 If the lives of the Tudor nobility were luxurious, they were dangerous in equal proportions, for the King who bestowed their riches could in a moment wipe them, and those who possessed them, out. So intimacy with the person of the King and with the whole Royal Family was prized and feared. They lived, these powerful and dangerous people, in their Royal Palaces, to which you must go or have no influence. Even worse, King and Consorts made Royal Progresses, staying here and there as guests of the high nobility. Imagine that, the King as your houseguest. A person, like any other, and yet not at all like any other: glamorous, dangerous, and involved with high concerns.
If the lives of the Tudor nobility were luxurious, they were dangerous in equal proportions, for the King who bestowed their riches could in a moment wipe them, and those who possessed them, out. So intimacy with the person of the King and with the whole Royal Family was prized and feared. They lived, these powerful and dangerous people, in their Royal Palaces, to which you must go or have no influence. Even worse, King and Consorts made Royal Progresses, staying here and there as guests of the high nobility. Imagine that, the King as your houseguest. A person, like any other, and yet not at all like any other: glamorous, dangerous, and involved with high concerns.
You could say this is a silly preface to my common discourse on citrate, but not so. I have written before about its powers in our little domain: It binds calcium, it inhibits crystals, giving it reduces stones. But I have not said how it gets into the urine.
It comes as a royal visitor to some Duke or Marquess, Earl, Viscount, or Baron.
For this molecule has high purposes. It is noble and powerful. What it does in urine is but a tiny fraction of its many actions and probably not one of the more important ones. But what we do when we take citrate calls into play a vast biology. For all our lives we eat a diet that imposes an acid load on our kidneys, our bones, and elsewhere. Our kidneys, especially, adapt to that acid load, so what we call our ‘normal’ state is actually at one extreme. The pills, being alkali, reverse this lifelong adaptation and thereby profoundly alter the physiology of the kidneys and bone. In general one might say the alterations are for the better.
This is a long article but one worth reading for those who prescribe or take potassium citrate pills.
I want to acknowledge the expert error checking of Dr Yangming Cao (UCSF – Fresno) in the section ‘Why are Potassium Citrate Pills an Alkali Load?’ He corrected a significant error in the original article.
A Picture of the Kidney
Many of you are physicians or scientists who know about the kidney, but a few reminders are always worthwhile. Others are neither and we need to have names in common. Human 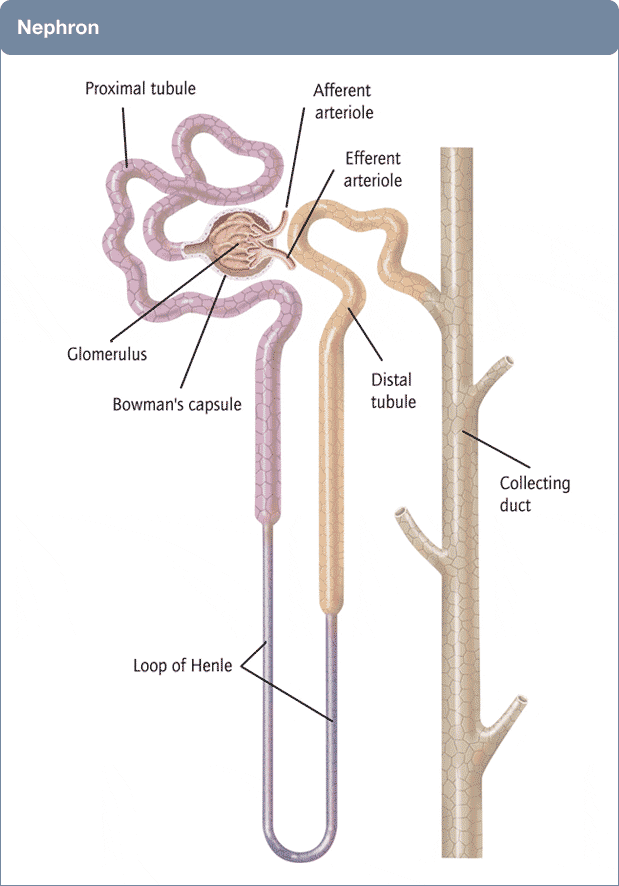 kidneys are made of about one million individual nephron units. The renal process begins with filtration of small molecules like citrate, or atoms like sodium and calcium, through the glomerulus, which is a complex of capillaries whose filtration pressure arises from the heart not a foot away.
kidneys are made of about one million individual nephron units. The renal process begins with filtration of small molecules like citrate, or atoms like sodium and calcium, through the glomerulus, which is a complex of capillaries whose filtration pressure arises from the heart not a foot away.
A majority of the filtered water, salts, and molecules is reabsorbed in the proximal tubule. The distal tubule (highly simplified here) performs tightly regulated absorption or secretion, so as to produce a final urine and maintain blood concentrations in their normal ranges.
These loops will come up again and again on this site so I should comment on the thin and thick portions. The long thin loops of Henle (Henle was the scientist who is credited with describing this part of the kidney) extract water specially well.The thick portions just below the ‘Distal tubule’ notation are called, appropriately enough, the Thick Ascending Limbs of the Loop of Henle. The thick limbs reabsorb NaCl, but not water, and in doing that entrain a marvelous system for – of all things – retaining water! In an article so long as this one, and concerned with citrate, I cannot pause longer here. But we will be back, someday.
Citrate is in the Blood
Kidneys Filter and Reabsorb Citrate
In one published study, concentration of citrate in blood is about 80 – 170 micromolar. A recent review places it at 120 micromoles/liter. If we use 120 micromoles/liter as a reasonable average, and a common value for glomerular filtration of 120 milliliters/minute, the filtration of citrate is about 21 millimoles a day. Of this about 1 – 4 millimoles appear in the urine, the rest being reabsorbed by the kidney cells. So the fraction of filtered citrate excreted is about 5 to 20%, and regulation of this fraction controls the amount of citrate in the urine.
Citrate in Blood Binds Calcium
The concentration in blood of calcium not bound with proteins is about 1 millimole/liter. Citrate concentration is about 0.12 mmol/liter, so in principle about 10% of non -protein bound – calcium can be bound by citrate. Because in calcium citrate crystals 2 citrate molecules can bind 3 calcium atoms, the the figure would seem to rise to to 15%. But in solutions like blood, other materials compete with calcium for a place on citrate – magnesium is one example. So the actual fraction is difficult to estimate. Normally blood citrate level is stable, so although significant, citrate binding of calcium is not likely to influence calcium metabolism by, for example, altering regulation of parathyroid hormone secretion.
