Kidney stone types
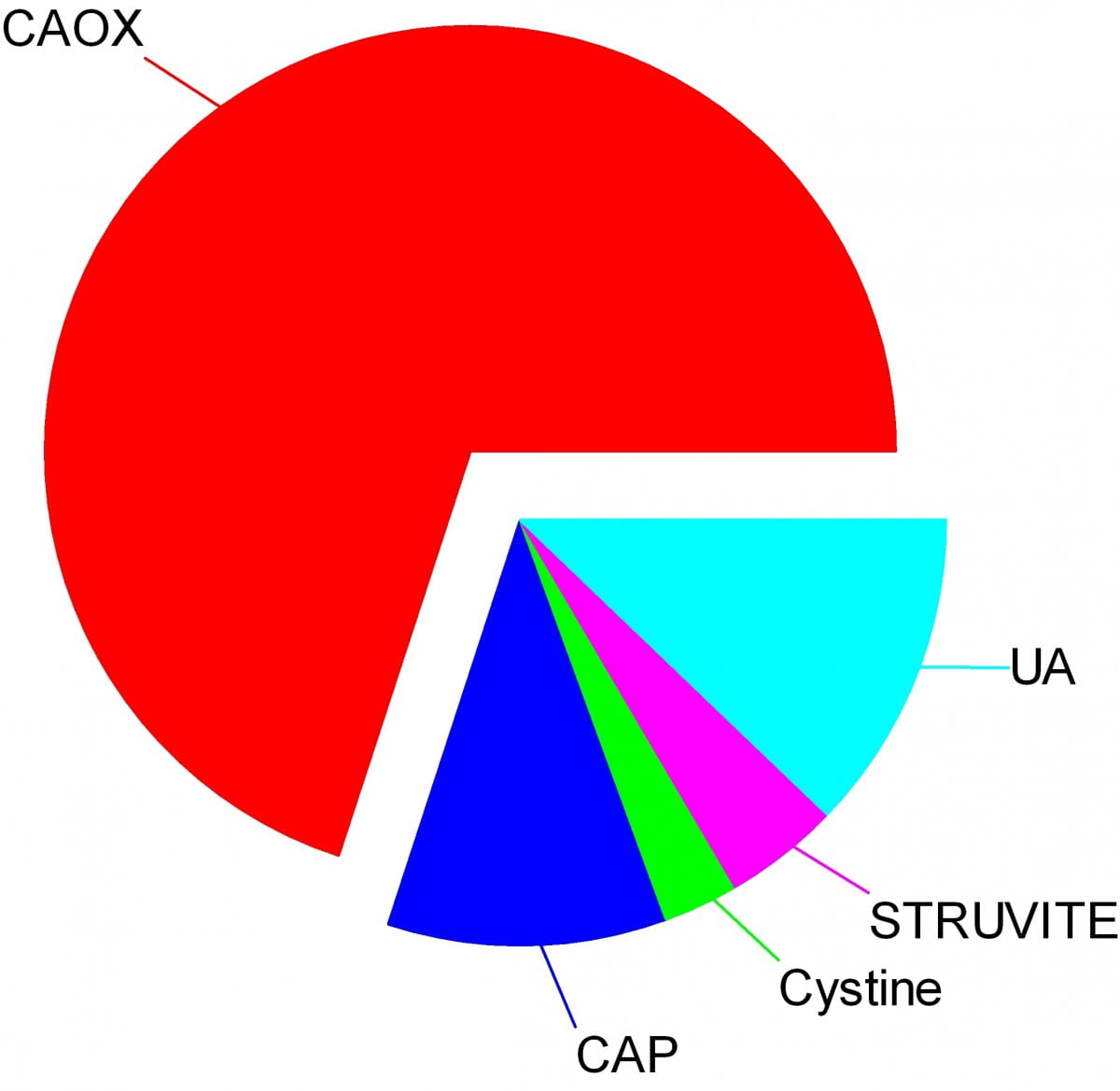
Crystals make stones and their names signify the kidney stone types. Here are the names of the crystals that make the stones: CAOX, Calcium Oxalate; CAP, Calcium phosphate; UA, Uric Acid; Cystine; Struvite.
The wedges on my pie chart show the relative abundances of stone types in our large population of stone forming patients. Calcium oxalate stones predominate by a wide margin in our clinic and in all others I know of.
The names, matter because the whole science of stone prevention focuses upon stone crystals. Each kidney stone crystal creates its own unique illness and requires specific treatment. That is why we name stones by the names of their crystals and why when stones are analysed the results are reported by these very same names.
Being a bold and rather large graphic, the featured picture does what I intended, brings the main facts into view as, at a circus, the great animals and the small animals circle the ring by way of an introduction. Come. I will show you all the common stones, like at a fashion show, or a circus parade. You can watch as they go by and remind yourself, or wonder, which ones might have been yours.
Here they are.
Which type do you have?
You might think your doctors know what stones you have formed, but don’t rely on it. People move, doctors move, health records are far from ‘all electronic’. That stone report from 4 years ago could lie in a dusty filing cabinet, your new doctors unaware it exists. Worse, it could hide in a dresser drawer and you forgot it you put it there. Perhaps even more worse, the stones might stay in that drawer, never analysed at all. Find the stones, find missing reports, urge analysis by your physicians. They can help you most if they know your stone analysis.
When they do not know, physicians can still mount prevention efforts but with less focus and probably less effect than when guided by a knowledge of the crystals. So always seek treatment. If a stone comes along the way, make every effort to get it analysed.
Why should you care to know all this?
Because you will conduct much of your own treatment, and over many years.
Since stones tend to recur, prevention requires treatment over long periods. These treatments work by altering urine chemistry in a direction that minimizes the risk of forming crystals. Such altering of urine chemistry requires control of fluid intake, lifestyle, and diet, and sometimes additional use of medications.
Just as the sailor who aims along a chosen track against the random, misdirecting, confusing sea and air maintains a constant way in proportion to that skill which comes from knowing the way of the boat, patients who aim to keep a certain kind of condition in their urine despite the demands and temptations of the world do so, I believe, in proportion to skills that come from knowing how their work and lives and foods affect their bodies, and how those crystals form which they so much desire to prevent.
Put another way, knowledge is power.
Why is this article so long?
I wanted to put all five main types of kidney stones. That makes a long story. But probably you will care to read about only your own type.
I should mention here, to save a lot of confusion, that stones often contain mixtures of crystals.
The pie chart refers to the most common crystals in a stone, for which the stone is usually named. Much of the time, minor crystal components are not crucial, but sometimes – to jump forward a bit – they are. Even a trace of struvite or cystine, for example, can have great diagnostic importance.
Calcium stones
Calcium Oxalate Crystals
In the great circle atop this page article, the calcium oxalate stone, being most common, occupies a lion’s share of the space. 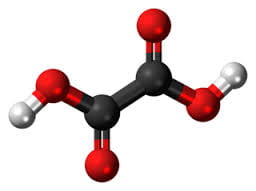
The calcium oxalate crystal forms when calcium combines with oxalic acid. Oxalic acid (at left), a dead end waste product that the kidneys remove, contains two carbon atoms (the large black spheres), four oxygen atoms, and two hydrogen atoms (silver).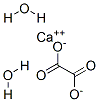
At the acidity of urine, the positively charged hydrogens leave their negatively charged oxygens. As a result the oxalate molecule carries two negative charges. In the figure at right one negatively charged oxygen attracts the hydrogen of a nearby water molecule (H – O -H) while another attracts a positively charged calcium atom.
