

This is our main article on Randall’s plaque, a papillary nidus that fosters growth of calcium stones.
Other articles on this site illustrate plaque, and discuss plaque as a mechanism of calcium kidney stone production. But these have used plaque as part of explanations for stones not as something in itself.
This article provides a full narrative about modern plaque research. Because our own research group performed much of the modern work we may seem perhaps partisan. But in fairness we do show what others have contributed.
A word about context.
From long before our time to now, scientists have recognized the massive numerical predominance of idiopathic calcium oxalate stone disease as contrasted with stones arising from systemic disease. Our concern here lies with plaque in such common patients. Elsewhere, and later on, we plan to consider plaque arising from specific diseases. Quite possibly its origins may not be the same in all cases.
In 2014 this review offered a detailed review of publication history concerning plaque. It accords with part of what we have written, partly not. But we are not historians, rather working scientists in the field. And, we did not set out to do a history of it. These authors certainly did not intend a scientific exposition.
The mysterious picture shows Randall’s Plaque embedded in kidney tissue with an attached stone. It is not the first picture of plaque but the most dramatic. I cannot give you access to the article because Journal of Urology is very restrictive.
What Must We Know?
Because our main objective is stone prevention, what must we know about plaque?
The article will tell you it is a common anchoring place for calcium oxalate stone formation, especially in younger patients and perhaps in men more than in women. All studies with more or less enthusiasm identify hypercalciuria as a factor that promotes its development. Our group adds low urine volume and pH. So in the course of stone prevention, control of urine calcium, and maintenance of high urine volume and perhaps pH may well act against new plaque. Urological surgeons can see plaque, and new grading systems permit a kind of rough quantification. Likewise with repeat procedures they may see less plaque in well treated patients.
That is the pragmatic nugget one can extract right now. You may content yourself with it alone, but so barren a statement will mean much more to you once you know the details. Thence this long but pretty text we offer as our contribution.
Who Was Randall?
A Perfected Scientist
Alexander Randall was a professor of urology at University of Pennsylvania.
In 1937, he published this paper that first described the plaque named in his honor.
He deserves this honor for many reasons. Certainly, he founded the theory of kidney stone formation on anchored papillary plaque. Even more, among all who have cultivated the field of stone research, he was, in this work at least, a supreme scientist. He began with a mandarin scholarship, reviewed all contemporary theories of stone production, and found them wanting truth. Perhaps as a result, he experienced the revelation of an inspired idea that he tested well and, thereby, discovered a useful scientific truth.
In other words, he performed the acts of a professional scientist and was rewarded by the joy of discovery. Nothing more can be asked.
His Work Was Long in Coming
Even before this monumental paper, in Feb 1936, we found him lecturing at the N. E. branch of the American Urological Association. Of the discussants Fuller Albright spoke, commenting about his cases of primary hyperparathyroidism with stones. Those stones, he mentioned, were mostly calcium phosphate, and he saw in the urine and kidneys crystal casts of the kidney tubules. To him, the open ends of these casts seemed ideal sites for anchoring.
He was right, Randall was right, but they seemed at odds.
Unabashed, Randall went on to his magisterial discovery, and Albright to his, also. No doubt that audience of distinguished physicians failed utterly to recognize that they had that evening witnessed the passing encounter of the two brightest stars in their universe. And like two stars both passed on their independent ways, drawing around them, each one, his own system of planetary followers. Each founded an entire school of scientific thought.
He Found Prior Theories Wanting
Stasis held that stagnant urine would crystallize. Indeed it will because supersaturated. Yet stones often occurred without stasis, so he rejected the theory as being narrow and mostly inapplicable. Although some believed that Infection might set up a nidus of abnormal tissue that calcified, he noted that sterile stones predominated. Vitamin A deficiency could produce stones in rats, but calcium phosphate, not uric acid or calcium oxalate – the most common one. Even so he gave this theory credence.
Colloidal Chemistry. Here he means excessive amounts of crystal making materials cause stones. In support scientists had given oxamide to rats to raise their urine oxalate, or calcium carbonate to raise calcium. He argues that many people with high urine oxalate form no stones – they knew that then. Likewise in cystinuria stones may not form despite excesses of that amino acid. But ultimately he agrees that supersaturation probably does play a role in determining the type of stone. Parathyroid Hyperfunction we now call primary hyperparathyroidism does cause stones, and he endorsed this as a main factor. He already knew it accounted for only 5% of stones.
He Formed A New Hypothesis and Tested It
Having rejected prevailing theories as either unconvincing or too narrow, he created his own hypothesis about how stones formed.
Hypothesis is a compound of two main items. The first is what I already spoke about – a vision, an imagining about how they might form – in this case on an anchored site not as yet known. His vision was that a site on papilla existed, and stones formed on it. The second part of hypothesis is deduction. If his vision were true, then what necessary prediction might he test in order to falsify it? The test was a search of papilla in cadaver kidneys for a hitherto overlooked site that stones could and did in fact grow on. He looked, he found plaque, and found stones growing on it.
Where Had Everyone Else Been Before Him?
These plaques were not disguised. Any pathologist could have described them. No doubt many before Randall saw them, perhaps made note, but passed by. Surely some observed tiny stones on these plaques. They were not rare nor hard to see. But if you have no idea about what a plaque might mean, how easy to pass it by as trivial, unrelated to what one is looking for. Is that not how things are? We see what we are looking for.
A Word About Science
What Hypotheses Are
I have written about hypothesis in science, and specifically about kidney stones. They consists in useful dreams or imaginings about how nature might have produced what one can plainly observe about the world. All imaginings can satisfy our desire to understand how things got to be the way they are. But useful imaginings do more than satisfy that desire. They make predictions about the world and, therefore, offer us a way to test if the imagining be true. By true I mean predictive of what is there but not known about until imagination pointed the way.
That sequence matters. Only in ignorance would someone imagine the workings of nature in conflict with what is already known. That is why even the most erudite cosmologist cannot imagine usefully about medicine, or botany. Although one always shapes an explanation about how things work around what is already known, most explanations stop short of true prediction about what is not already known but could be looked for.
Randall did not stop short. He imagined how kidney stones might form, looked for precisely what must be found if his imagining were true, and found it there, where he imagined it might be. That is how he discovered plaque as an anchored site for stone formation.
How Hypotheses Create Theory
One hypothesis is not a theory. A theory combines into a coherent story all of the hypotheses that have been proposed, tested, and not falsified. This collection of narrated usable hypotheses is what we ‘know’ about how nature brought about – in this case – kidney stones. For example, Randall sought and tested a new hypothesis to advance the theory of stone production and got us plaque. Later he sought how stones might assemble on plaque. In this his tools limited success. He sought what might create plaque and considered animal experiments that did not yield success. Clearly he hoped for a complete theory that accounted for how plaque formed and how stones grew on it.
He found the plaque, but could not do the rest.
What Exactly Did Randall Do?
A Novel Hypothesis Came to Him
He turned away from contemporary theories with a disdain one can sense 80 years later and focused himself on human tissue in the light of a brilliant hypothesis: “That there must be an initiating lesion that precedes the formation of a renal calculus”. As a further point he identified not the renal pelvis or calyces as host to such a site – too simple and too rugged. Instead, the papillae called to him. Complex, and known for some fragility. From this he made a powerful prediction: “That the initiating lesion was to be looked for on the renal papilla.”
His imagining was of an anchored nidus stones could grow on. His deduction was that such a lesion would be found on the renal papilla. By such a lesion he meant on papilla and with evidence stones grew thereon.
He Tested His Prediction in Human Tissue
If inspiration comes unbidden like a song, the proper test comes from a mastery of method and technique. He inspected autopsy kidneys looking for a site stones had grown on. Finding none would have falsified his hypothesis. As he most brilliantly said, instead of trying to produce a stone, how much better to “…find stone as it occurred in man, and to observe … its point of origin….”
He Discovered Plaque
Among 429 pairs of kidneys 73 (17%) showed papillary lesions. These lesions were cream colored, near the tip of the papilla, and definitely inside the urothelial covering. With a microscope it seemed a plaque of calcium in the interstitial tissue, definitely not inside the tubules. He saw that it arose in the basement membranes of the terminal tubule walls and involved the space between the tubules as it grew larger (interstitial means in between the tubules and vessels).
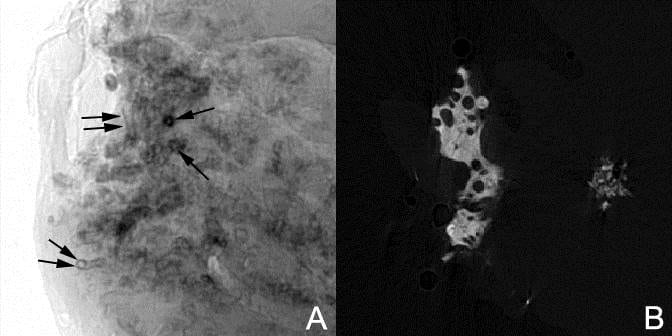
The First Picture of Plaque with Stone
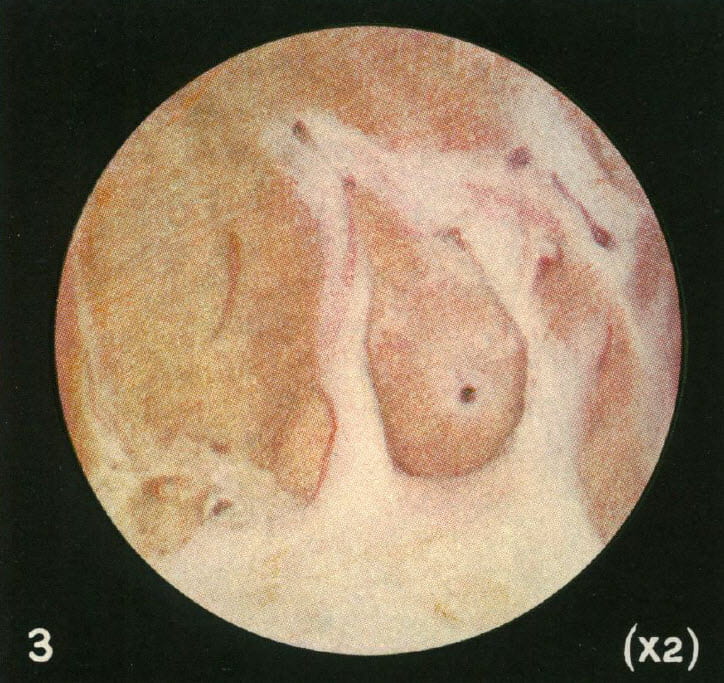 After a number of cases, he found a tiny black stone growing right on the plaque where the lining epithelium had given way so urine could bathe the tissue deposits. Here is his photograph from 1937. The tiny reddish black round dot is in the middle of a white or cream colored clearing on the papilla.
After a number of cases, he found a tiny black stone growing right on the plaque where the lining epithelium had given way so urine could bathe the tissue deposits. Here is his photograph from 1937. The tiny reddish black round dot is in the middle of a white or cream colored clearing on the papilla.
