kill people and dieting fails so often we need more. Lovely note, Fred
Did you consider that the MiraLax might have more directly contributed to the problem? Many kids have had strange neurological reactions to MiraLax, so many that the FDA chose CHOP to do an investigation of its use in kids. I had previously assumed it was due to changes made in the gut flora, but I’m learning that it may actually be due to oxalates. I’m sure you know that ethylene glycol causes damage by creating oxalates. The Mayo Clinic has a study listed online stating they are investigating whether or not polyethylene glycol may create oxylates in some people. My family has a microduplication of a gene that appears to affect B6 downstream. We were also unknowingly exposed to high concentrations of aspergillus, which produces oxalates. My kids neurological problems are being dramatically improved by cholestyramine, which binds oxalates. When my daughter was a preschooler, one or two doses of MiraLax (I can’t remember which) appeared to cause her to experience hallucinations, and I refused to give it to her again even though her pediatrician said Miralax couldn’t cause that problem. Anyway, I was looking for more information on the possible connection and ran across your post.
Hi Julie, Thank you for your comment. I did not link Miralax to increase in oxalate production or urine oxalate. But a search of PubMed for https://www.ncbi.nlm.nih.gov/pubmed/?term=Polyethylene+glycol+3350+(PEG+3350)+and+oxalate showed what you said – there is a trial pending about the matter. Ethylene glycol certainly is metabolized to oxalate. Right now no other research articles exist on the subject from this search. Very thoughtful! Best, Fred
I am almost relieved to read this information. I am a roux-en-y patient post op 2001. I began my surgery journey at 301 lbs. I lost about 20#’s prior to surgery then the “proverbial” 80#’s without even trying. In 2001 all there was was the Roux-en-y option and I hated my body & my food issues so badly that I don’t recall kidney stones even being mentioned as a side effect or a danger. (This is back when you went back to food starting with liquid food in the blender.) So weighing in at about 207#’s for years, I decided to join Weight Watchers where we now have “free foods!” Guess what is free? My favorite salad fixings starting with SPINACH! I add a couple hard boiled eggs but I have “limited” calcium rich foods consisting of only 2% milk in my coffee & (1) Greek Oikos yogurt a day. I take a whole bin of supplements including chewable Vitamin “C” & calcium chews. As of my most recent PCP follow up I received the news that I am testing in at a moderate level for “calcium oxalate crystals” in my UA labs. I really DON’T WANT TO CHANGE MY DIET! I reached “goal weight” a year ago last September! I have NEVER reached goal before, EVER!
I am 56 years old. 5’6” tall. I just discovered I’m finally past menopause & I haven’t been in this good of shape physically since high school. I now have an appointment scheduled for a urologist January 15th. My father had kidney stones but during his late 30’s & early 40’s until he got them “zapped,” as he called it. So please! Tell me what can I do to make these calcium oxalate crystals decrease or disappear before my urology appointment? And even MORE importantly, what can I consume, and still stay within my diet constraints for keeping my weight off? I have been a regular popcorn eater as well. I neglected to mention I now only allow myself to fluctuate between 143 & 152. I have had a full lower body lift-thighplasty & tummy tuck, as well as an upper body lift (bracheoplasty) including a breast lift as well without implants. For a total of 3 surgeries. I do suffer from Lymphatics issues of my right leg/ankle after the thigh lift & now must wear compression daily to avoid swelling. This may have been started when I first had the tummy tuck. Through all these surgeries and the side effects, I would STILL take the person I am today than where I was headed at an ever increasing weight of 301 lbs. I didn’t leave my house much & didn’t like myself either back then.
Please advise or direct me to information on how I can still enjoy salads, follow my WW eating plan, or make these crystals go away in the meantime! (My email is enclosed & required to submit kind sir!)
Sincerely,
Trudy Slatter
(DFW TX metroplex area)
Hi Trudy, These procedures do raise urine oxalate and the higher oxalate can lead to stones. Spinach is a particularly bad choice as it is so much higher in oxalate than most veggies. I would avoid it and use kale or other products less provocative of stones. Urine oxalate is gauged by 24 hour urine testing which I would advise if there are urine crystals. But perhaps just stopping the worst offenders will help. This article on low oxalate diet – not what you should do!!! – shows the very high foods, like spinach, and a few others. For the rest of the veggies, one need not overly care given there are no stones. Regards, Fred Coe
Hello Dr. Cole and all,
I have directly implicated Miralax in my supersatriation. Calcium oxalate crystals were seen in a urinalysis which I promptly looked up. I learned about oxalates, saw the foods that were prime offenders and questioned the medical marijuana. I immediately stopped it and did not have any resolution of my symptoms.
I always had angst when taking miralax since I’d had all my organic chemistry college courses and knew what (poly) ethylene glycol was.
I immediately stopped the miralax. I saw a lessening of symptoms within a week or two. Severe symptoms has lingered but I can see the path toward health and the path toward death behind me, I so pray.
I had been given your name as someone to see but I’m not well enough to see my drs that are 5 miles away. Much less, i wasn’t sure that you would see me since my kidneys are ok per creatinine. I’ve been searching for someone to help me get the oxalates out of my body in a humane manner. I am 90 % bedridden still. I take the occasional epsom bath which helps. I’ve tried different supplements with mixed reactions so I have pulled back to only necessary prescriptions and a multivitamin every other day.
Do you do tele visits?
I do live in Darien but I just can not travel far right now.