You can imagine how another oxalate ion (the name for a charged molecule in water) could attract the same calcium, or another calcium atom attract the bottom oxygen on the oxalate molecule so the chain extends and makes a crystal. You can see more about this in a video I made. Broadly speaking – though my more expert colleagues may bridle at such a simplification – the calcium atoms and oxalate molecules combine by the attraction of their opposite charges.
The calcium oxalate kidney stone comes in two varieties, calcium oxalate monohydrate and calcium oxalate dihydrate. The former are harder and therefore more resistant to fragmentation by lithotripsy. Likewise, the former appear more often when elevated levels of urine oxalate are present.
Calcium oxalate stone formers
From Systemic Diseases
Sometimes this kidney stone arises from a systemic cause, like bowel disease, primary hyperparathyroidism, or primary hyperoxaluria. Only physicians can establish that a known disease – like bowel disease – is the cause of stones. Only physicians can discover underlying primary hyperparathyroidism as a cause of stones. Patients cannot do much for themselves except provide as complete a medical record as possible.
Idiopathic
Most of the time this kidney stone arises simply from the interplay between inheritance, diet, and aspects of daily living. We call such patients idiopathic calcium oxalate stone formers, from Greek ἴδιος idios “one’s own” and πάθος pathos “suffering”.
Even though physicians discover the links between daily living and stone production, and select those changes that can prevent new stones, patients themselves must create and maintain those changes. I believe patients can so this in proportion to how well they understand what is needed, and why. When changes in daily life are not enough, physicians add medications, so even then patients remain active therapists for their own disease.
Stones usually form on kidney surfaces
About one million nephron units make up a normal adult kidney. The calcium oxalate kidney stone type does not grow in the tubules of the nephrons but ‘outside’ them, on the surfaces of the renal pelvis where final urine collects and drains through the ureter to the bladder. Here is a video that shows how they can form.
Calcium phosphate crystals
Phosphate ion and urine pH
Calcium phosphate stone crystals form when calcium atoms combine with phosphoric instead of oxalic acid and produce the calcium phosphate kidney stone.
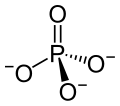 Phosphoric acid is simply a phosphorus atom (shown as the ‘P’ in the line drawing to the left) with 4 oxygen atoms bonded to it. One oxygen atom has two lines for its bond to phosphorus; this oxygen cannot provide any charge with which to bond calcium atoms to make a crystal. The other three have ordinary bonds that are shown by a line, and a dashed and solid arrow. These two arrows mean simply that the oxygens lie above and below the plane of the paper – so if you built the molecule with sticks and balls it would have a three dimensional shape.
Phosphoric acid is simply a phosphorus atom (shown as the ‘P’ in the line drawing to the left) with 4 oxygen atoms bonded to it. One oxygen atom has two lines for its bond to phosphorus; this oxygen cannot provide any charge with which to bond calcium atoms to make a crystal. The other three have ordinary bonds that are shown by a line, and a dashed and solid arrow. These two arrows mean simply that the oxygens lie above and below the plane of the paper – so if you built the molecule with sticks and balls it would have a three dimensional shape.
One of the three negatively charged oxygens never has a hydrogen on it in urine but only in exceedingly acidic solutions. A second charged oxygen is always occupied by a hydrogen atom in urine.
This makes the third oxygen, variably occupied by a hydrogen in urine, a tie breaker.
In a urine of average normal acidity (pH around 6), most of the tie breaker oxygens have their hydrogen leaving the phosphate ion only one negative charge. Not enough to make a crystal.
When the urine is abnormally alkaline (pH above 6.3 or 6.5), the variable oxygen becomes charged so the ion has two negative charges that can combine with calcium to make crystals. For this reason the calcium phosphate kidney stone tends to occur in people who produce a more alkaline urine than those who produce calcium oxalate kidney stones.
Brushite vs. hydroxyapatite
Much like calcium oxalate, calcium phosphate crystals begin simply as one to one pairings of doubly negative phosphate ions with doubly positive calcium atoms. This initial crystal is named brushite. Brushite, which is an equal mixture of calcium and phosphate ions, can convert to hydroxyapatite (HA), which has a more unbalanced proportion of calcium to phosphate. Hydroxyapatite crystals make bones hard.
Because less soluble than brushite, hydroxyapatite cannibalizes brushite. The organic molecules in urine modify this process.
Calcium Phosphate stone formers
From Systemic diseases
Primary hyperparathyroidism and renal tubular acidosis raise average urine alkalinity (higher urine pH) and foster calcium phosphate kidney stones. Many uncommon genetic diseases do the same.
Idiopathic
Idiopathic calcium phosphate stone formers share a common set of traits. Perhaps because urine contains far more phosphate than oxalate, they form more frequent and larger stones than idiopathic calcium oxalate stone formers. Often the stones originate as crystal plugs at the terminal ends of the kidney tubules. More crystals deposit over the end of the plug open to the urine, to make the final stone. Crystal plugs damage the cells that line the tubules and cause local scarring.
Uric acid stones
Uric acid crystals
Structure and charged sites
A breakdown product of DNA and RNA, uric acid forms crystals in abnormally acidic (low pH) urine. Obese and diabetic people, those with gout or kidney disease typically produce abnormally acid urine. I know how the urine becomes acid, but leave it for elsewhere on the site.
Uric acid, the molecule we are interested in here (shown to the far right), has two linked rings made of carbon atoms (they are at the angles where lines join), with 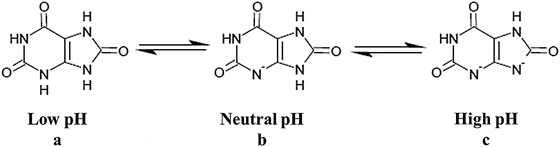 interposed nitrogen (N), oxygen (O), and hydrogen (H) atoms.
interposed nitrogen (N), oxygen (O), and hydrogen (H) atoms.
This molecule has only two charged sites, the nitrogen atoms at the bottoms of the rings. In urine of pH 6 or so, one nitrogen lacks its hydrogen and therefore carries a single negative charge. In more alkaline solutions both nitrogens lack hydrogens, but urine does not normally achieve such alkalinity (pH>8).
When urine pH is low (<5.5) and both nitrogens have their hydrogens, the molecule lacks any charged site, so water can no longer find a hold on the molecule. It crystallizes. It simply leaves the water as water droplets themselves form from the high and vaporous late afternoon clouds and fall from the air as the warm rains of springtime.
Relation to water
Water molecules are each a single oxygen atom (large ball) bonded with two hydrogen atoms (small balls) as in this picture from Wikipedia. The hydrogen side has a positive, the bare side of the oxygen a negative charge. So water molecules link to each other, 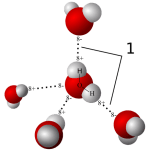 positives to negative surfaces, to make up the clear and seemingly continuous fluid we drink, swim in, and hold up umbrellas to keep off of us when it rains. They link by charge at angles, shown by the number ‘1’ so as to make up a three dimensional macrame.
positives to negative surfaces, to make up the clear and seemingly continuous fluid we drink, swim in, and hold up umbrellas to keep off of us when it rains. They link by charge at angles, shown by the number ‘1’ so as to make up a three dimensional macrame.