This historic picture should evoke some sense of wonder. The round shape of the image field is because of the old optics in use then for microscopes and magnifying glass. No one before him, before this, had made such an image. So this is discovery incarnadine, the ancient relic of that moment in which dream becomes undeniable reality.
We have both known such moments, rarely enough to remember each one. If life is a warp and weft of common stuff, they shine out like threads of gold. Amidst the endless and so often futile theorizing that litters this field of ours, his image stands forward as the very element of undeniable truth. Of science, the immortal diamond.
First Micrograph of Plaque with Stone Over it
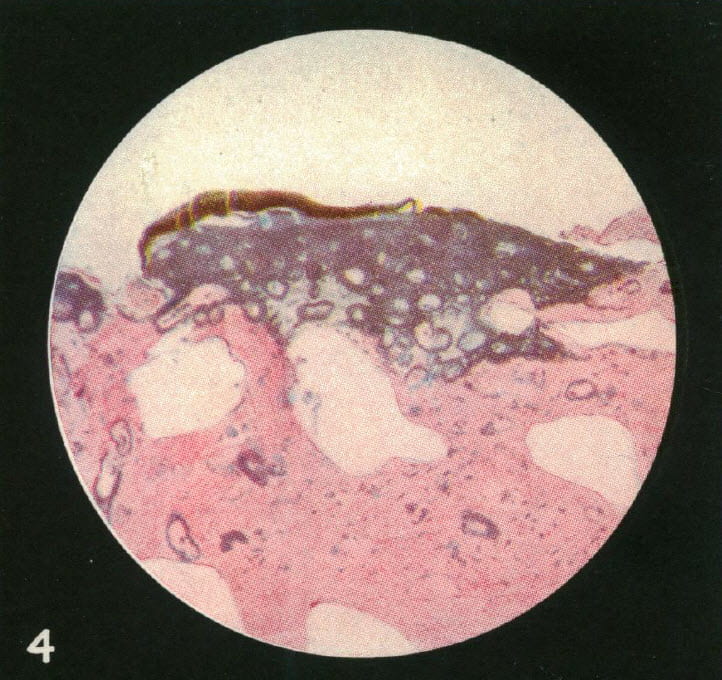 He had a microscope and succeeded in making thin sections of the stone and its underlying plaque. The stain colors calcium, so you can tell where it is.
He had a microscope and succeeded in making thin sections of the stone and its underlying plaque. The stain colors calcium, so you can tell where it is.
We are looking at tissue from the place where the stone grew over plaque. The papilla has lost its covering epithelium. The rings of color are around tubules whose lumens remain open. In other words, the calcium deposits in the collagen that supports the tubule lining cells, but does not break through into the tubule to form plugs.
For those not trained in anatomy a special word. Kidney tubules are lined by a single layer of cells. They rest upon a tube of condensed collagen that supports them, their so called basement membrane. Outside that collagen tube run the capillaries that nourish the cells and support their lives. Inside the tubule the cells face into the fluid that the kidneys are converting into final urine.
Plaque forms in those collagen tubes, the basement membranes. Thence, the calcium stain forms rings.
Where the brown stone material lies over plaque, the latter has become very dense. Even so, the open tubules are easy to see.At the end, where the stone layers over it, plaque gets so dense the tubule lumens vanish.
With collaborators, he established the plaque as a salt of calcium crystallized with phosphate and carbonate.
He Uncovered the Plaque Stone Junction
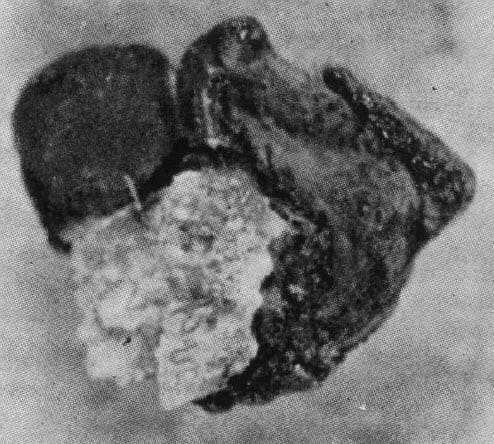 Well enough, you say. I see a stone like material lying over plaque. But, can you show me a real stone with plaque on it where once it attached?
Well enough, you say. I see a stone like material lying over plaque. But, can you show me a real stone with plaque on it where once it attached?
He did that. From chemical analysis the black part proved calcium oxalate, the white part – torn off plaque – proved calcium carbonate and phosphate. This proves that crystals of one kind have grown over crystals of quite another kind. That latter forms in the kidney tissue, in fact in the basement membranes of tubules
He Articulated Modern Understanding of Stone Genesis
“So here we have a definite renal calculus growing on an initiating lesion, and the lesion is a deposition of calcium in the walls of the renal papilla, and the two are of different chemical composition.”
After a brief exposition he goes on to his most remarkable summary:
“This lesion, innocent enough while buried in the wall of a renal papilla, can lose its epithelial covering and from then on be bathed in calyceal urine, and acting as a foreign body, it becomes the nidus upon which urinary salts precipitate. Here, likewise, we can picture the reason how and why renal calculi can remain stationary while increasing in size. Here we can account for a common origin of stone formation, which also allows of the known variation of salts so deposited to form stone, and one can assume that the salt which does crystallize to form a calculus is the one which, at that epoch, is most readily thrown out of suspension.”
Within those quotes lies the articulation of a full theory of kidney stone genesis. At the end, he had proved only a small portion of it but left as his legacy to us the honor of a further attempt.
Then What?
You would think that after his immense discoveries stone research would follow as schooners in the wake of an ocean liner, or rabbits in the wide path cleared by a passing bear. It was not that way. People remembered his plaque but failed to connect his work directly to their own. I mean by this he discovered because he studied human tissue, discarding as equivocal what could be found in animals or produced in animals by unnatural means. Likewise he discarded elaborate inquiries into chemistry or cells in culture that sought explanations not rooted determinedly in human tissue findings.
As a result we created animal models of stone formation that were utterly correct within their scope but divergent from human tissue for the most part as related to the common calcium oxalate stone. Likewise we successfully uncovered factors that could foster crystallization – supersaturation, regulation of urine calcium, oxalate, citrate and pH, principles of crystallization. But we did not link our discoveries to tissue consequences in the one relevant animal – ourselves.
Work After Randall
Long Before Us
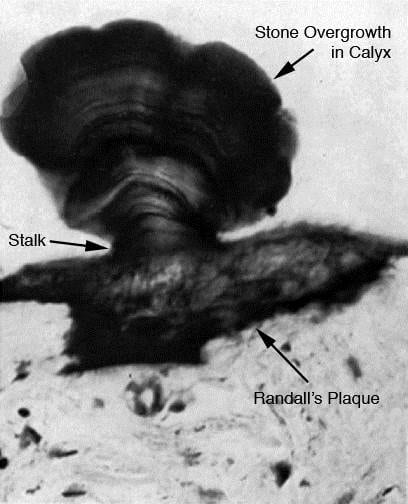
This dramatic picture was taken by W. A. D. Anderson in 1940. Although we have the original paper, neither of us can figure out exactly how he produced this image. It is obviously a histological section but how he solved the problem of cutting mineralized tissue and retaining anatomical contiguity is unclear. Even so this shows without question a stone physically growing on tissue embedded plaque. It is x600 magnification, and so is very tiny. The Von Kossa stain he used stains calcium selectively.
Like Randall, he studied cadaver tissue, and reports that he found plaque in 180 of 1500 autopsies. He could not link plaque to clinical history of kidney stones but noted that records often were sparse.
Vincent Vermooten in the South African Medical Sciences Journal 1940 6:48-49 describes Snow-capped papilla in human kidney. Neither of us can find this reference.
Before Us
Before us, others tried to identify sites of plaque origin and refine knowledge of its crystal structure. Khan and his colleagues identified plaque from a single patient as hydroxyapatite. A report by Marshall Stoller described plaque as extending far higher up in the medulla than Randall had found, and localized in basement membranes of collecting ducts and vasa recta. In this he agreed with Randall that plaque lay in basement membranes, but unlike Randall also placed it within the long hairpin thin loops of capillaries that descend into the medulla. As a clinical investigator he associated it with hypertension but not with kidney stones.
In a more clinical work, Stoller (I invariably name studies for their major scientist as opposed to casual issues of author sequence) contributed massively as the first person I know of to map plaque on papilla during stone surgery. He found it in a majority of stone formers and specifically showed it as more common among stone formers than people without stones. A second report of quite a few patients (143) linked plaque specially to calcium oxalate stone formers. Among them, those with high urine calcium had more plaque, suggesting an association between them.
What Did We Do Different?
If we contributed at all it was by linking ourselves closely to Randall’s work, and to the groundbreaking observations Stoller made, but with more modern and elaborate instruments and techniques. We did not so much discover anew as refine their work into modernity.
Instead of using autopsy tissues as almost all others had done, we perfected intraoperative biopsy of the papilla to get fresh tissue. Because we did that, our tissues lacked the inevitable degeneration that follows death. Likewise we had immediate clinical information from living patients to correlate with the tissues. We used electron microscopy Randall never knew, and high resolution crystal identification within the tissues themselves. We advanced Stoller’s work through biopsy.
What Did We Want to Know?
Instruments are senseless, mere objects. Science lives in the imagination that glimpses what might be and in the intellect that seeks to prove the worth or not of such a brief vision. We sought not plaque itself, but its source. We reasoned that kidneys produce plaque in the course of their physiological functioning, not as a product of some primary disease, and that the origin point of plaque in the tissue coupled with a deep knowledge of how kidneys worked would lead us to a hypothesis that linked plaque to kidney function.
So, we sought the origin point of plaque in biopsies from stone formers taken in the course of stone removal surgery.
How Could You Know The Origin?
By tracking backward to the tiniest specks, too small for any but an electron microscope to disclose. There, where almost nothing was, must be its origin.
What Did We Find?
Autopsy Kidneys
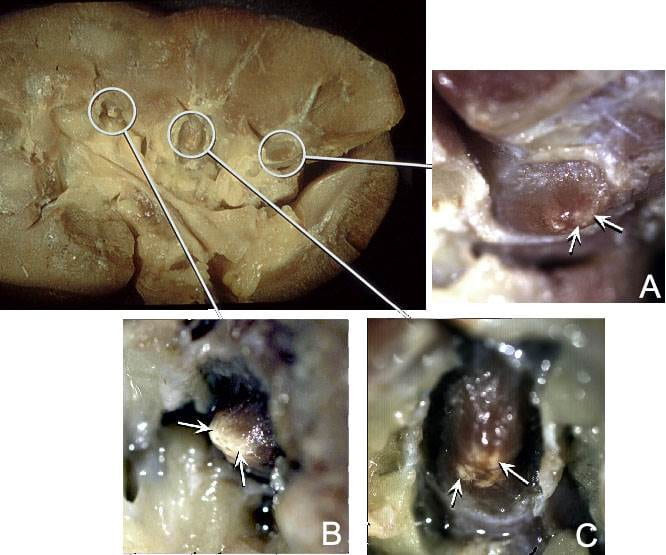
We never published this set of
images. The large one on the left of the panel shows a whole kidney with papilla outlined by white circles. We have removed the collecting system and the outer parts of the calyces so the papillae are easy to see.