Citrate has Signalling Roles
My purposes here are humble purposes, so all I wish to do is put here a tiny list of known effects of citrate on systems throughout the body without pursuing the details. Citrate concentration regulates lipid metabolism via malonyl-CoA. Citrate is sensed by the hypothalamus and thereby affects glucose intake and glucose metabolism by liver. To do these things citrate must enter the relevant cells, and it can do this only via a transporter that takes it across cell membranes.
The Citrate Transporters
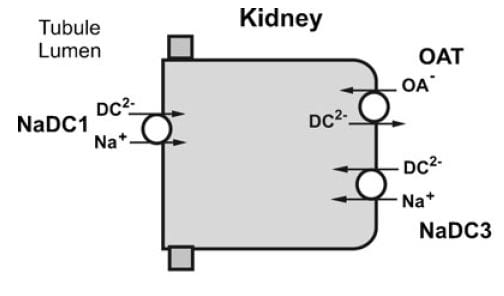
NaDC1 and NaDC3
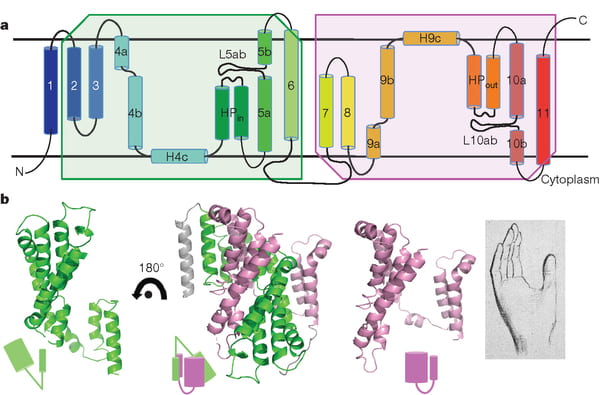
NaDC1 is on the apical membranes of the proximal tubule cells of the kidney – the surface facing into the tubule fluid – and regulates the rate of reabsorption of the citrate that has been filtered. Its gene is named SLC13A2. This same transporter is on the food side of the small intestine cells and permits absorption of citrate from foods. The featured image for this article shows the structure of the transporter.
The citrate that enters the renal cells can be used for metabolism, or transported out the other side – called the basolateral side, facing the blood – via another transporter called the Organic Acid Transporter (OAT). Yet another transporter, NaDC3, permits citrate to enter kidney cells from blood. Because it appears to regulate urine citrate, my focus is on NaDC1.
The citrate transporter DC1 couples sodium and citrate movement. Since not everyone who reads this will know, let me mention an almost universal property of living cells: they pump sodium out of themselves and pump potassium in. Because they do this, sodium will tend to move into cells if given an opportunity – a hole. DC1 and DC3 can be thought of as sophisticated holes, or channels, through which sodium atoms can move if they have a citrate molecules with them. The actual proportions are 3 sodium atoms move with one citrate molecule, and the form of citrate which moves is one we have encountered before. Recall how citrate binds calcium because each molecule can have 2 or three negative charges on it. The doubly negative (divalent anionic) form of citrate is the one that traverse the channel.
They Transport More than Citrate
NaDC1 permits not only citrate to cross cell membranes but also succinate, alpha ketoglutarate, fumarate, malate, and a variety of less biologically relevant molecules. One might ask why, and I presume it is because the named molecules are all part of the citric acid cycle, which is the main engine of cell energy production. NaDC3 transports all of the same molecules as NaDC1, along with glutarate and a very long list of other molecules not in the citric acid cycle.
This cycle is at the center of that metabolism which uses oxygen to produce energy from food. The reference is to an excellent textbook review that is free online. Another chapter in that book finishes the story of how the cycle produces energy. The antiquity and centrality of the citric acid cycle will become apparent to you if you even browse these chapters. If you read them, you will encounter some of the most important aspects of living cells.
Why are Potassium Citrate Pills an Alkali Load?
In the citric acid cycle citrate is metabolized as citric acid, meaning that 3 protons are taken up from blood with each molecule. Removing protons is identical to adding alkali. Typical dosing is about 20 – 40 mEq of potassium salt daily, but the amount can vary widely.
Commercial potassium citrate contains 1080 mg of the compound in a 10 mEq pill. Typically the potassium citrate salts have a potassium on each of the three anion sites on the citrate molecule. The MW of citrate anion is 189.1. Urocit K, a common commercial version, is a crystalline monohydrate salt so it has a MW of 3×39 (for 3 potassium ions) + 189.1 (for citrate) + 18 for the one water molecule, or 324.1 in all. Given 324.1 for 3 mEq of base, the 10 mEq tablet contains 10/3 x 324.1 or 1080 mg.
The Flow of Citrate
In an earlier era organ physiology was popular and scientists often gathered together 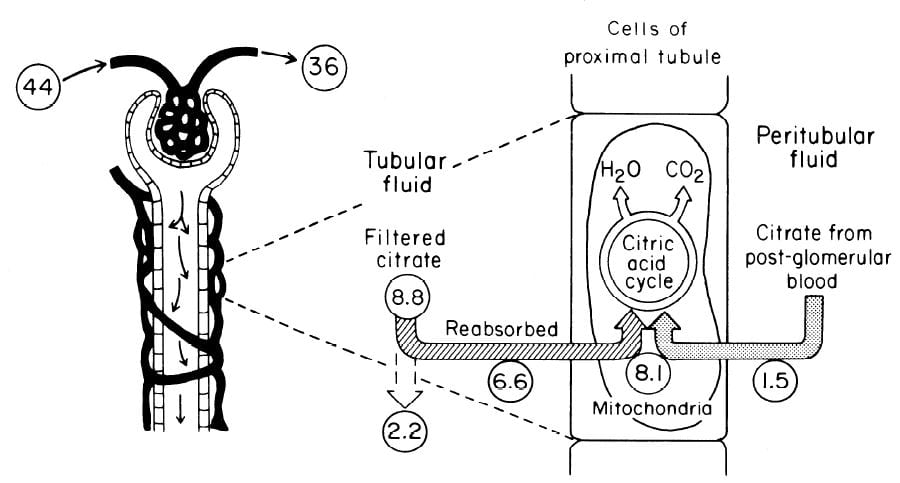 measurements to paint a picture of how things work overall. Here is such a picture from a wonderful review of renal citrate handling by Simpson. Values in small circles are micromoles (umol) per minute.
measurements to paint a picture of how things work overall. Here is such a picture from a wonderful review of renal citrate handling by Simpson. Values in small circles are micromoles (umol) per minute.