To be ‘in solution’ means to have some charge to which water molecules can link up with by attraction. Calcium atoms are positive and become surrounded by a shell of water molecules facing it with their bare negative surfaces. Oxalic and phosphoric acids have negative charges and are surrounded by water molecules pointing their positive or hydrogen sides to them.
Uric acid at neutral pH has its one negatively charged nitrogen water can grasp. But when pH falls, and neither nitrogen has any extra charge for water to bind with, how can the molecule remain among the water molecules? It cannot. The molecules stack into solid crystals and fall from solution.
Uric acid stone formers
The stones can be orange – red, large, and numerous
The stones can be red or orange because uric acid crystals absorb hemoglobin breakdown products that are red – orange pigments in urine. Sometimes uric acid crystals pass in urine as a red orange gravel.
Uric acid does not have to connect itself to some other atom or molecule to make a crystal, in the way that calcium must bond with oxalate or phosphate ions to make calcium oxalate or calcium phosphate crystals. When pH is low enough to extinguish its charge, uric acid can crystallize very fast, in seconds, and pass as an orange gravel in the urine. If retained, such crystals can grow rapidly into large stones. Because there is much more uric acid in urine than there is oxalic acid, uric acid stones can grow very large and rapidly. Some fill up the entire collecting system of the kidney.
Urine pH controls stone formation
But because the whole process depends almost completely on the acidity of the urine, uric acid stones are very easy to treat. Just a modest amount of supplemental alkali will make the urine of almost any patient alkaline enough that the hydrogen atoms are removed from the one crucial charged nitrogen. Water can bond there so uric acid remains in solution. Because so simple, treatment prevents stones with certainty. Relapse need never occur.
Mixed stones require special care
Unfortunately, however, stones commonly contain uric acid mixed with calcium oxalate. In this case, one needs to track down the cause of the calcium oxalate stones as well as make the urine alkaline enough to stop uric acid stones from forming. Calcium phosphate crystals mix with uric acid only rarely, because it takes a rather alkaline urine to remove the hydrogen atoms from phosphate so it has two negative charges and can bind efficiently with calcium atoms. At that higher pH, uric acid will have its charge site and remain in solution.
Struvite stones
Urea and the planet
Kidneys cannot make struvite. Bacteria make it. Not all bacteria, either. It takes bacteria that normally thrive in the soil, and they do it for ancient and compelling reasons. These bacteria produce the kidney stone named Struvite after Heinrich Christian Gottfried von Struve (1772–1851).
Animals deposit urea (at left) all over the planet when they urinate. Plants cannot use it.
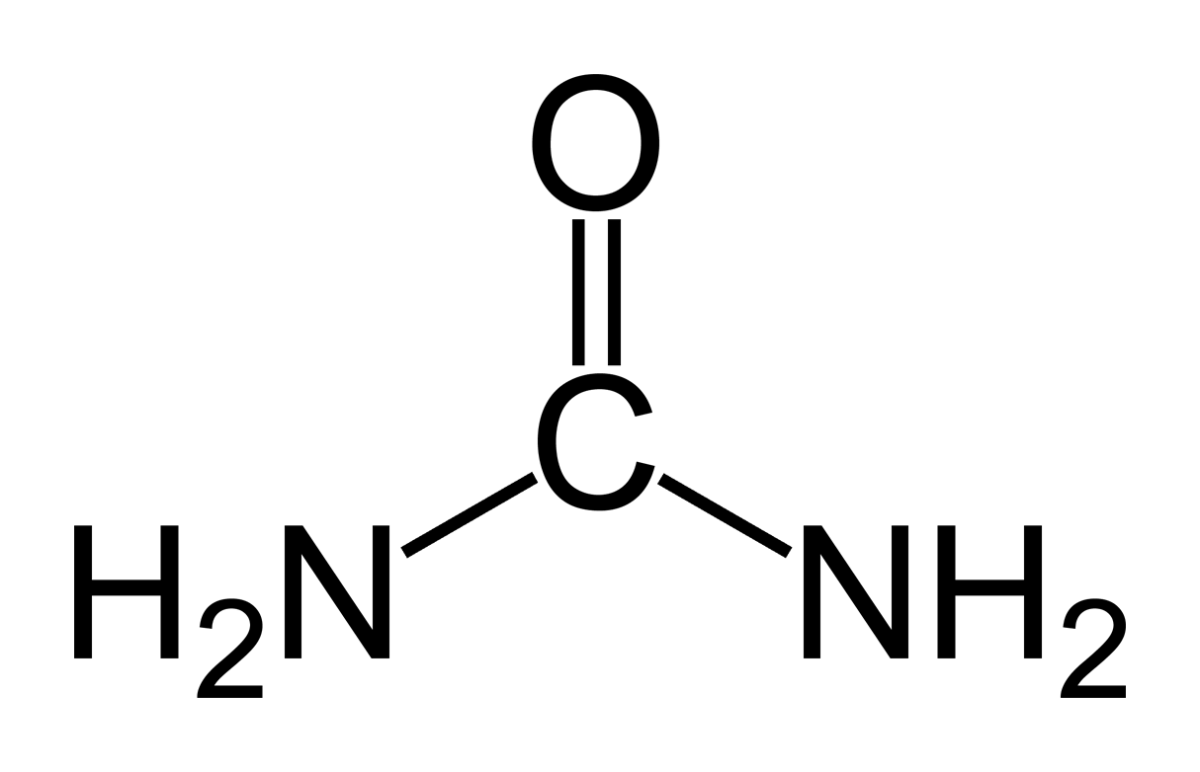 Like oxygen, nitrogen is an essential for life yet dangerous. It is integral to proteins, DNA and RNA. As these molecules are broken down and remade, some of their nitrogen slips by and can form poisonous compounds unless caught up in safe waste products. Of these, the main one, urea, contains 2 nitrogen atoms bound to a single carbon atom (‘C’ in the picture to your left).
Like oxygen, nitrogen is an essential for life yet dangerous. It is integral to proteins, DNA and RNA. As these molecules are broken down and remade, some of their nitrogen slips by and can form poisonous compounds unless caught up in safe waste products. Of these, the main one, urea, contains 2 nitrogen atoms bound to a single carbon atom (‘C’ in the picture to your left).
Uric acid contains 4 nitrogen atoms (look back at the picture of it). Birds and reptiles excrete most of their nitrogen as uric acid; mammals like us excrete nitrogen mainly as urea.
As the animals of the world urinate on the soil, their urea brings nitrogen to plant roots, but the plants cannot use it. They cannot release the nitrogens from the carbon atom that holds them. Those soil bacteria that make struvite crystals have an enzyme, called urease, that can release the nitrogen for plants to use as their nitrogen supply.
So, soil bacteria with urease maintain the nitrogen cycle of the earth.
Struvite crystals
As they release nitrogen from its carbon in urea, the nitrogen takes up a proton making ammonia (NH3). Ammonia is a powerful alkali and takes up another proton.
As it does so, the working bacteria surround themselves with spheres of very alkaline fluid enriched with ammonium ion (NH4) that carries one positive charge. Soil magnesium ( an atom with two positive charges) and phosphate sans all of its protons (an ion with three negative charges) spontaneously form their triple salt: three negative phosphate charges, two positive from magnesium, one positive from NH4).
The crystals anchor the bacteria and help create a porous nitrogen rich soul good for plants to grow in
The struvite kidney stone
Why they start
Because urine is filled with urea, soil bacteria that get into the urinary tract can break it down to ammonia and create struvite from the magnesium and phosphate urine always contains.