The leftmost papilla connects to insert ‘B’ through a white line. The arrows in B point to a white material at the papillary tip. This is plaque as Randall would have seen in his autopsy tissues.
The other two papilla have different amounts of plaque. The one labelled A has minimal amounts – at the arrows. That labelled C has more than A and less than B. Moreover in C the plaque lies in four small separate areas.
We noticed this variability of plaque amount throughout our subsequent surgical work, but here it is evident within a complete single kidney.
Studies During Surgery
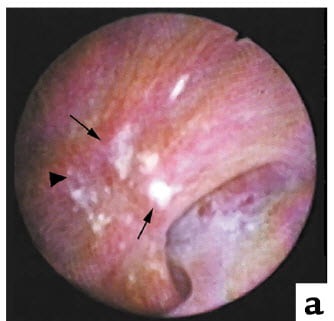 Like Randall, we found plaque with stones growing on it. Here is a picture taken during surgery. The white plaque stands out. The arrowhead points to an eroded area where a stone may have once lodged.
Like Randall, we found plaque with stones growing on it. Here is a picture taken during surgery. The white plaque stands out. The arrowhead points to an eroded area where a stone may have once lodged.
A low power view of the papillary biopsy shows more or less what he had shown. Our optics are more brilliant, our stain a sharper black.
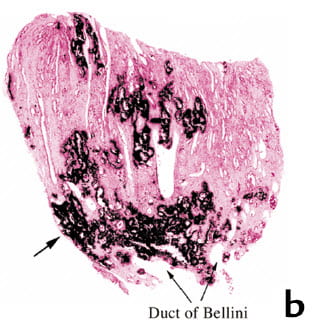
There are the rings of calcium surrounding open tubules. Some plaque ranges to the very outer rim of the surface. An arrow points to it.
So far, nothing differs from him. We found what he found, exactly. This was a stone former, who made calcium oxalate stones.
The tissues gave no evidence of injury or inflammation. He found the same. It was as if the crystals filled in the collagen that lies between the tubules and blood vessels and specially filled the areas around tubules making rings.
Nearly eighty years later, with better optics and fresher tissues, we replicated his work to a cipher.
Where It Began
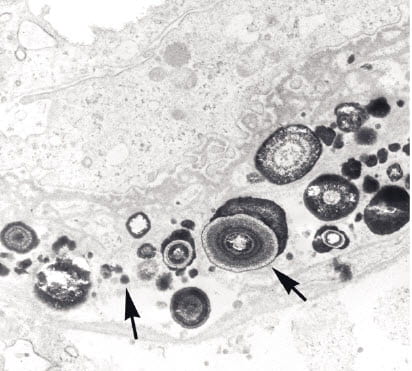
Here, in the thin limbs of Henle’s loop. It begins as tiny laminated microspheres of alternating layers of crystal (white) and organic molecules. An arrow in the picture at the right shows such a sphere whose layers stand out clearly. As this is an electron micrograph, the sizes are very tiny indeed.
What are the loops of Henle? For those not anatomists, we have nephron pictures. This is a simple drawing to orient you. Here is a fancier drawing. The loops run down the length of the papilla and help concentrate the urine. Can one imagine a better place to look for calcifications?
Spacial Orientation
With only planar microscopy it is hard to orient plaque in three dimensions within kidney tissue. To do this we used high resolution micro CT imaging of the tiny tissue biopsy material.
In the left panel of the plate below, the double parallel arrows point to a tubule seen along its long axis. A pair of rails lies perpendicular to the arrows, just like tram tracks. These are the outer edges of a tubule covered with a shell of plaque. The paired right hand image shows the plaque in white outline where the tubules pass though as voids. So the plaque collection is around the outsides of the tubules.
The two non parallel arrows at the lower part of the left image point to a tubule with an open lumen at its center and a shell of plaque
outlining its circumference. One cannot see it on the right side panel.
So plaque forms sheaths around tubules which can merge into one another to form the thin shell like structures seen on the right side. Nowhere did we see any mineral plug the tubule lumens. It was all outside.
You might wonder at the lack of a reference to publication of this lovely picture. In fact, one of us (AE) produced it but used it only in a chapter of a book yet to achieve final publication.
What Did We Do Next?
With an origin in hand, we began to test the main points of a complete theory of kidney stone genesis on plaque. What renal functions might produce it, how certain are we that plaque is necessary for stones, how stones form on plaque, all these became our focus.
We Measured Plaque Abundance
Scientists measure things, and we learned to measure the abundance of plaque in humans by making movies of their papilla during kidney stone surgery and calculating the fraction of the papilla covered by plaque by counting pixels inside and outside of plaque borders.
Asked What Urine Measurements Correlated with Plaque
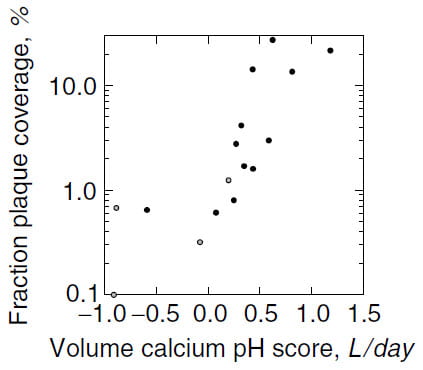 The standard kidney stone 24 hour urine includes measurements of volume, calcium, oxalate, phosphate, citrate, pH, sodium, potassium, magnesium, sulfate, ammonium ion and uric acid. Of these, three correlated with plaque abundance: urine volume, calcium, and pH. Plaque abundance varied inversely with volume and pH, and directly with calcium excretion.
The standard kidney stone 24 hour urine includes measurements of volume, calcium, oxalate, phosphate, citrate, pH, sodium, potassium, magnesium, sulfate, ammonium ion and uric acid. Of these, three correlated with plaque abundance: urine volume, calcium, and pH. Plaque abundance varied inversely with volume and pH, and directly with calcium excretion.
Plaque abundance spans a nearly 100 fold range, thence we log transformed the vertical axis. In a general sense as a score made up from urine calcium, pH, and volume rises so does plaque abundance. The score is an arithmetic derived from multivariable linear regression. Each of the three components had an independent relationship to plaque.
Association, you might say, gives little clue as to cause. We agree. But its lack tells of near certainty in a negative way. So these associations moved us forward into a possible way that by its effects on urine calcium, volume, and pH kidneys might produce the plaque we so tediously measured. This work, linking renal physiology to plaque genesis remains for another article.
One should pause here and remind ourselves that Stoller, long before us, correlated plaque with hypercalciuria. That he did raises our confidence in the association.
Asked If Plaque Correlates with Stone Numbers
If stones form on plaque then more plaque should, or could, mean more stones. In our initial series of 15 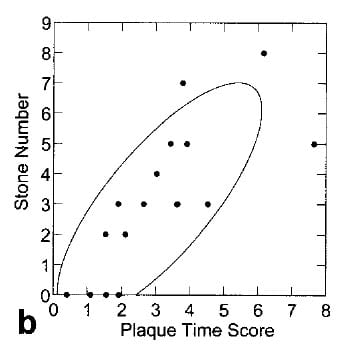 patients, this proved true. Counting stones depends on both what radiographs show, what patients tell you, and what you can glean from hospital records.
patients, this proved true. Counting stones depends on both what radiographs show, what patients tell you, and what you can glean from hospital records.
Despite the complexity of counting new stones and measuring plaque, the two did correlate as this simple graph illustrates.
The four points at 0 stones come from four control subjects who did not form stones. The ‘plaque time score’ arose just like the prior score, from a multivariable linear regression analysis of factors that correlated with stones. Clearly time matters a lot, but so does plaque abundance.
Attached Stones Specifically Attach to Plaque
A glance at the prior figure will tell you that although plaque varies over a huge range, it occupies only between 1 – 10% of the papilla surface in most stone formers. This fact offered an opportunity for a rigorous test of the idea that it is indeed plaque itself that underlies and supports growth of stones on papilla.
Such a test took the form of a trial in which attached stones are graded as attached to plaque or not both during surgery and at confirmatory views of movies made during surgery. The trial used a fixed-sample testing and group sequential design to limit the numbers of patients needed. We terminated the study at 115 stones in 9 patients. By then, we had found 81 of 90 attached stones attached to plaque and calculated that more measurements could not affect the final outcome of the trial. We calculated the probability that 75% (95% CI 58 – 93%) of attached stones would be attached in replicate studies. This despite that plaque occupies only a minor fraction of papilla surface.
The Unattached Stones Had Grown on Plaque
You might have noticed that of 115 stones we accounted for 90. What about the other 25?
Of these, 12 showed precisely what Randall had found. They had on their surfaces unmistakable plaque remnants. Four were lost. That leaves nine to consider.
Micro -CT analysis scans each stone using x ray and reconstructs their internal regions by opacity to the radiation quantified exactly. Such radiographic density accurately identifies the various stone minerals. The remaining 9 stones thus analyzed all possessed internal apatite inclusions. We assumed they had detached and grown new layers of mineral to cover the old attachment site thus internalizing it. Not so exacting as much else we did, this work, but adequate to the concern at hand.
How Stones Form On Plaque
Randall knew calcium oxalate crystals grew over a plaque surface exposed to urine by failure of the overlying epithelial covering. But he could not determine what crystals and organic molecules formed the junction between stone and plaque. We helped define these. The original paper deserves reading by those with technical background. Multiple articles on this site detail plaque formation.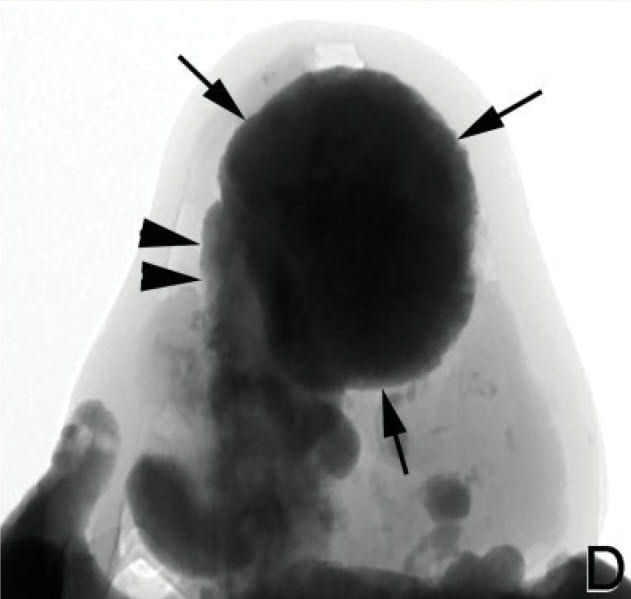
One is very detailed, the other presents overgrowth as part of a larger review. The third narrates the process in video form. None, however, show two rather dramatic pictures of the junction I much love.
The Stone Plaque Junction – From 30,000 feet
Here, in a biopsy from a patient, high resolution micro CT imaging shows a stone growing on plaque. Arrows outline the attached stone. Kidney tissue is pale because transparent to radiation compared to the calcium in the stone. Inside the tissue plaque fills considerable space, and a rising column of it meets the stone and forms the stone’s supporting pillar (double arrowheads).