Citrate is presented to the glomerular filter at 44 umol/min, and 36 umol/min leaves the glomerulus (8.8 umol/min filtered) in blood what will pass by the blood side of the proximal tubules. From that 36 umol/min, 1.5 umol.min are taken up by renal proximal tubule cells and metabolized in the citric acid cycle. Of the 8.8 umol/min filtered, 6.6 umol/min are taken up on the urine side of the same cells making 8.1 umol/minute for metabolism. The remaining 2 umol/minute (3.17 mmol/day) are lost in the urine. NaDC1 and NaDC3 had not been cloned and sequenced at this early time, but physiologists knew the transporters were there and toted up what they did.
Urine Citrate Varies With Acid Base Status
Acid loads, such as high protein diets, will increase citrate uptake into the renal cells and thereby reduce urine citrate. Alkali loads such as diets high in fruits and vegetables or potassium alkali supplements reduce uptake and increase urine citrate.
Alkali
Clinical Response
In a trial, calcium stone formers with low urine citrate excretion eating a constant diet were given sodium bicarbonate or  potassium citrate, 20 mEq three times a day. Urine citrate rose with both treatments, as did the urine pH. Not relevant here, but in later articles, the sodium alkali did not change urine calcium, but the potassium alkali lowered urine calcium. Alkali itself lowers urine calcium, sodium raises it, and their antagonism is the reason for the differences.
potassium citrate, 20 mEq three times a day. Urine citrate rose with both treatments, as did the urine pH. Not relevant here, but in later articles, the sodium alkali did not change urine calcium, but the potassium alkali lowered urine calcium. Alkali itself lowers urine calcium, sodium raises it, and their antagonism is the reason for the differences.
Mechanism May be Increase of pH
If the citrate transporter is placed into test cells, the movement of citrate can be studied, and such a study shows how powerful is the effect of pH.
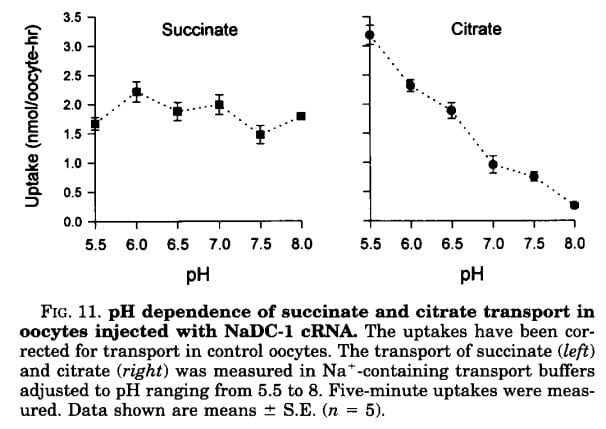 Succinate is a citric acid cycle intermediate like citrate, but its uptake by the citrate transporter is not affected by the acidity or alkalinity of the medium (pH). Citrate uptake is powerfully affected.
Succinate is a citric acid cycle intermediate like citrate, but its uptake by the citrate transporter is not affected by the acidity or alkalinity of the medium (pH). Citrate uptake is powerfully affected.
We have encountered pH before and remind ourselves here that urine values vary from about 4.5 to just below 8. Likewise, citrate has three sites that can accept protons, the acid component of water systems. As I mentioned in the paragraphs just above this point, the charge on the citrate molecule rises with pH as protons are progressively removed, and the sequence of pH values (the pKa values for the dissociating sites for those of you who know about such matters) are 3.13, 4.76, and 6.40. Obviously, in urine, the divalent (2 open negative sites) form will predominate until urine pH rises above 6 and will fall to about 1/2 of the total at 6.4. At about 6.4 transport of citrate was indeed just about half of that at the lowest pH.
pH in the Proximal Tubule
But it is not urine pH which affects citrate transport, it is the pH of filtrate in the proximal tubule of the kidneys, and that pH is not the same as that of the urine. At the end of the proximal tubule, the pH is about 6.7 to 6.8, and at that pH more than half of citrate is in the trivalent form and not available for transport. With alkali loads, as in the experiment in the table, the pH will rise, and citrate transport fall below normal, so citrate appears in the urine.
Problems with the pH Idea
Strangely, modern sources do not mention an older literature which raises questions about this mechanism. Simpson, in an important review from late antiquity (1983), mentions that the drug acetazolamide, which raises pH inside the proximal tubule and lowers pH inside the renal cells raises urine citrate only slightly and at first, but shortly after administration urine citrate falls despite a continuously alkaline urine and presumably tubule fluid. This suggests that even a high tubule fluid pH is not enough to counter the effects of changes in pH within cells or perhaps the blood. So it is not only tubule fluid pH that matters, but perhaps the pH inside the renal proximal tubule cell.
Acid Loads
Those unfamiliar with the matter may not realize that the diet we eat in the US and most of the other first world countries imposes an acid load that must be excreted daily in the urine. So the urine citrate excretion we find in our clinics and in experiments on ‘normal’ diets are those consistent with an acid load. When we give potassium citrate or other alkali we often do little more than neutralize this acid load, yet urine citrate usually rises. Experiments about acid loads add to the diet acid an extra amount of acid.
Tubule Fluid pH
As for alkali loads, a lower proximal tubule fluid pH will increase the fraction of filtered citrate in the divalent form which is transported by NaDC1. The pH of the tubule fluid will fall with acid loads for several reasons. Acid loads – for example a high protein meal – are buffered on blood bicarbonate which lowers the concentration of bicarbonate, and therefore the pH of the filtrate. LIkewise, the tubule cells are stimulated to increase their reabsorption of filtered bicarbonate which further lowers pH. All of this implies that kidneys sense the acidity or alkalinity of the blood, which they surely do.