You might wonder how soil bacteria get into the urinary system.
Because we eat them, with foods that are not cooked, and they become part of the intestinal bacterial population from an early age. In us and around us, they find a way into the urinary system, especially in women whose shorter urethra makes entry easier. No matter how skillfully used, any instrument put into the bladder can carry our personal soil bacteria with it.
What they do
Because they live among molds and fungi, soil bacteria easily mount resistances to antibiotics, so antibiotics given for a urinary tract infection will tend to kill sensitive bacteria and select out those that can resist them.
Soil bacteria can produce struvite stones de novo, or infect calcium stones to produce a mixed stone. Either way, struvite stones are infected by their very nature. They can become huge. Their bacteria can injure the kidneys, even enter the bloodstream and cause sepsis.
Treatment is a mix of thoughtful surgery and selection of antibiotics after such surgery to kill bacteria that remain. If the stones are a mixture of struvite and calcium crystals, new calcium stones need to be prevented.
Cystine stones
Inherited kidney abnormality
 Lemon yellow with a sugary coating these form only in people who have an inherited kidney disorder called cystinuria.
Lemon yellow with a sugary coating these form only in people who have an inherited kidney disorder called cystinuria.
Although the kidneys function well, they permit abnormal amounts of four amino acids to enter the urine. Three do not matter that we know of. The fourth makes crystals and the cystine kidney stone type.
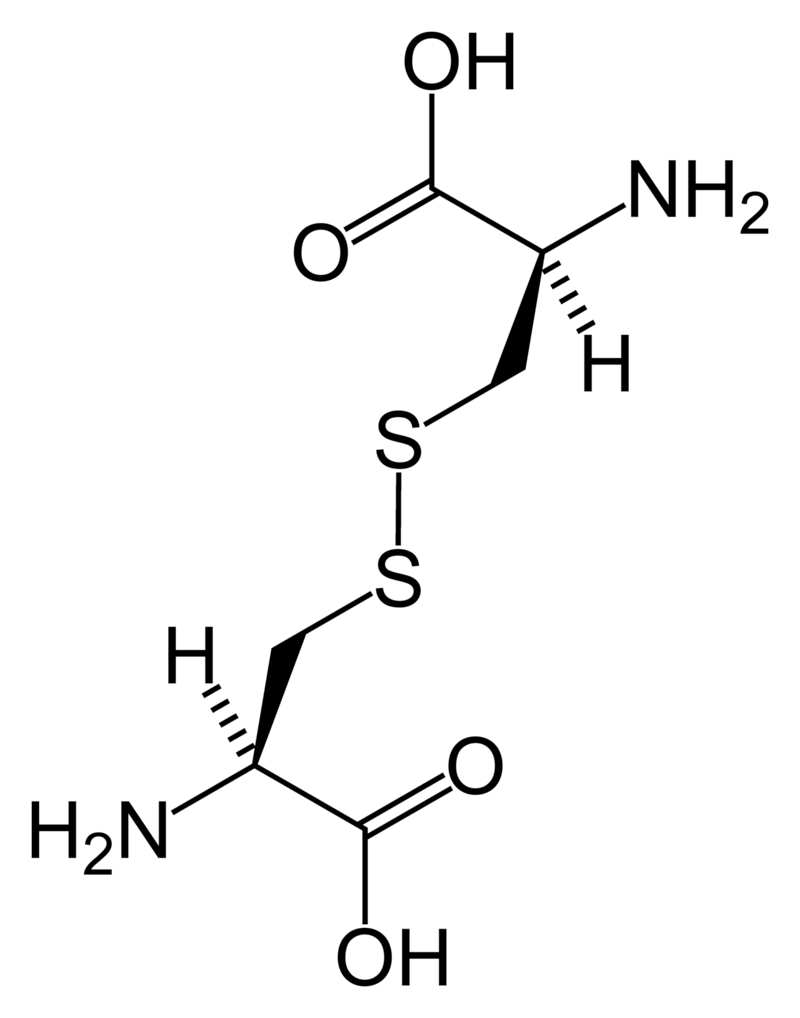
Cystine
Cystine (left) forms through the coupling of two identical amino acids – called cysteine – through their sulfur atoms (‘S’ in the line drawing).
Each cysteine contains two carbon atoms – not shown except as corners – bonded together (shown by the single long line that connects the two corners) as in oxalic acid.
One carbon atom has 2 oxygens bonded to it; the other has one nitrogen (which makes it an amino – nitrogen containing – acid), a hydrogen atom, and a sulfur atom. As for phosphate, the dashed and solid arrows simply mean the hydrogens and sulfurs lie above and below the plane of the page and a stick model would have a three dimensional shape.
Cystine Crystals
Cysteine itself is very soluble because the sulfur atom has an appreciable negative charge.
But the big, long cystine molecule has very little charge because the sulfurs bind to each other. So, like uric acid, cystine loses intimacy with water molecules and simply leaves the solution as crystals. Also like uric acid, the process is fast.
Cystine stones
Because people with cystinuria lose large amounts of cystine in their urine stones readily grow large, and fast.
Stones probably form in the urine itself. But cystine crystals can plug the ends of kidney tubules, as calcium phosphate crystals do, causing cell damage.
Since cystinuria is an inherited disease, stones may begin in childhood.
Effective treatment always requires very large amounts of fluids to dilute the urine. The few effective drugs resemble cysteine. Their sulfur groups bond with cysteine to form a ‘mixed disulfide’ more soluble than cystine. But their side effects can limit use.
Rare stones
Here and there we find patients who make uncommon crystals and require very special care.
Uric acid, as an example, can form odd crystals such sodium or ammonium acid urate, especially in people with bowel disease and chronic diarrhea.
Anti-viral drugs can crystallize in urine and form stones only recognized for what they are through stone analysis.
Very rare disorders of metabolism can produce molecules which crystallize in the urine, for example 2-8 dihydroxyadenine.
Although it can take a while before the right answer emerges, stone analyses will put physicians on the right track for these special cases.
The end of a very long post
That’s my parade.
The common animals and the rarer animals have gone by, and you have glimpsed the main ones, big and small.
The one point is what it was at the beginning. Each kind of kidney stone has its own ways, and treatment requires we know which one you have.
Likewise, for whatever that one may be, it is good to know as much about it as you can know. For long term prevention of stones is hard to come by and ultimately what the patience and and consistency of patients themselves matters most.
If you don’t know which stones you have made, find out.
Track down old reports and pull them together.
Keep copies and send everything to the doctors who care for you.
Fred Coe MD


Hello Dr. Coe: I am “new” to the world of kidney stones. In July 2022, without any pain symptoms, but fairly frequent UTIs, I was referred to a urologist in the Chicago suburbs. Turns out, I had a 20mm stone located in the lower lobe of my left kidney! I opted for lithotripsies (3 to date). The procedures were fairly successful and I was able to collect a lot of stone fragments (again without pain, thank goodness!). Lab analysis indicates the composition as : 50% Calcium Oxalate Dihydrate (Weddellite) and 50% Carbonate Apatite. My urologist said we could wait a few months since most of the stone looked like it was broken up and passing. Feb 2023: I just went back to have the KUB x-ray to see the progression and, unfortunately, the small remainder of the 1st stone was still present and 3 more medium stones have been created (still all in the lower lobe). What now?! P.S. Thank you for all you do to share information and provide knowledge! -Nancy
Hi Nancy, I apologize for the lateness – some comments became buried this past winter. You ave forming phosphate stones and should be fully investigated for cause and treated. Fred
Nancy, my lab report just confirmed I have the same exact composition stones that you have (hopefully ‘had’ by now), however all my stones were in my bladder, not kidneys. My urologist just removed numerous stones last week. My follow up appointment is in a couple days. If you are willing to share, did you figure out what caused your stones and how to prevent them from recurring?