Thank you very much, this article will be sent to my other drs because None of them knew anything. God Bless
Hi Christine, It sounds like you have had a lot of trouble but I cannot tell if you have kidney stones. I do telemedicine, and can see you that way if you have stones or some problem related to them. My secretary phone is 773 702 1475. Regards, Fred Coe
Hi Dr Coe, you have spoken with my physician, Dr Reddy. He has provided me with very important information and is providing great care thus far. I’m a bariatric patient. I had the RandY in 2004. I started at 293lbs with sleep apnea, back issues, GERD, and who remembers what else. I had the surgery and immediately after I had to have my gull bladder reoved and a blood transfusion because my numbers fell low. I’ve had good results but it is always a challenge to keep the weight off and not to have body dismorphia, as my kids think I have. I was told I had kidney disease back in the low to mid 2000s. I had a couple of stones that sent me to the hospital and when I couldn’t pass them they blasted them etc… Now I fell from stage 3 kidney disease to stage 5, like in 2 months time. This was a shocker. I was hospitalized and a biopsy done that indicated damage and oxalate. At this time, I’m being considered for kidney transplant and dialysis at the same time because there is no guarantee that the three family members that have come forth with a kidney will be a match. So it’s best to be proactive.
From reading your articles, it would appear that my condition was in fact caused by the gastric bypass. My mom got a bypass shortly after me and has had no stones etc… her kidney function has dropped a little but not enough to be concerned, she is 73. I’m not sure if I understand the need for increased salt intake. Kidney disease can make you retain water and have hypertension, it would seem to me that more salt would cause more problems. I’ve been put on blood pressure medicine 2x a day to keep my numbers down. Has there been a study to show how far into late stage 4 and early stage 5 that damage starts to affect other organs in the body? Everyone always say drink more water…what is an easier way to do so. Lastly, the real question is, when and if I get a new kidney, what do I need to do to prevent oxalate build up in that kidney? Is the same thing bound to happen with the new kidney? If so, should I even consider taking someone elses kidney or should I just do dialysis? I appreciate your time and look forward to a response.
Lisa
Hi Lisa, I did indeed speak with Dr Reddy and found him a thoughtful and very smart physician who fully understands what has happened to you. I am happy to respond here because others will perhaps benefit – you already have an attentive and completely reliable physician caring for you who does not need prompting from me. I do think the bypass was the main generator of kidney disease by raising oxalate delivery into the blood and thence into the kidneys. Your crystals are not in the urine but in the kidney tubules themselves. Higher sodium intake will reduce water absorption high up in the nephron where oxalate is secreted from blood into the filtrate. Less reabsorption of filtered water leaves more to dissolve calcium oxalate salts there. No trials support this thesis but many events of volume depletion – GI disease, for example – are followed by acute kidney injury in people with high oxalate loads like after bariatric surgery. The new kidney is also at risk from the same factors, so either your physicians might consider switch to a gastric sleeve or else take precautions such as maintaining a high diet sodium, avoiding diuretics and laxatives etc. This is complex medicine, and I completely trust your physician. Regards, Fred Coe
Thank you so much for your informative blog Dr. Coe.
I don’t know if you are still answering these or can help me? I would be so grateful!
I have CKD stage 3, with egfr in the 50’s for several years. I recently got diagnosed with thyroid cancer and had my thyroid, some lymph nodes and perhaps 3/4 parathyroids removed (surgeon).
After surgery I was put on a very high dose of calcium carbonate because the hospital only tested me once after the surgery. I had what I know realize was severe hypercalcemia for about 9 days after surgery with calcium levels of 3.5 when they should have been <2.0. My urine was actually cloudy and I could not eat or get out of bed. I lost ten ponds in a week. My egfr plummeted to 43 and creatinine went up to 128 (normally around 110). My calcium meds have since been reduced so I am now round 2..1 calcium levels, but obviously they need to be lowered still…
Please Dr. Coe, do have any idea if this would have caused permanent damage to my kidneys? I am really scared about my future. Or is the time frame not long enough? (I am hoping it' not long enough) My endicrinologist said he didn't know about kidneys and I don't have a nephrologist since my egfr was previously stable.
I am a 46 year old single mother.
I would be so grateful if you could answer. And thank you again for your beautiful writing and explanations.
Hi Christina, It would seem the calcium carbonate caused both hypercalcemia and high urine calcium with crystallization of calcium phosphate/carbonate stones. The high serum calcium could have caused kidney damage with loss of kidney function given values were so high as you report and your eGFR fell during the same period. It is important to be sure that crystals and stones did not cause obstruction of the kidneys, so a CT scan – no contrast – would be important. A nephrologist is indeed important here. As for the dose of calcium, it should be as low as possible – to prevent abnormally low serum calcium is the goal. Surely that can be done on the moment. As for the long term, that is conjecture, but a nephrologist and – I think – stopping the extra calcium seem very important. Regards, Fred Coe
Hello I had a gastric bypass in 2018 and I have been having calcium oxalate kidney stones. Two surgeries to remove 2 months apart and lots of little ones I have been watching my diet. Staying away from high oxalates. My doctor never told me anything about this side effects or right vitamins but I have changed a lot but my body is not abdsorbing the vitamins I need and they want to do a bypass reversal. They hope my body will go back to absorbing the vitamins and my GI tract absorbs the oxalates to help stop the kidney stones. I’m scared because I will gain the weight back but I wonder if it will help. In theory it should but reality scares me. Any advice
Hi Venus, The usual reason for stones in bypass is increased urine oxalate and reduced urine citrate and volume. Vitamins are not at issue. One can manage stones despite bypass but reversal will surely be effective as well. Given the new weight loss drugs, you may manage well without the bypass, but that is not my area of expertise. Regards, Fred Coe