Randall would have so enjoyed this remarkable image.
The Stone Plaque Junction – From a few microns
Here is a high resolution electron micrograph of the stone plaque junction.
The letter ‘B’ marks a protuberant fold of plaque in kidney tissue exposed to the urine. Black stained organic molecules from urine and perhaps tissue have coated the surface where the arrow points. The body of plaque in kidney tissue lies to the bottom left, the body of the stone to the upper right.
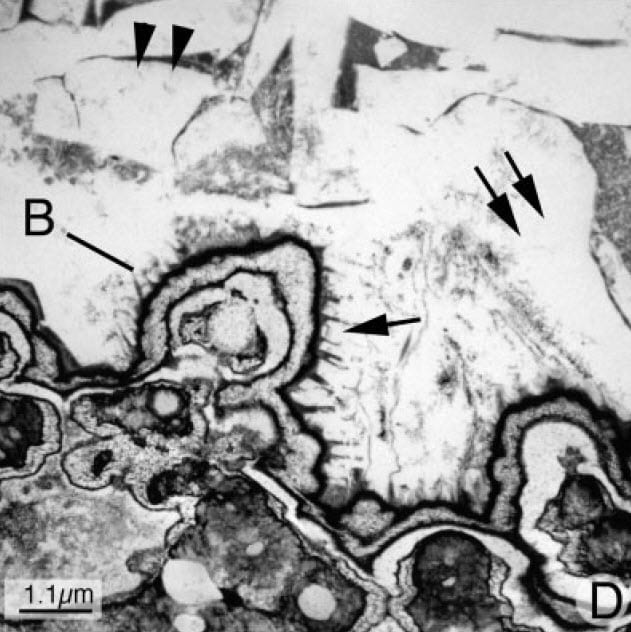
Inside the outermost black coating that faces onto the stone base, layers of crystals – white – and organic material – black alternate. That inside organic material is from the kidney, not the urine, and echos the layers of crystal and organic materials that make up the primary spheres of plaque we showed you just a few pictures back.
The single arrow to the right of the ridge labeled ‘B’ points to large rather edgy crystal plates that belong to the stone. The plates rest on and join the organic covering of the plaque that rides up between plates to form black spikes – one spike points to the top of the arrowhead.
The blending of urine crystals onto coated plaque is seamless and minute. That outer coating, some possible amalgam of urine and tissue proteins somehow interacts with the crystals, but in ways we do not know.
Once fixed to the papilla stone crystals proliferate looking a lot like ice floes in a freezing river. The double arrows and double arrowheads point to large crystal plates. Dark organic material shows through in places. But the main action has occurred. Anchored to plaque and bathed by supersaturated urine, the stone can grow at leisure until detachment.
Somewhere In Between
The studies at 1 micron like that above are so incredibly difficult technically as to virtually forbid the collection of multiple samples from different kidneys and patients. By contrast the micro – CT imaging of whole biopsies are facile but not highly resolved. This has led us to cultivate higher resolution micro-CT techniques such as this one.
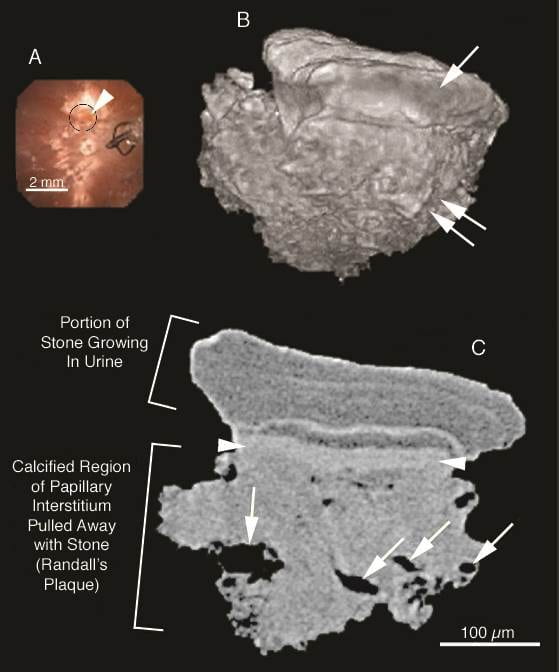
In the intraoperative image of panel A, a less than 1 mm stone growing on plaque lies within the black circle at the arrowhead. The image also shows other scattered plaque, and a stone basket at 3 o’clock. We biopsied the tiny stone with its underlying tissue and made a high resolution CT image (B) rendered to show the outer surface of the stone (arrow) and underlying plaque (double arrows).
Image C shows the interior. Labels identify the plaque and overlying stone. Arrows point to collecting ducts with open lumens that pass through the calcified plaque. Smaller holes, without arrows, are thin limbs with open lumens. The radiographic textures of the stone and tissue plaque obviously differ. The arrowhead points to the interface between the plaque and stone.
Clearly one can use specific analytical methods to determine the crystals and perhaps organic molecules present in each area. Because it is less demanding than histological work with calcified tissue, this technique offers a way to study the stone plaque interface in multiple patients.
In the discussion ‘we’ referred to the research group, but Prof Jim Williams developed this method as an independent project. He did not publish this figure but gave us permission to use it here. In addition, he has reviewed our remarks and accepts them as correct.
The Organic Molecules
Unlike plaque itself, the organic molecules in stones and urine concerned many scientists since Randall, and our own work has been a scant fraction of the total. But if we narrow the focus to molecules associated with human plaque, knowledge is slim as yet.
Osteopontin (OPN)
Well known to bind to calcium crystals and retard their growth, OPN rises in kidney after diverse kinds of injury. Kidney stones contain a lot of it. We chose to study its relationship to plaque, and especially to its orientation toward plaque crystals.
Kidneys with plaque did not show higher than normal levels of OPN, but plaque seemed to accumulate it, perhaps via crystal binding. Cells of the inner medullary collecting ducts and thin limbs produced OPN.
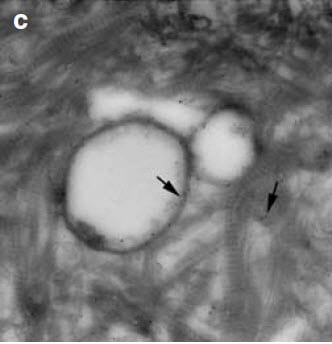
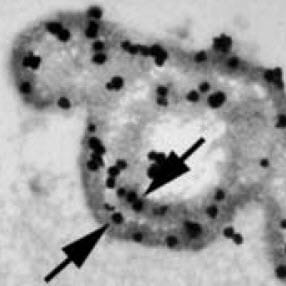 As you may recall, plaque particles begin as spheres of alternating crystal and organic molecule laminations. At 35,000 fold magnification, OPN labeled with gold particles localizes in this plaque microsphere (arrows, picture to the right) within the organic layers. A layer of crystals lies between – white speckles – the organic layers.
As you may recall, plaque particles begin as spheres of alternating crystal and organic molecule laminations. At 35,000 fold magnification, OPN labeled with gold particles localizes in this plaque microsphere (arrows, picture to the right) within the organic layers. A layer of crystals lies between – white speckles – the organic layers.
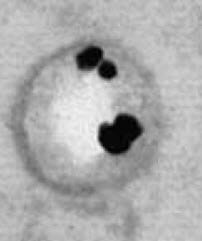 At even higher magnification (left hand picture, 50,000 fold), OPN touches the surface of the crystal (white circle). All of the black dots of gold bound OP touch the crystal surface.
At even higher magnification (left hand picture, 50,000 fold), OPN touches the surface of the crystal (white circle). All of the black dots of gold bound OP touch the crystal surface.
Such crystal orientation is what we would expect from a molecule well known for its ability to bind to crystals and affect their formation and growth.
Inter alpha Trypsin Inhibitor (IAT)
Why Study It?
In urine samples from many stone formers and non stone forming relatives Kristin Bergsland, one of our researchers, found this protein two of three whose levels correlated with stone formation. I say this because it is a compound protein made of three heavy chains (H1 – H3) and one light chain (bikunin) that can be linked in multiple combines. Bikunin (BK) and either the dimer of bikunin and H3 or the ITI trimer (BK + H1 + H2) correlated with stone forming.
Others had found that the combine molecule of BK and H3 (pre-α trypsin inhibitor, PαI) increased in animals given excess oxalate to promote stones, and in cells exposed to oxalate. Hyaluronan (HA), a complex sugar protein molecule, was known to bind H3 and other heavy chains to make complexes that stabilize the renal collagen matrix. This long chain of reasoning and prior measurements led us to look for HA and PαI in papillary biopsies. We expected to find it in and around plaque.
What Did We Find?
As always we studied biopsies from calcium oxalate stone formers with plaque. Normal tissue had almost no BK or H3 or HA, just OPN. The only exception was H3 and HA in the interstitium, that space between tubules and vessels. Not in the cells of the interstitium but between them, on the collagen.
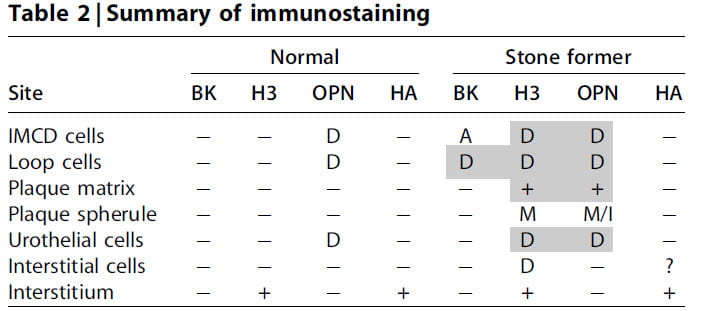
The table says it all. D means the protein was diffuse, in the cell cytoplasm. A means it was just on the membranes facing onto the tubule fluid. M means it was in the organic layers of plaque particles. + means present, – means not present.
You can see the hot spots are loop cells. Remember plaque begins in the basement membranes of those cells. Likewise, the collecting ducts have them all. Plaque has H3 and, as we already know, OPN. By special double antibody staining OPN and H3 lay together, so called co-localized.
 But in plaque microspheres, H3 was not like OPN. It did not localize to the mineral part but remained in the matrix – organic layers of the plaque. This means that whereas OPN may well get into plaque by binding to the mineral, H3 gets there as part of the organic materials that form around the mineral.
But in plaque microspheres, H3 was not like OPN. It did not localize to the mineral part but remained in the matrix – organic layers of the plaque. This means that whereas OPN may well get into plaque by binding to the mineral, H3 gets there as part of the organic materials that form around the mineral.
We made this image at 15,000 fold enlargement using ultra fast freezing to prevent certain kinds of artefacts. So the tissue outlines are not like those in other pictures in this article. But gold stained H3 (the prominent black line at the arrow in the central plaque particle) lay far from the white colored crystal in the middle.
What Did We Learn?
We worked very hard to do this work and even to write the paper. Given what we know, the H3 abundance may reflect attempts by kidney cells to stabilize the collagen around plaque. We think this because it operates that way in joint diseases where collagen is inflamed. We found no evidence of inflammation, but our tools are blunt enough we could pass it by unrecognized.