Thank you!
Mike Anderson
Hi Michael,
Make sure to get a 24 hour urine collection ordered from your doctor so you can learn WHY you are forming stones and get on treatment to stop! Read more about that here:https://kidneystonediet.com/resource-list/
Jill
Mr. Coe, I was reading your article about phosphorus stones and read the part about combination stone makers and what you end up calling them.
I am 40% calcium oxalate
40% calcium phosphate
20% struvite
Does that mean I am a struvite stone maker, or did I read your article incorrectly?
Hi Janet, The presence of struvite suggests infection may play a role, but labs sometimes confuse struvite and other calcium phosphate stones Here is my best on infection stones. I would proceed as if you have calcium phosphate stones but also search the urine for relevent bacteria (in the article). Regards, Fred Coe
Mr. Coe, I was reading your article about phosphorus stones and read the part about combination stone makers and what you end up calling them.
I am 40% calcium oxalate
40% calcium phosphate
20% struvite
Does that mean I am a struvite stone maker, or did I read your article incorrectly?
My latest stone analysis shows Calcium Oxalate Monohydrate at 40 %, and Calcium Phosphate (Hydroxyl) at 60 %. I have, recently, been bombarded with multiple stone passing. Sometimes for successive days at a time. Please advise!
Hi Kevin, you are forming calcium phosphate stones, and they are a thing of their own. You really need a full evaluation to find the cause of these stones and get treatment focused on their prevention. I cannot advise this too strongly. Regards, Fred Coe
Hi, Dr Coe
I continue to have flank discomfort 2weeks after lipotripsy & partial retrieval of a 4mm L uretral stone & a brief stent. I’m concerned about the analysis Calcium Oxalate Dihydrate (Weddellite) 25%, Calcium Oxalate Monohydrate (Whewellite) 50% & Carbonate Apatite (Dahllite) 25%. I’m also an MS patient with a slightly compromised immune system (lymphocytes in the basement) and a long history of substantial incontinence issues (so I’m necessarily careful about not over hydrating). I’m not aware of having recurrent infections but acknowledge that I’m at high risk. Interestingly, one of my long term meds is topirimate. I’ve read that drug is implicated in stone formation. Am I overthinking this and need to be more patient about recovery? Or is there something to be concerned about here?
Hi Gina, topiramate causes stones a lot and – however difficult for your physicians – you need to get off of it. I would blame this drug ahead of almost anything else i saw. I suggest it will cause low urine citrate and high urine pH, ideal for calcium phosphate stones. Regards, Fred Coe
Hi Dr. Coe,
Thank you for a very well written article. I have a pediatric patient with recurrent proteus UTI’s who was found to have 100% calcium carbonate phosphate stones. Normal Ca24, Ox24, and P24. pH 6.3. Only finding was the citrate low at 230. Anything to do after the stones now removed if asymptomatic and follow up imaging normal?
Hello Dr, the carbonate apatite could well be metabolic in origin and the low citrate despite high pH may be a clue. When your patient is fully recovered I would do another 24 hour urine looking for consistent low citrate and also at calcium phosphate supersaturation – which may indeed be high. On the infection side I would be sure urine ammonia is not high for the sulfate (usually it is 2/3 of the sulfate up to equal. We have found in adults that CaP stone formers have high urine pH with low citrate and suspect it is due to gene defects concerning the citrate transporter – we have not studied the matter further. I looked in PubMed for papers on the NaDC1 gene defects and could not find anything much – but it was a rather brief foray. Best, Fred
So I recently had to have a procedure done because my kidney stone had grown so much from December 2022 to March 2023 and it was causing a lot of problems for me (I’m currently 32 weeks pregnant, was 20 weeks when they put a stent in and 31 weeks when we did the procedure) my stone analysis came back as:
60% calcium phosphate
20% struvite
20% calcium carbonate
This is my second stone I’ve gotten (1 for each pregnancy), is stone formation more common during pregnancy?
Hi Morgan, You should be fully evaluated for the cause of your stones so proper prevention can be planned. I would wait until you have fully recovered from pregnancy and are not breast feeding. Regards, Fred Coe
If I have a kidney stone analysis of 40% calcium oxalate monohydrate
40% calcium phosphate (apatite)
30% calcium oxalate dihydrate
What kind of stones do I have and what are the causes and treatment?
Hi Shannon, You are most like a calcium phosphate stone former and it is important for you to be evaluated fully for cause.Do not hesitate to get this evaluation as it will point to what you need to prevent more stones. Regards, Fred Coe
Hello – thank you for breaking down the differences. Question, I have history of passing lots of orange cyrstals and frequent uncomfortable urination. My PCP told me to drink more water with lemon. Finally complained because passing crystals all the time. Came back with Uric Acid Dihydrate 80% and Calcium Oxalate Monohydrate (whewellite) 20%. I have constant flank pain, lots of uncomfortable urination, pain my feet and have been told only to drink more water and lemon. Any suggestions would be greatly appreciated as to what’s causing this. I don’t eat a lot of meat and I drink tons of water. Thank you!
Hi Jessie, Uric acid stones are totally preventable. You should be on treatment as soon as possible. Water and lemon is completely inadequate. Read the article and get a proper amount of alkali so uric acid crystals (orange) will not form any more. Regards, Fred Coe
Mine are also orange.
Would potassium citrate be the answer for uric acid stones and how does zinc help?
Hi Ed, Orange almost surely means the stone contains uric acid and that urine pH is too low. But analysis is readily available and the stone may also contain calcium salts which require their own prevention. Likewise one should be evaluated completely for uric acid stones have underlying causes which can benefit from treatment.
Hi,
I had my calculus analysed and received the following results:
Whewellite (Calcium Oxalate Monohydrate) – 33 %
Weddellite (Calcium Oxalate Dihydrate) – 33 %
Carbonate Apatite (Calcium Phosphate Hydroxyl Carbonate) – 34%
What kind of stone do I have? My doctor was meant to call me to give me the results and explain what it means, but he never did…
Thank you!!
Hi Vee, You form calcium oxalate/calcium phosphate stones. The former is predominant but the phosphate is high enough to be concerned. Here is how I would propose you prodeed.Whatever is abnormal in your blood or 24 hour urine and could cause stones like you form – that is what you target your treatments to correct. Regards, Fred Coe
2 months ago I had a 4mm stone that blocked my ureter that came back as hydroxyapatite 100%. I now have a 7mm stone same side stuck in my ureter along with smaller stones on both sides. Is it common to make such a large stone in 2 months? I assume it’s the same type, but obviously have to wait until after the procedure to find out. My urologist told me it was pretty weird to have formed this thing so fast. I don’t know about that but it sure is painful. And I’d like to put a stop to these stones if I can. They are recommending a metabolic study. Would this be an ideal way to find the underlying cause if there is one? Not much info out there that I’ve found about those stones.
Hi Jennifer, calcium phosphate stones can grow rather fast, so prevention is very important. I would do the full study as recommended to find their cause and create a reasonable prevention program. Regards, Fred Coe
60 calcium oxalate monohydrate 30 calcium phosphate is what my kidney stone came back as, how do i prevent these? Why am i making them?