Transport Adaptation
Over time – many hours to days – the NaDC1 transporter and its gene (SLC13A2) increase 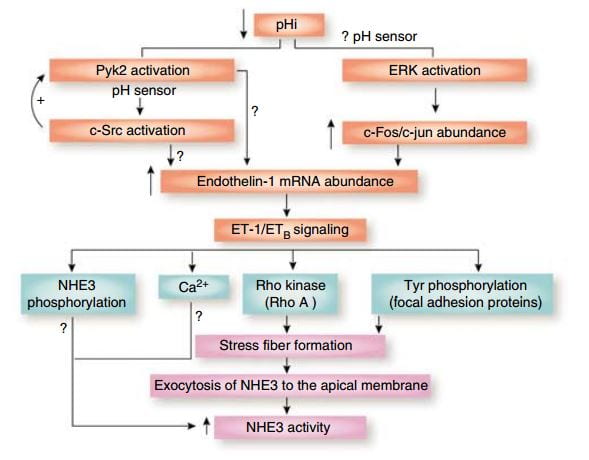 their abundances. This increase is mediated by endothelin – 1 (ET-1) through the endothelin B receptor (ETb).
their abundances. This increase is mediated by endothelin – 1 (ET-1) through the endothelin B receptor (ETb).
This figure from the above reference shows thinking about acid and endothelin as it was in 2007 and seems to be still. A fall in pH in proximal tubule cells can be sensed by a protein named Pyk2, which activates by adding a phosphate to one of its amino acids (tyrosine) and, interacting with another protein (c-Src), increases the abundance of the mRNA of ET – 1 which then signals through its ETb receptor to increase renal acid excretion – bicarbonate reabsorption – via NHE3, a transporter that reabsorbs sodium and secretes acid into the proximal tubule fluid.
This same ET -1 and its ETb receptor also signal increase of NaDC1 transport. Here, mice engineered to have (ETb+/+)or have not (ETb-/-) the receptor were challenged with an acid load. 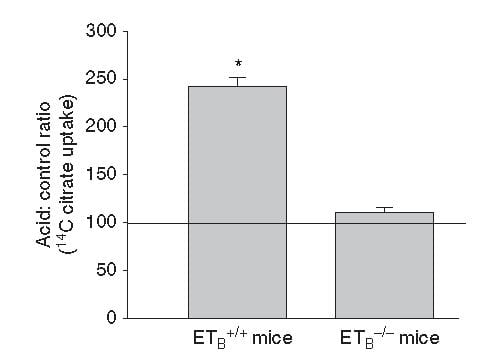 Citrate uptake by isolated NaDC1 transporters in the deficient mice do not respond to acid.
Citrate uptake by isolated NaDC1 transporters in the deficient mice do not respond to acid.
So one and the same effect, acid sensing and endothelin – 1 signalling increases acid excretion and citrate conservation.
But, you may ask, why am I grouping these two together?
It is because both concern acid base balance.
Citrate is metabolized as citric acid, taking up 3 protons per molecule metabolized, which is the same as saying it provides 3 molecules of alkali – like bicarbonate. Loss of citrate is therefore loss of potential alkali. NHE3 is a main driver of acid – protons – out of blood into proximal tubule fluid which reclaims filtered bicarbonate – conserving alkali.
So urine citrate, which we are interested in because it binds calcium and inhibits crystals, has a much larger role to play – part of the grand system which maintains a constant blood pH against the acid or base loads of diet.
Which pH?
I have spoken about pH of the proximal tubule fluid, of the blood, of the urine, but the one that is central to regulation of NaDC1 is the pH inside the proximal tubule cells. That pH appears to respond to acid or alkali loads, but the manner of its response is not simple. The signalling is through the Pyk-2 sensor already discussed and a parallel pathway via ERK (same diagram, above) which I did not discuss. But how sensing works, what is sensed, this remains very much an open research issues, and I will leave off here as this article was about urine citrate and the conversation has already taken us through many byways, beautiful if exhausting to follow.
Potassium
But – that awful word – one important fact remains to be uttered. Depletion of potassium lowers the pH inside kidney cells and lowers urine citrate. I will not pursue the details of this well worn story, except to point out its extreme clinical relevance. Diuretics that are used in stone prevention, or for hypertension, deplete cell potassium stores. It is the potassium citrate we give to patients.
Ammonium, and the Rest of the Story
How can I leave off without filling out the details of how kidney cells respond to acid challenge with production of ammonia that balances acid load with acid excretion?
Bicarbonate
A Better Buffer than Most
A buffer keeps pH relatively constant by taking up protons when they enter a solution and giving them up when alkali enters. It is a kind of shock absorber.
At the beginning, evolution favored bicarbonate. It is a buffer of considerable virtue in that it can take up protons or release them, like common buffers do, but has a special trait.
Bicarbonate is forever in equilibrium with carbon dioxide gas (CO2). When bicarbonate takes on a proton to become carbonic acid, much of that acid becomes carbon dioxide gas. When protons are taken out of blood, CO2 gas forms new carbonic acid which donates a new proton to the solution, and essentially bicarbonate appears in solution ‘out of thin air’. That it flows from solution into thin air and back makes bicarbonate a more stable buffer than those which live only in solution so it was an excellent choice.
What Kidneys do with Bicarbonate
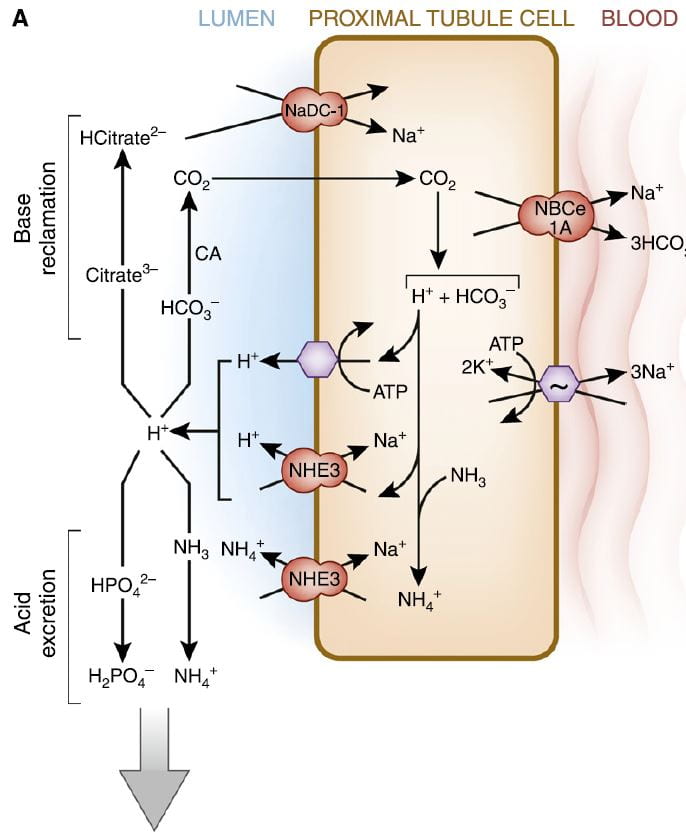 It is this very molecule, bicarbonate, which the kidneys traffic in when they respond to alkali or acid loads, and it is, of course, CO2 the lungs regulate in blood under the control of the brain.