Bone Genes and Plaque
An obvious if subtle theory held that plaque arose from processes kin to bone. Presumably medullary interstitial cells differentiated into bone forming cells to do this work. We tested this idea and falsified it in one experiment. The occasion for the experiment was study of medullary sponge kidneys. Data from cell culture had suggested that bone genes were expressed and therefore that the many calcifications of MSK arose like bone does. To test this we measured two critical bone genes Osterix and Runx2 in tissues from MSK patients using immunostaining.
Indeed some cells expressed both genes. But none of the cells were in areas that contained any calcifications. On the other hand, many areas contained calcifications, yet no cells in those areas expressed the genes.
This led us to use the very same measurements in calcium oxalate stone formers with plaque. No cells from these patients expressed either gene even though plaque was abundant.
More or less these experiments falsified the bone gene plaque hypothesis.
Papillary Erosions
We speak glibly about stones forming on plaque, breaking off, passing, and causing in this manner the miseries of stone disease. But what about the papilla? If stones break off plaque and take some plaque with them, and if plaque is embedded in the kidney tissue, does this not mean that successive stones may gradually wear away or abrade away the papillary tissue?
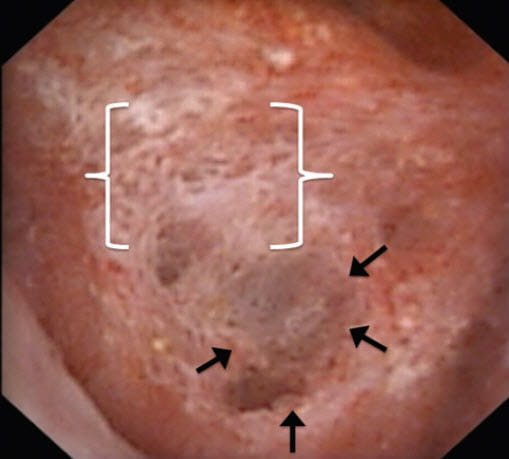
After long silence, we returned to plaque using endoscopic visualization and a reliable scoring system to answer this kind of question. We could score not only plaque abundance but also what we called pitting – a kind of trench where tissue has gone away, outlined by arrows. A bed of plaque lies just above it in brackets.
Unlike the feverish and minute work on molecules, or the groundbreaking discovery of where plaque originates, this work is leisurely statistical inference using fine surgical observations. Discovery, though limited by what we can measure has assured relevance.
Briefly, using standard associational tools, we found plaque abundance correlated with pitting. How many stones had passed, how many shock wave lithotripsies, both also correlated. Multivariable general linear 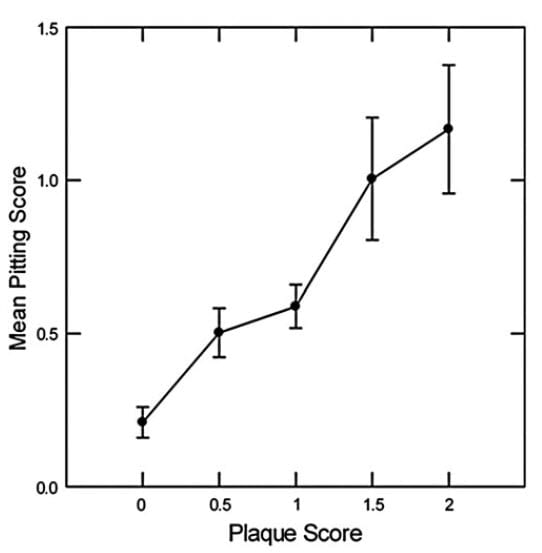 modeling revealed that the primary association, adjusted for the rest, was between plaque and pitting.
modeling revealed that the primary association, adjusted for the rest, was between plaque and pitting.
This very conventional graph shows adjusted pitting scores plotted against increasing plaque scores. The p value for the trend upward is very low (<0.001) and therefore unlikely as a chance observation.
So we believe that more plaque may mean more stones that break off from plaque taking plaque and papilla tissue with them. As a result, papilla show pits where plaque was. Is this true? We do not know. It is an imagined view of what happens where we have no cameras going by day and night.
Native Immunity
Molecules including OPN and IAT belong to a this ancient system that defends us against pathogens. Crystals resemble such pathogens whose outer coatings contain regular mosaics of surface charge such as bacteria and viruses. Kristin Bergsland wrote this useful review of the problem, but we have not pursued it experimentally. Many other so called crystal inhibitors belong to this same pathway, as she points out.
Our Plaque Work
What did we add to what Randall had left us?
Of course, its presumed site of origin. More, strong evidence that calcium oxalate stones grow on it, and a reasonable view of just how they do so. Rummaging about inside the tissues we have identified some likely molecules. Ranging into physiology we found, as Stoller before us, an association between plaque and urine calcium excretion. Perhaps more than anything we reignited interest in plaque and made clear that calcium oxalate stones in humans may well form without a necessity for crystals to adhere to tubule cells.
Why that last sentence?
Decades of work prior to us used animals given surplus oxalate. They plugged their tubules with calcium oxalate crystals. Stones clearly could grow over the open ends of those plugs bathed by the final urine. Massive researches disclosed details of cell injury, means of crystal attachment to cells, and the like. But in humans plugging of tubule cells with oxalate does not occur except under very uncommon and specific conditions such as primary hyperoxaluria.
Perhaps it is that we found no such plugs most benefited stone research. More, even, than our positive findings. Perhaps that, like Randall, we studied only human tissue and made progress – that fact alone – benefited others.
Plaque Work By Others
We have already mentioned the splendid work by Stoller before us, but want to review what others have done during our era. We exclude all work in rodents or with cells as being thus far not proven relevant to the common idiopathic human calcium oxalate kidney stone disease. Likewise we exclude all work having no original data but consisting in reviews or theoretical proposals. We have been thus stringent for ourselves, omitting all mention here of our many reviews.
Our search of PubMed for human plaque research was simply: randall[All Fields] AND (“plaque] OR (“plaque”[All Fields]. So it was highly inclusive. Because plaque links to amyloid formation, we specified exclusion of that term.
Letavernier’s Group
Using kidneys removed for cancer, Letavernier’s group performed a study of plaque localization. In other words they did not study stone formers but quite the contrary patients whose kidneys became available for other reasons altogether. They expected to find plaque in its earliest stages and, as good scientists often do, repeated much of what we did using this different tissue source. But with the addition of tubule segment specific antibody staining they could go further than us to resolve some issues of localization perhaps more perfectly.
Like us they found plaque at the papillary tips, with crystals of calcium phosphate. Plaque lay around thin limbs and vasa recta but almost never collecting ducts. They show microparticles that much resemble our plaque microspheres. They believe their observations most favored nucleation of the particles in the interstitium. Of most importance to the field, their findings match well with ours despite focus on nascent plaque in people without stones. Stones themselves and what causes stones could affect plaque. This work suggests the opposite.
Taguchi et al
What They Wanted to Know
I do not know who is the lead scientist in this group and therefore list it by first author. They harvested tissues from 7 normal people and 23  calcium oxalate stone formers. They biopsied papilla from the stone formers during PCNL. The ‘normal’ people had either ureteroscopy for cancer screening or nephrectomy for adhesion of adrenal tumor. The purpose was to discover gene expression differences related to plaque.
calcium oxalate stone formers. They biopsied papilla from the stone formers during PCNL. The ‘normal’ people had either ureteroscopy for cancer screening or nephrectomy for adhesion of adrenal tumor. The purpose was to discover gene expression differences related to plaque.
What Tissue they Used
This operative picture shows white plaque at the arrowheads and an obvious plug of a bellini duct at the arrow. Our article has avoided plugging, but others on this site give considerable background. Plugs, unlike plaque, produce obvious tubule cell death, and fibrosis and inflammation in the interstitium around plugged ducts.
The authors say they extracted RNA from whole papilla tissues to perform genome wide expression profiling. This means that they took whole biopsies, and measured differences between patients and normals in RNA abundances for all available genome elements. They noted that in some tissues plaque and plugs coexisted. Presumably, papilla from their ‘normals’ had no plaque or plugs. Although, the preceding paper points out that plaque can be found in exactly the kinds of patients this group took for their ‘normals’.
What They Found
In all they had data for 50,599 genes. They could compare papilla with evident plaque (and plugs) from stone formers to papilla with no evident plaque (plugs not stated) – from their ‘normals’.
As usual the result is an immense list of genes up or down regulated with – to us – no apparent relationship to how plaque might form or to stone disease. Inflammatory pathway genes seemed elevated. This makes us suspect that plugs affected some of their tissues. Plugs destroy lining cells and lead to obvious inflammation. The authors summarize their main findings in relation to renal injury, oxidative stress, and vasoconstriction.
No matter what one does with elaborate theorizing and speculation from a mass of nearly irreducible data, the basic tissue seems potentially faulty. If plugged tubules contaminated the so called plaque samples then they looked at the simple ravages of cell death from crystal nephropathy. Responsibility for proof otherwise lies with them.
Letavernier’s Group
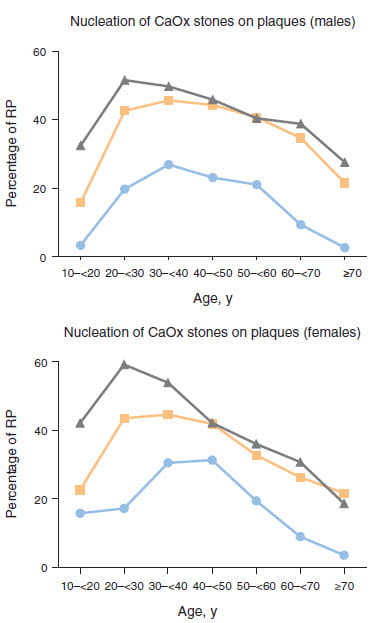
We have said that stones often carry with them traces of the plaque on which they once had grown. This group found 10,282 of their 30,149 stones analyzed between 1989 and 2013 had such traces. Of interest to us the main mineral was calcium oxalate monohydrate in virtually all instances. I leave aside here issues with method as this group does know how to identify plaque. So we can take their results as certainty.
Their large series let them chart the fraction of all stones from the period noted against time, by sex. The results shows quite an increase over time, one they tallied as unlikely by chance. The three plots are 1989-1991 for the circles, 1999-2001 for the squares, and 2009-2011 for the triangles.
Among the younger patients, age 20 – 30 the fraction of plaque related stones rose very high indeed, to nearly 60 percent. By sheer area under the curves, men produced far more plaque related stones than women. Also, plaque wanes drastically with age.
They measured some possible vitamin d receptor gene variants by way of explanation but found nothing significant statistically.
To us, these results have many uses. Indeed time has altered things, and perhaps the reason why would enlighten us as to mechanisms of disease and improved treatment. But also, a large fraction of stones do seem to form on plaque.