Hi Isabel, You make calcium oxalate stones with a sizable amount of phosphate. A proper evaluation will probably reveal high urine calcium and high pH and that will help plan treatment, but that is a guess. Be sure to get that evaluation. Regards, Fred Coe
Thank you for the valuable information above. My stone is Calcium Oxalate Dihydrate (Weddellite) 65% Carbonate Apatite (Dahllite) 35% . As asked above, what is the prevention and cause of this stone’s analysis. Thank you.
Hi Margaret, the high phosphate content (Delhiite) usually reflects high urine calcium and pH which need treatment. Your best course is a complete evaluation with especial attention to the above two abnormalities. Regards, Fred Coe
My kidney stone came back as 90% Calcium oxalate monohydrate. 10% Calcium oxalate dihydrate. I’ve passed 1 and have 3 more to go. I get them frequently, what do I do to prevent them.
Thank you.
Hi Thelene, Diagnosis is the best guide to prevention. Here is my best on that subject. Regards, Fred Coe
Hi, My results are 90% CARBONATE APATITE10% CALCIUM OXALATE MONOHYDRATE what should I be doing?
Hi Mike, You are forming stones mainly of calcium phosphate and that is a special problem. Here is my best on that. Regards, Fred Coe
Dr. Coe,
My son is special needs and this is the type of composit his stone was diagnosed with. He is formula fed and on seizure medications as well as on a ventilator.
Calcium Oxalate Dihydrate (Weddellite) 20%
Carbonate Apatite (Dahllite) 80%
Thank you for your time.
Kim
Hi Kimberly, His stone is predominantly calcium phosphate and these often reflect underlying disorders of urine acidification with high urine pH and low urine citrate. Renal tubular acidosis is not rare in young people with these stones. Formula feeding is perhaps the prime suspect and it can be adjusted if urine pH is too high. LIkewise some seizure medications cause renal tubular acidosis and can be replaced by alternatives. Regards, Fred Coe
Hi, I’ve had 10 stones between both kidneys since 2011. Passed my first one in 2012 10mm and was told it was struvite, that there was no reason that I was getting them, the rest were tiny, less than 2mm at that time and had been watched since.
In 2018 I was tested for parathyroid and was negative, passed small stones showed 5 remaining. In 2019, had kidney infection in ER for IV antibiotics.
Oct 2021 CT scan showed 5mm left kidney and 5mm right kidney both non-obstructing (2019 comparison). from ER (emergency room) passed stone while there.
COMPONENT: Carbonate Apatite (Dahllite)90% Calcium Oxalate Dihydrate (Weddellite)10% weight 0.49
Nov 2021 us retro showed 9mm non-obstructing left stone and 3mm non-obstructing stable lower pole stone.
Sept 14 2023, ER, Obstructing kidney stone left ureter (UVJ), 6mm, ureteroscopic laser lithotripsy with stent.
COMPONENT: Calcium Oxalate Dihydrate (Weddellite) 20% Calcium Oxalate Monohydrate (Whehellite) 60%Carbonate Apatite (Dahllite)20%
Is there a reason to have 3 different types of stones? I’ve never had any more than the original 10, they’ve just gotten bigger then passed on their own except this recent one. Do they change from one type to another?
Thank you in advance,
Tamara
Hi Tamara, You are indeed complex. You may have had infection related stones. You are forming calcium phosphate/carbonate stones which means too alkaline a urine pH and probably high urine calcium. YOu need comprehensive evaluation. Here is my best take on that. You need detailed prevention based on the results. Regards, Fred Coe
Calcium Oxalate Monohydrate 68%
Calcium Oxalate Dihydrate 5%
Calcium Phosphate (Carbonate form) 5%
Calcium Phosphate (Hydroxyl form) 20%
Protein 2%
I had 2 smaller stones. One removed, the other lasered, collected and analyzed. I have horrendous hydrating habits. Little to no water. Mainly soda and energy drinks and I still do not drink enough fluids as I need. I am trying to improve my intake (my doctor begged me to drink fluids of any kind 🙁 )
Have a wonderful remainder of your week!
Amanda
Hi AManda, Your stones fit with both low fluids and high urine calcium, but that is guessing. Be sure you have been properly evaluated and know all of the factors that could cause you to form stones. That should improve your chances of avoiding more stones. Regards, Fred Coe
Dr Coe
My sister has been fighting kidney disease for several years due to malabsorption stemming from gastric bypass surgery. As far as I understand it is due to oxalates. She has not had kidney stones. What is the difference between oxalates and oxalate stones?
thank you.
Frank
Hi Frank, BYpass procedures can raise urine oxalate and stone risk. Oxalate is the anion of oxalic acid and readily combines to cause calcium oxalate crystals and stones. It can also injure kidneys if very high by producing oxalate crystals in the kidneys themselves, not just in the urinary system. Her physicians might want to consider means to lower urine oxalate or given recent improvements in obesity management perhaps the bypass is no longer ideal for her. Regards, Fred Coe
Hi Dr. Coe,
I just received the results on a stone that my Urologist removed during a laser litho procedure. The composition is:
Calcium Oxalate Dihydrate (Weddellite) 10% Calcium Oxalate Monohydrate (Whewellite) 45% Carbonate Apatite (Dahllite) 45%
I would appreciate any feedback you can provide.
Thanks,
Barry
Hi Barry. The stone is very rich in calcium phosphate and this usually means a urine with high calcium loss and elevated pH. I believe calcium phosphate stones need a bit more urgent prevention that begins with a search for the causes. Regards, Fred Coe
Hi Dr. Coe,
I recently had my 1st stone. Analysis shows 20% calcium Oxalate Dihydrate, 60% calcium oxalate monohydrate, and 20% Carbonate Apatite. I had been on topomax for about a year and have weaned off of it. Do you think the topomax was a contributor and what other foods should i avoid or add to help prevent another?
Thank you!
Kristy
Hi Kristy, I am reasonably content that Topamax could cause such stones. But the percent CaOx is a bit higher than I would have expected. Get full 24 hour urine testing along with some fasting bloods and be sure.
Hi Dr
i had this report
CommSize. 1×1 R mm
R: Multiple pieces received. Dimensions of the largest piece
reported.
Weight. 6 mg
Calcium Oxalate Monoh 80 %
Calcium Oxalate Dihyd 20 %ent *
best regards
Hi Taha, the stone is of the most common type. You might well benefit from an evaluation as to cause so prevention can be effective. Regards, Fred Coe
70% Calcium oxalate monohydrate. 20% Calcium oxalate dihydrate. 10% Calcium phosphate (apatite).
I had stones in both my right and left and came on rather quickly Sept-Nov I thought it was menopause as I never have had any issues with kidneys never even a UTI. After I noticed some blood in my Urine I went to physician UA , ultrasound then found my self quickly to OR to remove on my right they went thru my back stone was too large and then my left stents placed both sides. 24hr urine complete. The above was my analysis of stones.
I have had stomach issues since last summer. N/V not much of appetite as it uncomfortable to eat. Pain in my joints hips legs mostly and back ?
Never really sick , concerned as no one is listening feel like I’m just complaining all the time I’m sure.
Any suggestions ?
Thank you for your great info in stones above and all the comments !!