It is this very molecule, bicarbonate, which the kidneys traffic in when they respond to alkali or acid loads, and it is, of course, CO2 the lungs regulate in blood under the control of the brain.
The figure is from the ‘A’ panel of a lovely drawing in a lively and engaging review. Being small, bicarbonate is filtered, and being the main buffer of the blood almost all of what is filtered must be reclaimed. So the proximal tubule cells, which do most of that reclamation, busy themselves forever with that task.
The way they do it is the simplest way. They add protons (H+) to bicarbonate in the tubule fluid, which becomes, as I have said, carbonic acid that transforms into carbon dioxide (CO2), which gas passes through the cell walls into the interior. Note, ‘CA’ is carbonic anhydrase an enzyme which speeds up the process of the transformation. In the cell, the CO2 becomes carbonic acid. Because protons are being pumped into the tubule fluid, protons are stripped off the carbonic acid so it becomes bicarbonate. The bicarbonate enters the blood with Na via the NBCe1A transporter.
There are two proton pumps. One uses ATP for energy to move the protons. The other (NHE3) uses the low Na in the cell as a gradient; sodium moves in through a channel like a revolving door, which makes one proton go out for every Na that moves in. At the blood side of the cell, the ancient ‘Great’ ATPase pumps Na out and potassium in, as it does in most cells that live on Earth. NHE3, the exchanger, is the molecule we met a few paragraphs above. It is increased by Endothelin 1 via the ET1b receptor.
At the top of the left side of the picture is citrate, our little slice of this massive structure. A few scraps of proton add to citrate so it has 2, not 3 negative sites, and can be reabsorbed. Its gene is regulated by endothelin 1 so when NHE3 is increased so is NaDC1.
Phosphate
Reclaiming bicarbonate is Sisyphean work. Nothing happens to get rid of acid loads from meals. But more protons are secreted than are needed to reclaim bicarbonate. Some are buffered on phosphate. But all the protons buffered on phosphate produce bicarbonate from carbonic acid inside the cell, and that bicarbonate enters the blood via NBCe1A.
Ammonium Ion
Ammonia is produced in the proximal tubule by removal of nitrogen from glutamine, pictured at left. As always, 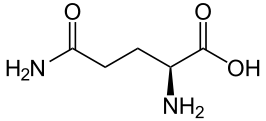 kinks are carbon atoms in this kind of drawing. The first one on the left has an oxygen and NH2 group, and a bond to the next carbon. Carbons typically form 4 bonds each. The next 2 carbons are merely linked to one another. The fourth has another NH2 and the final one at the right 2 oxygens. The left hand one is removed by an enzyme to produce NH3 and glutamic acid. The second one is removed to produce α-Ketogluteric acid which lacks any NH3. The 5 carbon skeleton remains unchanged.
kinks are carbon atoms in this kind of drawing. The first one on the left has an oxygen and NH2 group, and a bond to the next carbon. Carbons typically form 4 bonds each. The next 2 carbons are merely linked to one another. The fourth has another NH2 and the final one at the right 2 oxygens. The left hand one is removed by an enzyme to produce NH3 and glutamic acid. The second one is removed to produce α-Ketogluteric acid which lacks any NH3. The 5 carbon skeleton remains unchanged.
Ammonia (NH3) can tale up a proton to form NH4+, ammonium ion, which has a pKa of 9.3 meaning that at the pH of proximal tubules and cells, it is fully protonated. Loss of this ammonium ion in urine represents net acid excretion because the protons that were taken up came from carbonic acid which is converted to bicarbonate and transported into blood. Unlike titration of phosphate, excretion of ammonium ion does not increase urine pH because the pK is far above the pH of urine.
Under normal meal conditions, about 40 – 60 mmol/day of acid are excreted, of which about 2/3 is ammonium. Large acid loads, as for example, a ketogenic diet for weight loss, would induce a large increase in ammonia production so acid excretion can keep pace with acid production.
α-Ketogluterate
One might think this byproduct of glutamine metabolism, the 5 carbon skeleton, might be metabolized and done with, but no. A significant amount is metabolized. But some is not.