Çiftçioglu et al
This person and colleagues attempted to identify a new class of calcifying nanoparticles with enough life like properties to warrant the name of nanobacteria. In collaboration with the Stoller group these investigators found evidence for nanobacteria in plaque harvested from human papillae. The results depend critically upon the specificity of antibody used for their detection. We found no subsequent confirmation of this work by others, and are inclined to a skeptical view until that occurs. Our group collaborated briefly with them and could not substantiate the validity of their antibody.
Mayo Clinic Group
John Lieske and Amy Krambeck have contributed massively to our knowledge about prevalence of plaque. I have already reviewed their work in another article, along with illustrations. They studied large groups of unselected stone formers and indeed found plaque as well as tubule plugging. But the latter far outweighed the former. Their large series was more predominantly female, whereas our high plaque group was the opposite. In a smaller series focused on idiopathic calcium oxalate stone formers, they described patients much like ours in having considerable plaque and being mainly younger men.
The French series reviewed above and the Mayo series can seem a bit at odds, in that they illustrate differing rates of plaque related stones – low in the large unselected Mayo Clinic group, large in the French study. But age may explain a lot. The French data show plaque related stones as a feature of youth, peaking in the 20 – 30 year old range, whereas the Mayo group age average approximated 60 or more. Sex also matters; women droop more at older ages, so that plaque stones are about 20% vs. 40% for men.
Integration Between Ourselves, Letavernier, Stoller, and Mayo
Science delights in offering different views of a single topic, a single problem. Where all concerned seem, as if by chance, to agree there one can build with great confidence.
All of us have found plaque, howsoever much we quibble about amounts. Likewise most stone patient studies associate plaque with calcium oxalate stones, male sex, youth – our main cases were younger males, and when measured hypercalciuria. By consensus, plaque lives in the papillary interstitium, at least in part. The French study of non stone formers make one believe that perhaps all kidneys can produce plaque albeit in amounts too small for detection without highly resolved imaging.
The marked fall in plaque related stones with age makes one wonder where it goes. If it builds up to form the nidus of stones in young men and women, do kidneys perhaps have ways to dispose of it? If one looks back on the clinical history of, let us say, the older women Lieske studied with Krambeck, who had scant plaque, did they form stones in their youth? Could one possibly track back actual stones produced over time in people asking if the frequency of plaque stones falls within individuals? Now that modern ureteroscopy permits high resolution images of papilla, perhaps repeated studies over time may show a waning of plaque in favor of other causes of stones – such as plugging.
Right now, one can say if high urine calcium fosters plaque then lowering urine calcium may reduce it. So proper stone prevention may well prevent plaque. Likewise, at least we found low urine volume as a factor, and all agree that high urine volume is good for preventing stones.
So be it. Time will bring more work, and we will add it here.

Hi Dr. Coe – my most recent CT results came back with the following impression: “a faint calcification in the left renal pelvis may represent a calcified Randall’s plaque or a tiny stone.” Does this mean that I’m waiting for the other shoe to drop with another kidney stone? And is there anything that can be done to reverse a Randall’s plaque once it has formed?
Hi Adrienne, The radiologist cannot distinguish stones from plaque, and usually CT cannot resolve plaque. If you form calcium oxalate stones chances are good that you form them on plaque, so prevention measures against stones are what you most need. I do not know how to prevent plaque, but I suspect it works the same way as stones. Best, Fred Coe
Hi Dr. Coe
I’m writing this to you because I was diagnosised with Randall plaque by a professor of Urology at University of Miami back in 2008. I have had over 300 kidney stones and still I’m producing at a rapid rate .I’m 51 now and don’t know how much more of this I can take seems I’m always in the hospital in tremendous pain due to my stones are very large and the doctors get mad or label me a drug addict when I am warranted in pain my stones are from 5mm to over a centermeter in size not one urologist want to help me get rid or help me find a way to cut this problematic issue down they just want to do lithotripsy on me leave me in pain and kick me out of the hospital how do I find a doctor as smart as you or who cares to help me with preventing stone formation my body is exhausted from years of this problem I live in Palm Beach County and I have a child with special needs that needs me where can I find help ? I don’t think my body can do another hundred stones and the complications I go thru with all these procedures is drastic as well .I’m hoping you can lead me in a direction of help and hope for this condition carries such pain in my life if there’s anything I can do to help myself cut down on these stones id do anything at this point ..Thank you for sharing this artical I learned a lot but I’m no doctor just a patient in need of some much needed relief and like I said hope …Renee Villano
Hi Renee, so many stones sound awful but I have found such cases are rather easier to treat, sometimes, than less active ones. You need full evaluation. Then get the right treatment for what is wrong and do followup urine testing to be sure it is fixed. Stones should much abate. Regards, Fred Coe
I have Randall’s plaque! I live in Clermont Florida I’m a 21 year old female living the same life as you!!!!!!!
Nicole omg i know ur nightmare and at 21 that scares me i dont want u to end up like me if u need to reach out email me Raysrose33@gmail.com ANYTIME REALLY
Dr Jeffery Thill a urologist in clermont Florida I have Randall’s plaque so bad
Dr Dr Coe
i am desperate even worse now its stone after stone after there growing at alarming rates i have eswl or laser stents i have same issues as both these 2 women have as well i also had gastritis bypass because doctor said it would cut down stone formation nope it dont it made me worse my stones are calcium ox and ive been told i produce all types of stones as well i dont know how bad my Randalls is getting cause that diagnosis was in 2008 i have been to every urologist in town no one cares to help me per usual and im a nurse and educated Dr Levellee was at UM Miami but hes no longer there he diagnosed me with Randall in 2008 since i wrote u a year ago ive atleast have had 40+ or more stones eswl laser cut out nephroscopy tubes a severe vit A deficiency losing my eye sight no one seems to tell me how much A to take so i googled the mayo clinic on Vit A deficiency and what to do also have Hypothyroidism and then Hyperthyroidism it fluxuates im anemic and now have orthostatic hypertension pass out fractured my skull facial bones i take meds for that now too my last ct states alot of kidney stones bilaterally ranging from 2.mils to 2.1 cent i cant get a Iv no more for i have no more access or if they do get access it takes hours pics mids central lines they stopped cause of reoccurrence of blood clots in my neck sub claven arms its a nightmare for me i am even having issues while under anesthesia im crashing or lung collapses PLEASE i need help these stones are killing me literally i know my body can not go thru this anymore i am gonna die statistically i should be dead also suffered a bowel obstruction resection and a mild heart attack in July im dizzy nausea pain every day im on medical marijuana now it helps cause i dont want to take pain meds im down to 2 percocet IR a day one for pain in am one for pain at night the pain i can handle due to the vast amounts of pain ive suffered from obstructed stones which i cant express enough how much pain that is it inhumane i need someone to go see for help like i had said i have a child with Autism she needs me my husband passed away at 47 3 years ago i have copd i have hypercalcemia hypocalcuria nephrocalcinosis its in my tubes i knew all this in 2008 but i had a sick husband for 3 years now im just getting worse i hardly pee i pee once at 5am and at midnight i can go on and on i fired all my doctors i have a good primary a good pain management whos saved my life but i gotta get this Randalls looked at this stones addressed these Urologist do not care they just wanna do litho on me thats it im exhausted like that other girl wrote you i fear for my life this is not a joke i can not even get litho now if i needed it which its coming again i have a 1.2 cent above my left top ureter so here comes the pain again i have to stop producing stones or its gonna kill me Please guide me here i live in West Palm Beach Florida where do i go who do i see ? Im begging for help im afraid to complain anymore in fear of the doctors i do have know will drop me as a patient which has happened in the past OBVIOUSLY they NEVER had a stone they can not relate to me or my pain ..I need a brake here someone has to care isnt it why they became Doctors id think …You seem to care so there has to be more that care …Please help me
Hi Renee, I do not remember your contacting me in the past, and I am very concerned to read all this happening to you. Dr Levellee is a very fine urologist so his loss is a serious one. You need far more than kidney stone prevention, from your list of problems, and I know little about the medical scene in Florida. However, the chairman of medicine at University of Florida, Dr Roy Weiss, is a very fine physician and a compassionate person. Feel free to use my name as an introduction. Regards, Fred Coe
I am in desperate need of help. I have been battling kidney stones since 2001. I’ve had numerous surgeries to remove stones and I have had lithotripsy as well. During my last visit to my urologist he suggested another surgery to blast the stone; however, he was unable to do so due to my ureter being so restricted that he couldn’t get the camera past it. I had a stent in for 6 weeks to expand the ureter then proceeded with the surgery to remove the stone and stent. After surgery, I was told that I had a lot of Randall’s Plaque in my left kidney. He decided not to look nor remove the stone in my right kidney in fear of having both kidneys compromised. I agreed with that. This past Monday I had a Mag3 Renal Scan with Lasix performed. During the test I started to experience severe pain in my left kidney. Renal scan came back good with no obstructions and 50/50 function. However, I continued to be in extreme pain after the test with a significant decrease in urine output. Fast forward to today, I was experiencing severe pain, vomiting, chills, and episodes of fainting. I was transported to the hospital by ambulance. EMS said that when the fire department arrived I was unresponsive and appeared to be having a seizure. During the ride to the hospital, EMS stated I fainted 3 times. I live about 30 minutes from the hospital. My core temp when EMS got to my House was 94 degrees and BP was 71/48. My temp once I arrived at the hospital was 99 and my BP was 186/89. CT of head was normal. CBC showed WBC 14,000. UA showed RBC 386.7, WBC 175.0, Bacteria 5,076.2, and Occult Blood to be moderate. They were able to get my BP down to 113/56. I was sent home with Levofloxacin. A month ago, I had to go to the emergency room due to flank pain and my CBC showed 13,000. My UA showed RBC 866.6, WBC 33.9, Bacteria 1,448.8 and my Occult Blood was large. I was sent home with levofloxacin. I was hospitalized during the summer for sepsis due to UTI. I am in pain daily. I have recurrent episodes of severe pain in the left kidney which seems to correlate to high levels of bacteria in my UA. My question is this… is there a procedure to remove the Randall’s Plaque from my kidney, is the Randall’s Plaque causing the frequent high levels of bacteria in my UA, and could Randall’s Plaque be the source of my severe pain in my left kidney as it hurts considerably more than my right kidney which has an actual stone in it? Any help would be greatly appreciated as I am at my wits end with this. I have no quality of life right now as this is affecting my employment and daily abilities to do the normal stuff like grocery shopping, house chores, caring for my kids, and any time of social activities. I have become a hermit due to the pain. Please help!!!
Hi Heather, You seem to have major issues, both surgical and infectious, and also recurrent stones. This site is all about preventing more stones, but right now you need surgical intervention and given all that has happened your urological surgeon may wish to recommend consultation at a major university stone center. The plaque is benign, and does not prejudice kidney function. Your problems are infection and perhaps some ureteral damage. If your surgeon needs some help in locating a proper secondary source for you, I could try to help – but I would need to know where you live in order to make practical suggestions. Regards, Fred Coe
Thank you for responding. I currently see a Urologist at Emory University in Atlanta, GA. I travel back and forth from Tennessee. Like I stated in my previous post, I am desperate to find someone that can help me. I am able to travel if necessary to get the best care. Again, thank you so much for responding.