Hi Anna, APparently you formed considerable amounts of stones around of after menopause. Your urine calcium – other comment – was 360 mg/d, pH is high at 6.9, citrate is high at 1616, and one of your stones was 40% phosphate. I gather stones were never present before. I am sure your physicians will think as I do that primary hyperparathyroidism, rapidly progressive osteoporosis, or some drug or supplement newly acquired is the cause. Here is an outline of a complete evaluation which if done correctly will sort these alternatives out. There is surely a new cause at work that needs expert treatment. Regards, Fred Coe
60% Calcium oxalate dihydrate. 40% Calcium phosphate (apatite).
This is results of my right kidney stone above was left.
→
Urine Volume (liters/day)
• 3.86
SS CaOx
• 4.13
Urine Calcium (mg/day)
360
Urine Oxalate (mg/day)
• 39
Urine Citrate (mg/day)
• 1616
SS CaP
• 1.85
24 Hour Urine pH
• 6.926
SS Uric Acid
0.08
This is my 24 hr urine ..
Hi ANna, I included this in my note on your other post. Fred
Thank you for all your feedback and input. I take no supplements only a daily thryroid.
Why is uric acid so high in my 24 hour urine with higher ph.
I will see my physician for post op follow up 1/111/2023.
Any suggestions for testing? My symptoms are still having back pain (it went away for only 2-3 weeks) I’m concerned I’m forming stones again my stones were very large which is why percutaneous surgery. My fear is they will just put me on meds and not get to cause. I want to feel better
Hi Anna, I do not see uric acid in your 24 hour urine as you presented it. You need a bone mineral density to see if you are losing bone and serum calcium levels, fasting, in the morning, so see if there is primary hyperparathyroidism.Your physician needs to do all this. Given the timing of your stones it is likely there is an underlying disease as their cause. regards, Fred Coe
Yes 24 hour urine
1.054 UA
225 Na
360 Ca
1.135 P
I also had some other labs
Ca 9.8
Phos 2.6
PTH 139
D2 <2.00
D3 22
Total D 22
Bone density normal
Hi Anna, Very high urine calcium, low serum phosphorus, very high serum PTH and normal serum calcium and rather marginal 25 vitamin D. This is complex indeed. Possibly you have a gene variant involving the NaPi phosphate transporter, possibly a low calcium diet + routine familial hypercalciuria with secondary hyperparathyroidism. From this distance I can only guess. But I am sure you need care from a very sophisticated expert in mineral metabolism. Such people tend to work within universities. Perhaps there is a university stone center near enough for you to use. Telehealth is also feasible with limitations concerning state licensure. Regards, Fred Coe
Oops sorry typo follow up 1/11/2024 in just a few more weeks.
Thank you
My 1,25 DHVD was 64.
The uric acid in my urine is it likely to be in blood also. I have such joint pain? Should I see a Rheumatologists. They want me to start allopurinall ? I want cause I don’t just want another pill that could potentially mask what is really going on.
Does Exocrine Pancreatic Insufficiency have a role in any of this?
You don’t think Primary HPT like previously stated?
Hi Anna, with your high 1,25D low phosphate, high PTH and high urine calcium there are only 3 diseases that stand out: a NaPi gene abnormality, rapidly progressive bone disease, and primary hyperparathyroidism. Of the three the last is most crucial but your serum calcium is not high. On the other hand you have abnormally low serum 25D. I would suggest your physicians look for the gene abnormality and treat your vitamin D deficiency as it may ‘mask’ primary hyperparathyroidism. But I believe most important here is a physician who is extremely expert in bone and mineral work because whatever you have is serious and not obvious. Furthermore in making some suggestions I worry because I do not know your entire medical story and should be careful to not to influence the work of your physicians. In reviewing our correspondence I have already perhaps potentially done the latter and strongly suggest you arrange highly specialized consultative care at a university center near you or via telehealth. Regards, Fred Coe
Thank you. You have my history I’m otherwise very healthy nothing major had post partem hyperthyroiditis then euthyroid with by second and chose an ablation 10months after giving birth to my second child. Up and down thyroid management to this day.
A knee surgery, shoulder surgery (old sports injuries) a hysterectomy in 2012
2021 latter part a lot of GI stuff diagnosed EPI tried the enzymes made me feel even worse with vomiting …not taking them now.
Continue with bloating, headaches, the n/v and “just not feeling right” all stated to me menopause. then these stones out of nowhere and here I am …. Had labs today calcium up to 10.5 and probably rising.
I’m currently at Mayo having nuclear studies and ENT consult .. my very concern is what you mentioned above “masking” I am not a fan of taking out a body part and not having all possibilities studied. Do you have a colleague in AZ, referral. Can I tele health with you ?
Hi Anna, Sorry for the delay – my new site hid older questions out of sight! Mayo is great and I imagine they have evaluated you for primary hyperparathyroidism properly. As for telehealth it is possible between states but a physician in your home state would have to ask for me to consult. Mayo has a branch in Arizona, incidentally. Please let me know what else I can do. Regards, Fred Coe
Well….. I had parathyroidectomy on 3/26 with almost instant relief in my bone pain. Now at about 2 weeks in symptoms back with some new symptoms of “the sweats” and more N/V stomach feeling so Full all the time ?? My PTH is right back up to 130. There was an adenoma on my one parathyroid which was the only one removed. Intra operative PTH down to 50 all other glands appeared normal per Dr Hinni surgeon.
Now what they don’t know what to do with me. Also I know I have those kidney stone back causing me much pain in my back as well. This is complicated and a horrible cycle getting discouraged and depressed really.
Hi Anna, the surgeon who treated you remains your principal physician now. It might appear that your PHPT was not cured given the high PTH – you do not give a serum calcium to go with it. At this point he/she needs to move on: Do you still have PHPT (high PTH and high serum calcium)? If not, is the high PTH just calcium moving back into bone (Bone marker changes, fall in urine calcium, etc). The stones you mention, I presume they have been there for some time. Put another way, things are not settled, your physicians have a lot to do, and I am far away and know nothing of the complexities that one finds only in your medical records and, of course, from you in person. Best, Fred Coe
Yes just had 24 urine calcium and was over 300 .. PTH now back down to 75 and will address stone with repeat ultrasound 5/20? They are now checking for
MeN1 , which I’m doubtful.
I’m taking a break for awhile and see surgeon again 6/3. It doesn’t really seem to be figured out I’m not wanting to race back to get my neck cut again … feeling very passed around right now with no real plan ?
First paragraph under “Which type do you have?”:
“Perhaps even more worse, the stones”. “more worse”??? You did NOT really write that did you? A Phd? University of Chicago? Just tell me it’s not so.
Hi Pete, Indeed. And thanks for your clear eyed read. I shall fix it. Fred
Hello, I get kidney stoones chronically and just had to have 2 stents placed and had stones removed. They tested them and the results came back as :
25% Calcium Oxalate Dihydrate
50% Calcium Oxalate Monohydrate
25% Carbonate Apatite
My health DXs are: Insulin Resistant PCOS, HTN, Obesity
*I fell out of a tree when I was 5 and injured my right kidney (which is the side this stone was on and that I always have issues with. The only thing I remember when I injured the kidney is that I was urinating blood.
Family HX of multiple types on cancer, Type 2 Diabetes, Crohn’s Disease, HTN, Heart Disease
What does this mean and what should I be tested for?