What is not metabolized traverses the kidney to cells in the later nephron, the intercalated cells in the collecting ducts, which usually pump protons into the tubule fluid to create the final urine p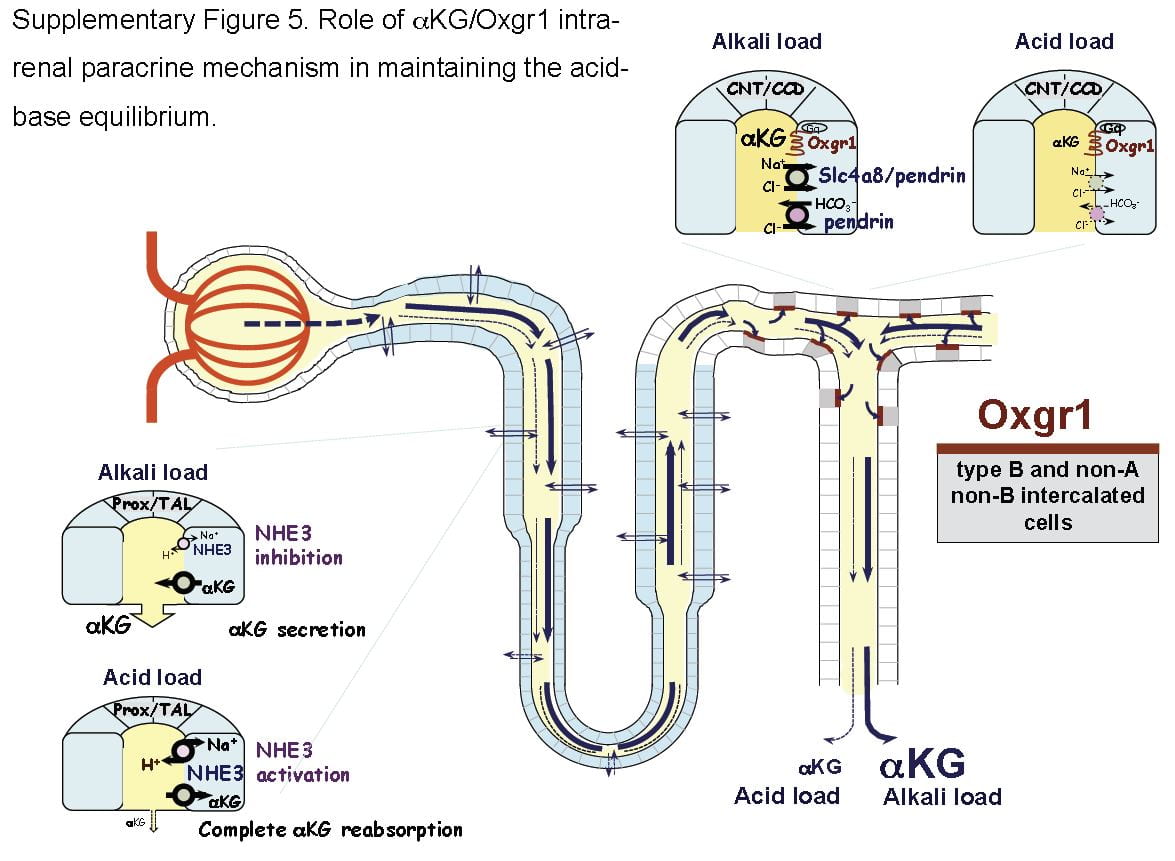 H which is critical to supersaturation and stone formation. But these same cells can reverse themselves and pump bicarbonate into the tubule fluid and protons into the blood, and they do this when confronted by an alkali load.
H which is critical to supersaturation and stone formation. But these same cells can reverse themselves and pump bicarbonate into the tubule fluid and protons into the blood, and they do this when confronted by an alkali load.
It turns out that α-Ketogluterate is itself filtered and reabsorbed in proximal tubule, and its reabsorption is profoundly reduced under alkali conditions so that more is delivered distally to a receptor (Oxgr1). When occupied by α-Ketogluterate this receptor signals the reversed intercalated cells (B and non-A cells) to increase their secretion of bicarbonate. The transporter for α-Ketogluterate is NaDC1. The net effect is to enhance bicarbonate – alkali – loss which offsets alkali loads.
The same receptor signalling stimulates pendrin, a complex exchanger which moves bicarbonate and Na together with chloride to effect NaCl and NaHCO3 reabsorption. Because acute acid challenge increases and acute base loading reduces proximal tubule NaCl reabsorption, this action would tend to maintain salt balance in that the intercalated cells would increase salt reabsorption as proximal tubule reduces salt reabsorption. Of note, although chronic acid challenge increases NHE3 abundance and activity, it reduces NaCl reabsorption via effects on other transporters. For these reasons the α-Ketogluterate – pendrin link is probably more important in minute to minute or hour to hour regulation than in adaptation to acid or base loading diets or treatments.
Citrate and Oxalate
You would think I had exhausted the topic by now, but no. NaDC1 and slc26a6, the citrate transporter and the anion 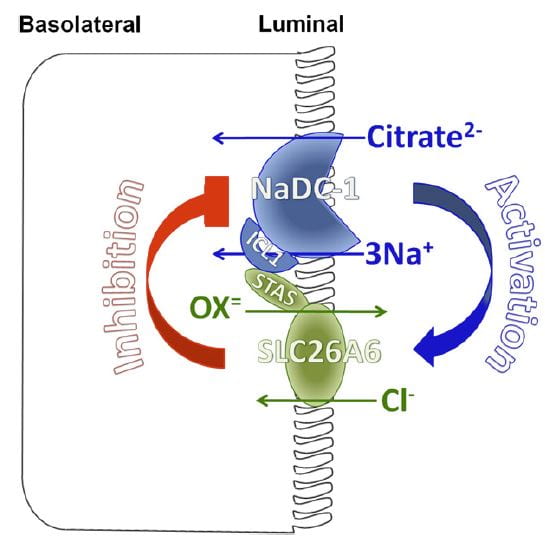 transporter (oxalate is an anion it can transport) which disengages NaCl transport from NHE3, themselves interact in relation to kidney stone formation.
transporter (oxalate is an anion it can transport) which disengages NaCl transport from NHE3, themselves interact in relation to kidney stone formation.
At least in animals and in cell experiments, the two transporters – which are present in a complex within the renal cell membrane – interact as in the figure. Slc26a6 inhibits NaDC1, so that when actively transporting oxalate into tubule fluid citrate reabsorption is reduced, urine citrate rises, and binds urine calcium to reduce risk of calcium oxalate stones. When oxalate secretion is minimal, NaDC1 increases to salvage citrate.
These animal and cell experiments imply that in human urine citrate and oxalate excretions should show parallel changes; this has not been tested.
Putting it All Together
Just Diet
Our urine citrate is an outcome of our biologies, which are variable, and our diets. Most of us eat a diet that imposes a net acid load, so our kidneys tend to conserve citrate and α-Ketogluterate, our intercalated cells pump protons not bicarbonate in to the final urine, our proximal tubules produce considerable ammonia and our urine pH is about 5 – 6.
Some of us, vegetarians whose diets do not have a proper balance of protein, very massive fruit eaters, as examples, have low citrate reabsorptions and high distal deliveries of α-Ketogluterate; our intercalated cells are reversed and stimulated to put bicarbonate into the final urine, our proximal tubules do not make much ammonia.