Hi Heather, Emory is an outstanding institution so you already have very expert surgeons. But with all your issues perhaps it would be worthwhile to go further. Dr James Lingeman at IUPUI INDY is world famous in stone surgery and I am sure your physicians would consider his help of possible benefit to you. Dr Monoj Monga at Cleveland Clinic would be in the same level. This is not to say your own surgeons lack excellence for I do not mean that. But these two are remarkably skilled and might just make a difference. Perhaps you might want to ask your present surgeons their opinion on the matter. Regards, Fred Coe
I had a uteroscopy today. Was told I have many Randall’s Plaque in my right kidney. I am presently following the Kidney Stone Diet and am signed up for Jill’s class. Do Randall’s Plaque ever go away? If I am following the Kidney Stone prevention plan do I still have a high probability of stones forming due to the increased plaque.
Hi Michelle, Outstanding questions! We do not know about the fate of plaque, but suspect more plaque means a higher risk of stones. So you should indeed pursue prevention with passionate intensity. If diet does not abolish urine stone risk, medications may well be a value added. Your physician can tell. Regards, Fred Coe
No stones at present but I have Randall’s Plaque. Do the Plaques ever go away? I am following the Kidney Stone Diet.
See my other response. Stones can form. Fred Coe
I was diagnosed with my first infection/stones at 15 years old (1995). It wouldn’t be until 2003 that I was told I had Kidney Disease. They were not specific, but for the next EIGHT years I would have repeat surgeries, to remove stones almost monthly. In this time I had so very blood transfussions from an unknown cause, attributed to my Kidneys. I have undergone 130 surgeries to date, gone to 17 different urologists, nephrologist, specialists. I have been diagnosed with CKD 3a-? , Nephrocalcinosis, Renal Arterial Stenosis, APRT def., FHHNC, DENT, cystinuria, nephrolithiasis,, hypimagnesemia, hypocalciuria and most recently Randall Plaque. I am sure I am missing a few because there have been so many over the years! I am now 38, have lived on the renal diet for 15 years, have a regimen of pills on a daily basis that would knock a zombie out; yet no real answers!!! They say your GFR can lower, but never rise back… I have had my GFR lower to 30 and return to 62., I have gone into suspected first stages of Kidney Failure, have ports put in, to return to healthy kidney function. I have had biopsies done on stones and tissue, and doctors become so baffled they told me they needed me to find alternate care in a specialty level. I have 2 young children, one of which has already started creating stones and had frequent infections, and a baby who was born with an infection and spent the first year of his life on meds, finally killing the infection after 10 months. I have had infections NON-STOP Since 2012, they have subsided only 3 times, all after being septic and on regimens of 4&5 antibiotics intravenous at the same time in the hospital. The organisms are never the same and always increase to over 100,000 quickly.
I would give both my legs to be able to live a week without pain, or without infection or worry!!! My case is supposedly so rare, but, because I am so young, when I go into a new doctors office, they do not take things seriously (or so it feels) and I feel like a lab rat and like I’m under scrutiny and a million questions that I don’t have answers too- EVERY TIME. They then want to dissect me and learn from my kidneys…. I just want answers.. I don’t want to feel like I have done something wrong, being born with such crazy Kidneys!!!
I know you can not provide answers to the unknown- but, any direction would be AMAZING, more than amazing!
I would love to be part of a study, but am always afraid of the aforementioned. I don’t want to feel like a criminal of genetic inheritance, my children have already started showing signs of the same diseases and I don’t want them to endure the life pain and anguish I have.
Hi Andrea, I am so sorry to hear about this much trouble. Most likely you need care at a kidney stone center based at a major university hospital. It may just be too challenging for physicians in general to go through all of the complexity in your case. If you tell me where you live I could try to recommend a place for consultation. Regards, Fred Coe
Passed one stone, Four removed in April for recurrent UTI’s. After surgery , surgeon said I have many Randall’s plaques and many embedded stones, both kidneys.Are all the stones embedded and do they drop out into the poles, or form in the poles. Is it possible with diet, lemon juice,Vitamin D (now normal), I can keep the Plaques and embedded stones in there. And pass no more? About to do my Litholink testing but all my labs and PTH labs were normal. My urologist thinks that’s a big big maybe. I was on low carb for two years, too many nuts, spinach and almond flour plus my D, and no where near enough water, that is probably what got me in this situation.
Hi Karen, Do the 24 hour testing, at least two different ones, and I bet it is a high urine calcium excretion causing your plaque and stones. The nuts etc do not cause plaque. Real treatment arises from testing for cause; the rest is all confusion. Here is my favorite article on this. Regards, Fred Coe
Could you recommend anyone in or near Northwest Arkansas? I have a very dear friend who suffers with stones. She has had numerous surgeries to remove stones, stints put in, left in too long, destroyed her left ureter. Most recently had stones lasered and the Dr actually lasered some of the Randalls placque. She has seen numerous urologists, has an upcoming appt with nephrologist. She gets infections often and is on pain meds. She is at her wits end. Been suffering since 2006. Really needs someone knowlegable to give her some answers. Thank you.
Hi Monica, The nearest is the university medical school. This link is to the urologists. Here is their kidney stone center that seems prevention oriented. I would recommend she go to the latter one first. Regards, Fred Coe
I found this article very helpful in answering many of my questions. I recently moved from Rochester, NY to Brunswick, MD. I found out from my urologist that I have Randall’s Plaque after having 2 kidney stones in less than 6 months. I also had bariatric bypass about 2 years ago. I have asked my primary care doctor for a name of a specialist in our area to see me for the Randall’s Plaque and another kidney stone. He never heard about Randall’s Plaque and could not help me. How do I find a doctor in my area? Thank you for any help you can give me.
Hi Barbara, The bypass is a common cause of stones and requires careful management. It can cause kidney disease. As for physicians, the closest medical school seems to be Johns Hopkins. Dr Brian Matlaga is a brilliant stone surgeon and general intellectual, and I would recommend him. Feel free to use my name as a reference. Regards, Fred Coe
Hi, very interesting article. I am a sad participant in the kidney stone scene. I have passed close to a dozen stones in the past few years. However, recently the stones are much larger and horrendously painful to pass. I have successfully captured two of the last 4 stones from 2019, one was Calcium Oxalate Dihydrate (Weddellite) 95%, Calcium Oxalate Monohydrate (Whewellite) 5%… the second was Calcium Oxalate Dihydrate (Weddellite) 80%, Carbonate Apatite (Dahllite) 10%, Uric Acid 10%. CT scan says I got a bunch more bilateral 6+mm stones. What is plaque, how’s it get there, what tests determine if and how much plaque is on/in the kidneys and where does one find out more information about kidney plaque? Meeting with my Urologist in two days and would like to discuss it with him. Thanks.
Hi Pat, The dihydrate stones usually form when urine calcium is abnormally high, so 24 hour urine testing is very important. Plaque is fostered by high urine calcium, low urine volume and low urine pH. The article you write on tells about plaque. Your urologist can see it if she/he does ureteroscopy for stone removal, or PERC. You cannot get rid of plaque, it is a place stones grow on, and the secret is organized prevention. Try this article, my favorite for the purpose. Regards, Fred Coe
Dr. Coe,
According to my urologist (after my last surgery) my left kidney has a “large amount” of Randall’s Plaque. Does plaque itself hurt in the kidney? Can pressure, such as flying or scuba diving, cause pain? I ask because the last time I flew, my left kidney hurt more by the time I landed. It could easily be a coincidence due to the fact that I pass 2 to 4 stones a month, mostly from my left kidney. Thanks for all the work you and your colleagues have done!
Hi Troy, Plaque lies within the kidney tissue, in the spaces between the tubules and vessels. Like the place in the walls between the pipes and conduits. It does not cause pain that I know of. It is a prime surface for stones to grow on. High urine calcium, low urine volume tend to promote plaque production. Regards, Fred Coe
I was 16/17 when I was diagnosed with randalls plaque after a year and a half of what we thought was water infections and 2 months of just kidney infections I finally got sent to the hospital were they told me I had it. It’s been a hit and miss with passing stones but this year it has been really bad and they told there is no cure and I can be in really bad pain passing them. I’ve found it does hurt in the kidneys I basically have pain pretty much every day in and out all day really
Hi Jodie, Plaque fosters stones, but stone prevention can much counter it and perhaps reduce plaque formation. Start here, and try to pursue prevention. Regards, Fred Coe
Hello sir,
I’ve been reading your articles on kidney stones and found them extremely helpful. I have a couple of questions, will you please answer them?
Which theory, as per you has been closest in describing the formation of kidney stones?
Secondly, is Finlayson’s assumption of considering papillary ducts and renal pelvis as MSMPR in series reflect the actual hydrodynamics inside the kidney? If not so, which experimental model, as per you, has been best in describing the reality?
Thank you in advance and I hope you continue to share such amazing contents.
Hi Ashish, Formation of CaOx stones on plaque is a certainty but its relative frequency vs. tubule plugging remains uncertain. Tubule plugs seem exclusively calcium phosphate apart from the rare primary hyperoxalurias, and overgrowth on plugs somethat like overgrowth on plaque though the details are not as well specified. Supersaturation is always crucial and that for CaP – as brushite – seems most important. I hope this helps. Fred
I have a severe life-ruining life-threatening kidney stone problem, 4 parathyroid tumors, confirmed connective tissue disease, a super rare immunodeficiency/autoimmune disease and undiagnosed genetic mutations.
I live in pure agony day and night now. Ever since the parathyroid surgery 1 year ago I have tried eating a normal diet with normal minerals and calcium consumption, after 6 years of avoiding ALL FORMS of calcium in order to reduce the stone formation frequencies the EXCRUCIATING pain and the never ending infections, but nothing helps me.
I am in grave danger and in desperate need of highly educated doctors who know these conditions are real and who want to help patients like me.
I urinate blood about 85% of the time now constant hospitalizations from neverending infections and even with all these genetic diagnosis ‘, it is impossible for me to locate knowledgeable doctors like the ones who write these articles.
I am still alive because I fight for it, and I am begging anyone to please tell me where to find the doctors who can help me before I lose this kidney.
My connective tissues disease causes very fragile tissues and organs and my organs actually get injured, get an infection that won’t heal until the infection does years of damage until the sick organ just falls apart.
I am dying for help, in Houston.
Hi Brandi Layne, Hyperparathyroidism is a major cause of stones and systemic disease, and surgical cure crucial. Given 4 gland disease, treatment is very complex. I gather you had surgery but did not say if it brought your serum calcium to normal. Avoiding calcium for stone prevention is a terrible idea, whatever has happened, and you need a totally different approach. Might I suggest that Houston has both the Baylor medical school and UT Houston. I would suggest you find suitable consultants at one or the other as your care is beyond what one usually provides in non university settings. Regards, Fred Coe
Hi- I am seeing random black specks in my urine which has only started happening since my urine has become more concentratated. I typically have a 1.025 or greater urine specific gravity each urinalysis (often greater than 1.030). My urine concentrates at the hole of the toilet nearly every time I go to the restroom. I have greatly increased my water intake yet it still concentrates. I’ve read that elevated ADH levels can cause this, which may be a thyroid issue that I am going to have checked out. So, as a result- the tiny black specks (almost like a grain of pepper)- are these stones themselves or something else?