Hi Teresa, I gather you form stones in your right kidney that was injured in childhood and presumably has some abnormalities that promote crystal retention and stones. Even with your other illnesses as noted, the proper next step is a complete evaluation for causes. The blood and 24 hr urine findings may well point to what is wrong and therefore to reasonable prevention. Regards, Fred Coe
thank you for your response. My provider actually prescribed a 24 urine test for more answers.
Hello,
Great article, very informative, but I am looking for information about kidney stones made of calcium carbonate. I am having a very hard time finding any information as the web says they are extremely rare. Any information or resources would be greatly appreciated.
Thank you,
Misty
Hi Misty, they usually are made of calcium phosphate mixed that includes carbonate – so called carbonate apatite. Here is the correct article on phosphate stones.Women make them more commonly than men. They require some expertise for prevention. Regards, Fred Coe
Hi,
I had a 2.2cm along with a 5mm stone removed August of 2023 100% Carbonate Apatite. I had my followup and currently have several smaller stones. How quickly do they form? Is there a way to prevent other than water or critic intake or at least slow them down? We are waiting to have another child and as a 33F I am nervous about them growing too quickly. There is very little information on them. I have no family history or personal history of kidney stones before this.
Hi Kristin, I am concerned that you have high urine calcium genetically and are taking prenatal vitamins that contain a lot of calcium and alkali which is fostering stones. I suggest proper testing to see if that is true so your physicians can adjust your treatment accordingly. Carbonate apatite stones are calcium phosphate stones growing in a rather alkaline urine. Regards, Fred Coe
95% Calcium Oxalate Monohydrate (whewellite)
5% Carbonate Apatite (dahllite)
24hr urine test has come back inconclusive.
Any thoughts?
Hi Bob, ‘inconclusive’ usually means the data have not been examined closely enough. Here is a guide you can use. See if it helps. Regards, Fred Coe
Calcium Oxalate Dihydrate (Weddellite) 30% Calcium Oxalate Monohydrate (Whewellite) 35% Carbonate Apatite (Dahllite) 35%
These are my test results I started to get them after my pregnancy, I always have blood in my urine which they have never looked into.
Hi Beatrice, This is not an uncommon stone related to pregnancy. It suggests high urine calcium losses have been present. I would hope you obtain proper evaluation and prevention measures to prevent chronic stone forming. Regards, Fred Coe
Hello! I’ve had 4 babies and each pregnancy I formed stones. They are over 60% hydroxyapatite and the one before was 80% hydroxyapitite. They said I may have MSK. What’s your advice for moving forward? I am done having children. Currently have a 4mm stuck above the UVJ
Hi Alyson, I think that you harbor underlying causes of stones. Pregnancy raises urine calcium as do pregnancy vitamins, and the latter will make urine more alkaline. Your stones preferentially form in alkaline urine. Your physicians might want to offer you comprehensive evaluation – here is my take on what that might look like. I suspect clues to prevention will turn up. Regards, Fred Coe
I really don’t understand what this means as far as composition:
Carbonate Apatite (Dahllite) 90% Calcium Oxalate Dihydrate (Weddellite) 10%
Also 37 weeks pregnant (at the time of stone discovery).
Thank you
Hi Taylor, it means a calcium phosphate stone that would have formed in a urine with high calcium and high pH – alkaline. You should be fully evaluated as to cause to prevent more. Best, Fred Coe
Hi doc, love your site. I am a type 1 diabetic for 34 years but I have been excellent control 5.5-6 a1c for the last 20. No kidney issues from blood labs or micro albumin.
Moving on I have had stones over the last 10 years with the recent analysis. No 24 hour yet but working on it.
I had EWRS two years ago because I had a stone in my right kidney that was ball valving. And I had that stone analyzed since then I have past one smaller stone maybe less than 2 mm. I am not real good at drinking a lot of fluids, but I have tried to increase. I do measure my urine calcium with occasional urine dipsticks, and I do find that I have medium to mild calcium from the urine dipstick. Any thoughts for now?
1.1mm Calculi composed primarily of: 70% calcium oxalate dihydrate, and 30% calcium phosphate (hydroxy- and carbonate- apatite). INTERPRETIVE INFORMATION: Calculi (Stone) analysis
Thanks for the help
I have been taking supplements more so in the last 10
Years for sure.
Hi Jacob, Your stone has a lot of calcium phosphate (30%) suggesting that your supplements may contain alkali, possibly calcium alkali. If so that could be the cause of your stone. You are also aware of low fluid intake. Given long standing diabetes and despite excellent control, I would surely get fully evaluated for the cause of the stones. Do the evaluation while taking the supplements as that has been in effect for a decade. Regards, Fred Coe
Hello. These are the results I received from my stone analysis of my 50mm stone. What type of stone was it.
Calcium Oxalate Dihydrate (Weddellite) 30% Calcium Oxalate Monohydrate (Whewellite) 35% Carbonate Apatite (Dahllite) 35%
Hi Valerie, Your stone contains considerable phosphate.You should have a complete evaluation as to causes. Prevention is based on what is found. Fred Coe
Can I get a telehealth appt with you? They are now saying I need another surgery. Surgeon did not visualize all glands since I had the one gross adenoma. Parathroidectomy 3/26 felt great immediately bone pain, gI symptoms and brain fog somewhat resolved. About 3-4 weeks later bad night sweats, bone pain back.
Letting them know about symptoms all along.
My calcium is 10.3 , urine calcium >304, PTH Intra op March 54, then post op April 113 , 75, June 85. Now my Hgb 16.0, Hct 47.4, Erythrocytes 5.37 all elevated. Is there a correlation. I have been staying very hydrated to avoid kidney damage and recurring stones which at this point only one small one in right Recall golf ball sized ones removed bilaterally in November retroperitoneal? Renal function test WNL
Hi Anna, It would appear the PT surgery failed to cure the disease. An appointment is best through 773 702 6134. Best, Fred Coe
Hello, I just completed my fourth surgery to remove stones and was told by the Urologist that the only medication to help is Potassium Citrate, which I am allergic to. It caused me to go into anaphylactic shock. I just received the test results from the most recent stone removal and here is what they said: Calcium Oxalate Dihydrate (Weddellite) 25%, Calcium Oxalate Monohydrate (Whewellite) 50%,
Carbonate Apatite (Dahllite) 25%. I am drinking a cup of Lime Juice every morning, I just started taking Chanca Piedra and am drinking anywhere from 100-130 ounces of water a day. I am wondering if there is something else I need to be doing. I am tired of being told by the urologist that even though I have the stones, they shouldn’t be bothering me like they do. Then they reluctantly go in and remove and have to break them up as they are to big to pass. This last one she had to cut one out as it was “stuck”. Any advice would be greatly appreciated.
Hi Donna, You need to find out the cause(s) of these stones- here is my best at what to do. When the cause is clear, take all possible steps to reverse it (them). Best, Fred Coe
Calculi composed primarily of:
50% calcium oxalate monohydrate,
20% calcium oxalate dihydrate, and
30% calcium phosphate (hydroxy- and carbonate- apatite
I had a 10 mm stone stuck between my kidney and bladder.
Kidney stones run in my family and I was diagnosed as having medullary sponge kidney.
Doctor is ordering the 24 hour urine collection.
What type of stone is this considered?
Should I be concerned about anything?
Hi, Stephanie, formally it is a calcium oxalate stone but it has 30% calcium phosphate which makes me suspect your urine is perhaps overly alkaline and urine calcium high. The 24 hour testing will disclose what is wrong. Do not fail to treat what is found so stones do not continue. Regards, Fred Coe