But the words ‘most’ and ‘some’ are misleading. In the US, certainly, chronic acid loading is the overwhelming rule, and the same throughout Europe and considerable parts of urban Asia. So our ‘normal’ poise centers on adaptations to acid load. It is not that we live in a neutral acid base condition, demanding from our kidneys little excretion of acid or of alkali. Life long we demand acid excretion. That is where we start. It is to that task our kidneys – and our bones, as I shall someday speak about – apply themselves all the days of our lives. However it is, for good or for evil, that lifelong adaptation to acid load affects us, that is our state, our permanent condition.
What Does Normal Mean?
When we give potassium citrate or any other alkali in doses of 40 to 60 mmol/day we neutralize a large fraction of diet acid. This is best considered not so much as an ‘alkali load’ as it is the removal of that acid load to which we have long been adapted.
Of course, urine citrate rises. Because we give alkali over months or even years, renal cells will adapt fully to the changes. But by ‘adapt’ I mean they give up the adaptations to acid loading. In the case where the dose of alkali just matches acid production one might best say the kidneys are relieved of their burdens in either direction, and reveal the way they would function if not driven to either extreme.
Like the small sailor plying the simple waters of a bay fills its sails sometimes southerly, sometimes northerly, making little way, dancing before a playful breeze, the cells shift their powerful machinery a bit here or there as one meal gives way to another. What shall I call this state of freedom? Why is this not the ‘normal’ from which point we register the responses to extra alkali or acid?
I have read where it was in Eden a condition of fruit, as the animals were not for them to eat. Perhaps I am wrong, and if Eden was as it says in our books the ‘normal’ state was alkali load. Perhaps Milton is wrong. After all he was not there, merely a poet making into life what he read in a holy book.
Potassium Citrate Pills
Raise Urine Citrate and pH
The expected changes are a decrease of proximal tubule reabsorption through reversal of the effects of chronic acid load. ET-1 signalling must fall, citrate reabsorption must fall because NaDC1 is no longer stimulated by ET-1 and because proximal tubule fluid pH will rise and with it the fraction of trivalent negative citrate.
Urine bicarbonate and urine pH will also rise. Partly, blood bicarbonate will rise and with it filtrate bicarbonate concentration and pH. NHE3 transport will be decreased vs. chronic diet acid loading, the baseline in the first world countries, and much of the proton secretion will be used in reclamation of bicarbonate. Naturally, NH3 production will be greatly reduced because the Pyk-2 sensing system will be signalling a higher pH.
Increases in Citrate and pH Vary Among People
But the biology is complex enough that in some people the main response will be citrate, and in other bicarbonate. Given all of the regulatory steps and signalling pathways involved a variety of responses is inevitable. Clinically this means one must measure and determine if the main effect is mainly increase of citrate excretion or of pH and therefore of CaP SS.
What is the Ideal Dose?
A nimble answer is enough to match net acid production – urine sulfate excretion is a decent index. I suspect that answer because of the problem of high urine pH in some people, and because as a clinician I never find it perfectly suits most patients. Yet it is a good starting dose because it aims at neutral acid base balance.
A Simple Pill with Powerful Effects
Physicians who treat kidney stones may well be the main ones who prescribe alkali loads to people with normal kidney function over months or even years or decades of life. This is indeed a remarkable physiological and clinical experiment, and that we do it makes the physiology and cell biology of acid base balance a central topic in clinical practice of stone prevention.
Likewise patients who take this humble medicine undergo what amounts to a reversal of cultural norm, which is a condition of chronic acid loading.
Thence, and for this reason, I have written a very long article about the topic, for physicians and their patients, and especially for scientists who know more about this topic than I do but may not see things from exactly the same view point.

Has someone forgotten about Chang Piedra? My patients drink half their weight in water with the juice of four lemons and take the tincture of Chang Piedra three times a day plus Potassium Citrate. It has kept all of them out of the surgical suite.
Hi Betty, I have not forgotten the remedy, just waiting for a good time to write about it. In brief, there is little evidence that it works, and a lot of evidence it is simply being promoted to make money. But, in fairness, I have not done my article, so I have to temper my remarks. Thanks for bringing up this topic – perhaps it will get me to write what I should have written last year. Regards, Fred
Not being a scientific sort of person I find all the information difficult to assimilate. All I want to do is to raise the bicarbonate level in my blood as recent blood tests have shown that my bicarbonate levels are very low. I have read that taking half a teaspoon of sodium bicarbonate 3 x daily (and maybe 3 x teaspoons of cider vinegar with each dose) helps. I am also taking magnesium citrate tablets each day – for some reason it seems to be helping? Am I doing the right thing? Would taking other supplements help??
Many thanks
Paul Soare
Hi Paul, Low serum bicarbonate levels mean something wrong with your kidneys, intestinal tract, or other important systemic physiology. What do your physicians believe is the cause?? Treatment depends upon cause here and we are far outside the common kidney stone domain. For example stone formers can have renal tubular acidosis with low serum bicarbonate, or it can arise from bowel disease or kidney disease. Whatever the cause, the cause takes primacy. Ask your physician why you have this condition. How you treat it is secondary to the cause. I am sure he/she is fully aware of that cause and all it implies. I am a scientific sort of person and urge you do this promptly before experimenting with any remedies of your own choosing. All causes of low serum bicarbonate are of serious medical concern, even those that can be perfectly controlled. Regards, Fred Coe
Thanks for the article. It challenges me to revisit my organic chemistry from 12th grade. I am a CS and Math major and professional. I’ve been on the Potassium Citrate at 20 mEq three times a day since 2004. First stone was in 1969 as a 12th grader. I’ve had no stone that caused pain since 2004. I did pass one tiny stone, but I now avoid the Texas heat to avoid the stones.
Hi Donald, I am glad you likes the article, and even more that you have been stone free. Stay in it. Regards, Fred
Hi Dr. Coe,
What an interesting article.
However, as someone with a background in physics and chemistry but very little biology, it is difficult for me to understand the biochemistry in play here at the system level. “Binds” seems to mean “chemically reacts with” or “facilitate the chemical reaction of,” perhaps? A “transporter” is an ion that reacts with whatever is to be transported, thus forming a compound that dissociates after the “transportation” has taken place? A “pump” refers to the movement of ions across a membrane due to an electric field created by an imbalance of charge across that membrane? I’ve read a little about pumps and the inherent “electron transport,” by membrane enzymes, the resulting molecule formation on the other side of the membrane, the appearance of certain ions on that side of the membrane, etc. although I have trouble following these explanations because for one thing, QM tells us that it’s not meaningful to distinguish between particular electrons. Instead, I have always had to consider the chemistry in detail, including the reaction with the complex protein at hand, where the protein thus temporarily modified goes on to facilitate another reaction further along the process, and so on. And perhaps the protein eventually returns to its original state by way of another reaction and so is considered to be a catalyst, etc.