Hi Hope, they might be calcium oxalate crystals. The very concentrated urine bespeaks a low fluid intake, and you might be able to change that. If you can get any of the specks, however small, their composition can be determined. Regards, Fred Coe
Hello. Thank you for your very interesting and informative articles. Considering the importance of plaque on stone formation I would like to know if Randall’ plaque or pluggings can be effectively and safely removed using endoscopic procedures.
Thanks for your answer.
Hi Albert, I am afraid not. But proper stone prevention may slow their formation – that has yet to be tested. Regards, Fred Coe
Can Randall’s plaque injure the kidney just with its existence? I am asking separate from actual stone formation
Hi Sarah, So far as we know, no. Fred
Hello Doctor. I am a 53 year old woman who went through menopause 3 years ago. My health issues have been a tendency to low blood pressure and a long history of Irritable Bowel Syndrome, otherwise healthy and active, 5 feet tall and consistently about 105lbs in weight. I had my first kidney stone in November of last year. It was 8mm and was causing an obstruction in my ureter. It was treated with Extracorporeal shock wave lithotripsy. A CT scan in July showed multiple 0.1cm to 0.3cm calcified foci noted in both kidneys. Stents were put in and a month given to see if stones would pass. A follow up CT scan at the end of August showed no change in situation. Last week I underwent a surgery for laser treatment that was unsuccessful, the stones were too ’embedded’ in the walls of the kidney to be removed either with a basket or with the laser as an attempt at this produced bleeding and was thus stopped. The surgery did show the stones were growing on Randall’s Plaque in both kidneys. In October I am due for a 24 hour urine test and a blood test. I had collected the fragments of my stone last year, so that will be sent for analysis as well. I have been told to reduce my diet salt intake in the meantime and, since I had already upped my water intake to 3 litres a day since my last stone, I was told to continue this as well. Is there anything further or another course of action that you would recommend? Any advice whatsoever would be appreciated. I live in Trinidad and Tobago, in the Caribbean and we don’t have many highly experienced Urologists or specialty clinics here.
Hi Vicki, You do not mention the stone composition or the 24 hour urine results, so it is hard for me to say much. Perhaps when you get them you might write again and I will try to help. Regards, Fred Coe
Is there a diet I can follow to help with this disease?
Thanks for your help,
Saundra
Hi Saundra, Plaque often arises from low urine volume or high urine calcium, so you would be wise to get 24 hour urine and blood testing as for any calcium stone former and treatment based on what is wrong. Regards, Fred Coe
Hi Dr. Coe, I started following the Kidney Stone Diet and Prevention program with Jill Harris after my first surgery for a stuck stone in my left ureter last May 2019. I just recently had my 2nd lithotripsy last week, this time for a 6mm stone that was stuck in my Rt ureteral side. I was reading my post operative report and it had mentioned that Randalls plaque was noted at the upper pole which brought me here to your article to understand it. Thank you for your very informative article. My surgeon noted that I had several small stones about 2mm in my rt kidney and she felt they were passable. I worry about them growing and then having more problems passing them down the road. Does having Randalls plaque contribute to the growth? My stones are Calcium Oxalate Dihydrate(50%) and Calcium Oxalate Monohydrate(50%). I drink min 96ounces water per day(most), take Hydrochlorothiazide and am maintaining a low oxalate, low sodium and reduced sugar diet. Until the program I did not drink enough water for sure. Is Randalls plaque something that can be reversed? Any advice would be very helpful as I do not want these to get bigger and cause a problem later. I will not be seeing my surgeon again until February and I just want to make sure I have all of the right questions to ask her. I appreciate your time, thank you!
Hi Michelle, Plaque is a common substrate for calcium oxalate stones to grow on. I do not know if it regresses, but stone prevention as you are doing it will work in calcium oxalate stone formers, many of whom have plaque. I do not know if stone prevention measures reduce plaque enlargement, but suspect they do. regards, Fred Coe
Have you been evaluated for hyperparathyroidism, Michelle? If not, I would think that this is a must for you. Good luck!
sir, I write to thank you for writing this article. it has immensely contributed to my understanding of renal calculi and it’s formation.
I am glad it has been helpful. Regards, Fred Coe
Hi, I was diagnosed with Randall’s many years ago. I’ve been through a huge series of hospitalizations, over 50 lithotripsy’s, multiple infections resulting from said stones and have constant issues. Please, could you guide me to any info and/or doctors that can help? I’ve tried every diet, every recommendation, every test, etc. Any info at this stage would be most helpful!
Thank You,
Jennifer
Hi Jennifer, You need prevention, and here is my best introduction to how. Have you had adequate testing, treatment, and followup?? If not, you really need to pursue it. With all of the procedures it is of especial importance for you. If you let me know where you live I can try to identify possible expertise. As well, telemedicine is a reality, and we can help from here if there is nothing closer by. Regards, Fred Coe
Hi Dr. Coe,
My stones are 80% Calcium oxalate monohydrate with 20% Calcium phosphate (and 24-hour urine pH is high, ~7.5). During ureteroscopy, my urologist removed five stones 4mm or less, some loose and several attached. I’m still forming some new stones despite careful adherence to preventative strategies.
My questions are, how firmly are stones typically attached to plaque/plugs?
And most importantly, any ideas on what I might try proactively to help them break free while still small-so they will pass with minimal pain?
Thank you and hope all is well with you!
Kind regards, Al
P.S. These may have already been free, but more than once I’ve observed blood in my urine and passage of a tiny stone after driving several miles over washboard dirt roads to a remote state park.
Hi Al, With more stones and surgery, and calcium stones you really should be on thiazide + low sodium diet as minimum. I do not know your treatments, perhaps these are already in effect. Regards, Fred
Hi Dr. Coe,
Thank you for your thoughtful reply. I have an excellent nephrologist and we’ve carefully applied chlorthalidone, low salt diet, reduced protein, and I’m passing 3-3.5 liters of urine per day. But I still have a new CT-confirmed 3mm stone that seems to be attached. From what I have read, attachment points seem to be pretty small, and some amount of plaque and tissue remains attached to stones that have broken off. I doubt anyone has actually observed one breaking free naturally.
Would you please share any ideas you might have on what factors might govern how and when calcium stones break free and what may trigger then to do so?
Kind regards, Al
Hi Al, If the urine has low saturations for calcium salts and uric acid and there is a new stone my best guess is that a tiny stone on plaque grew large enough to be seen on CT. Growth requires minimal supersaturations compared with new stone formation. If you count new stones, are they indeed common or become rare? Fred
Hi Dr. Coe,
Thank you for your interest and help. SS CaOx=3.70, SS CaP=2.14, SS UA=0.02. My pH-24 of 7.5 elevates SS CaP. My nephrologist has no explanation, but we remain laser-focused on tried-and-true prevention.
Stone count: This new 3 mm stone is the second CT-confirmed stone since all were removed by ureteroscopy in 2015. The first was small and thankfully passed unnoticed.
Please try to help me understand how/why/when attached calcium stones break free from the plaque? -Al
Hi AL, Stones break off of plaque carrying some of the plaque with them, so the plaque breaks off of the kidney tissue. I do not understand the high urine pH. Are you taking alkali?? Are the new stones calcium oxalate or mainly phosphate. Regards, Fred Coe
Hi Dr. Coe,
Thank you very much for your reply. I’ll answer your questions in a separate reply for clarity.
I don’t see a scale, so I have no idea how large the stones in the pictures are. I’m assuming one factor may be that kidney stones grow attached to the plaque until they get heavy enough that physical activity imparts sufficient momentum to knock them off.
Can you please offer any rough ideas on the range of sizes that a kidney stone need to reach in order to be heavy enough to be able to break free from the plaque, or other factors governing when they break free? Kind regards, Al
Hi Al, it is more complex. They break off because the tissue gradually remodels and the plaque the stone grew on detaches – you can find the plaque on the stone. Stone size does not predict detachment. Regards, Fred Coe
Hi Dr Coe,
Thank you for your interest. My doctors are all baffled by my high pH-24. It seems to be genetic and kidney related.
The last stone checked was 80% Calcium oxalate monohydrate with 20% Calcium phosphate.
I’m not taking any alkali. I eat a balanced omnivorous diet with moderate protein. I do take K gluconate and Mg maleate with CHD. No K Citrate or bananas now.
Veinous pH is 7.41. I excrete a lot of acid for a urine pH-24 that is now 7.5, (NH4-24 of ~50 with CHD and ~30 without CHD), and one theory is that I then excrete an even greater amount of base to compensate. I tried stopping CHD, K gluconate, and bananas for a year. pH-24 dropped from 7.3 to 6.9, but bounced back. Very confusing. Thanks, Al
Hi Al, as your stones are mainly calcium oxalate, the high pH is not a primary problem. The high ammonia can raise urine pH. One reason for it may be low potassium. Is your serum potassium normal? Is your urine potassium above 60 mEq/d? Fred
Hi Dr. Coe,
Very interesting and helpful! Thank you. I can’t seem to reply directly to your repl. But you wrote, “They break off because the tissue gradually remodels and the plaque the stone grew on detaches – you can find the plaque on the stone. Stone size does not predict detachment.”
Can you please try to give me some estimate of the range of time a kidney stone has to develop before breaking free? Weeks, months, years? Kind regards, Al
Hi AL, Unknown. Fred
Hello Dr. Coe, Your article was very informative. I am 53. I have stones in both kidneys. I had two lithotripsies on the left side. 8 yrs apart. Last one was 1219/22. My current Urologist diagnosed Randall’s Plaque. The largest stone I grew was about 0.5″ and recently a 6mm that blocked my left ureter as well as other stones that were removed. My new Urologist referred me to a doctor who specializes in endocrine surgery and will determine if I need parathyroid surgery. My blood tests showed blood calcium and PTH levels are elevated/high. My blood calcium has been high for many years, but my previous primary MD & previous urologist stated it was nothing to worry about. My new Urologist and new Primary Dr are both concerned about my blood calcium level. Currently, my urine ph is normal, and I drink plenty of liquids. Eight yrs ago, Magnesium was not found in my 24 urine test but was told to take supplements, (everything else came back normal in the urine test) and I was taking hydrochlorithiazide. My questions: Is Randall’s Plaque/stones related to tumors/overactive parathyroid glands? If yes, when tumors are removed, will it stop stone forming on the plaque? Will the stones that are embedded disappear or will I need to wait for them to drop and pass through my ureter?
Hi Adrienne, Thiazide raises blood calcium so be careful about the evaluation. Before commiting to surgery be sure your physicians stop thiazide for at least 2 weeks and collect at least 2 or 3 fasting morning bloods for calcium and PTH. I suspect you indeed have the disease and the bloods will show high calcium and normal or high PTH. I also presume your 24 hour urine calcium level is high or at least normal, not low – a crucial point. As for plaque, it is within the kidney tissue and cannot be removed. It fosters stones. If you have primary hyperparathyroidism and it is cured by surgery and your urine calcium falls from high to normal afterwards, one would expect stone production to fall. Your physicians are responsible for all of the above and certainly will do everything you need. Regards, Fred Coe