Anyway, I did my best to understand what is clearly a very insightful article. Thank you for writing it.
By the way, I was motivated by my own situation, which has been adverse for about a year now and has stumped my doctors, although I finally found a very good nephrologist who “took my case” just yesterday and who says that he corresponds with you in writing on occasion. I will omit his name though, as I don’t have his permission to cite it.
So, here’s my situation in brief: cloudy urine, with what seemed like chyle along with phosphate during the first month or two, although the chyle was never chemically verified; followed by excess phosphate ever since then, as high as 2.01, along with oxalate as high as 10.01; and urine pH of more than 9 at times. Bicarbonate levels are high, not low. The phosphate and oxalate levels were measured by Litholink three times; pH values of 9+ have been measured by other labs.
Well, despite lots of cranberry juice, a low oxalate diet, potassium citrate and calcium supplements, the aforementioned levels are still high and my urine is still cloudy. I developed a kidney stone between the onset of the symptoms and four months later, then another stone during the six months that followed. Just before the onset of my symptoms, I had an MRI to evaluate the state of my kidneys, as a small, clear cell RCC was excised from one of them three years prior. That MRI revealed no kidney stones, or metastases, for that matter.
I will also tell you about my other symptoms, just in case you feel that they are related:
(1) Excess bone density reduction over the past six years, from mild osteopenia to borderline osteoporosis, as determined by a bone density scan done six years ago and another one done earlier this month.
(2) Excessive hearing loss over the same period.
(3) Opacification of the mastoids.
(4) Facial paraesthesia.
(5) Rheumatological/immunological conditions such as Reynauld’s Syndrome, chronic aphthous ulcers and other mouth sores that present vasculitis, gliosis brought on by small vessel disease.
(6) Excess mitochondrial DNA determined through muscle biopsy.
(7) Apparent intracellular potassium deficiency, since potassium citrate prevents what was long-standing muscle soreness and weakness after mild exertion. (That condition was the motivation for the muscle biopsy.)
I would be most grateful for any ideas that you have as to the underlying etiology or etiologies.
I will send an email to you with my contact details.
Thank you.
Robert
Hi Robert, Thank you for thinking so well about the article. As for your own situation – very complex – you have indeed written privately to me and I will try to be helpful via a private rejoinder. For others, however, the pH of 9, if indeed true, means the urine is infected with bacteria that raise pH by hydrolysing urea to ammonia, and crystals can be struvite. Kidneys cannot produce a urine of pH 9. Regards, Fred Coe
Doctor, although neither a chemist nor physician, I found your paper very helpful. Unless I missed it, it doesn’t address the dissolving of uric acid stones already in place. Do you find that potassium citrate in daily dosage of, say, 30 meq can be effective in this regard? Thank you.
Hi Kent, Here is a more clinical article on uric acid stones. Some may dissolve, but prevention of new ones and of growth is absolute if the citrate raises urine pH above 6 on a steady basis. Regards, Fred Coe
Thank you!
I will let you know if staghorn dissolves entirely, a little or remains the same and requires operation…
History, large uric acid kidney stone left side created 15 years ago (age 49), when had poor diet and 3 restaurants. Passed lots of small stones, no pain, two larger stones with moderate pain. No insurance. No treatment.
Cholesterol over 248, type 2 diabetes, overweight 314lbs all in belly, 6’6″ tall.
Retired at 62.
Heart attack at 64, stent on widowmaker.
March 2018, No insurance
Excruciating flank pain right side only, thought i was passing a large stone, but have not passed any in 10 years.
Difficult urinate,
Multiple stream
Cloudy and smelly urine
Could barely move.
Present April 2018,
weight 262.5, goal 220. Cholesterol 103, Diabetes & Blood pressure controlled by meds. Better diet, eat less, walk 30min to 60min daily.
April 2018, turned 65, Medicare with Senior Advantage
Left side CT scan
4.7×3.7cm, 2×1.5 inches, staghorn left kidney, uric acid.
No stones on Right Side
Uric Acid 4.2 mg/dL
Infection found:
Staphylococcus greater 100,000 cfu/ml
Treatment for infection
Cephalexin 500 MG 1 cap 4 times a day, 14 days
Seems to have worked, no pain, urine clear
Treatment for Staghorn Stone
Potassium Citrate 10MEQ, 2 tabs, 4 times a day
Also drinking 64 onces club soda with sodium bicarbonate daily.
Complicated by leaving country for 6 months, May 1st, 2018.
If no change in 6 months,
Operation through back to remove stone.
Hi Charlie, If your uric acid stones are growing or recur your urine pH simply remains too low. Here is my best article on this subject. There is no real reason for uric acid stones to exist once pH is above 6, so be sure and check repeated 24 hour urines for pH and add alkali pills until that goal has been reached. Regards, Fred Coe
I have been on potassium citrate 1080mg two pills daily for about a year. In the past month I have noticed the pills are passing through my system and being expelled completely undissolved. So much so the writing on the pills is still readable. This has got me questioning whether or not I am getting any good out of the pills. Is this normal or should the pills be dissolving?
Hi Larry, you are seeing the wax matrix from which the active material has leached out. Not a problem, Regards, Fred Coe
Thanks Dr Coe on a balanced and discerning insight. Many eons ago, I was taught the ‘Henderson-Hasselbalch equation’ which made no sense at the time. Decades later I began to test pH, redox, and electrical conductivity of various bodily fluids (and various samples of ‘water’). I assumed that bodily fluids, and especially water, would have stable and direct relationship between pH and dissolved solutes—especially alkalai minerals. I was suprized to find the redox state of the fluid influenced the pH more dramatically than solute concentration. My professors reminded me that this was precisely what the H-H equation was describing. Could you provide a biological insight liking Citrate, AKG to physiologic imperative of ‘daily healthy de-acidification’ via Redox?
Hi Tom, I am afraid medicine has not yet incorporated redox measurements into patient care. What we can manipulate in the lab is not always available in patient blood and urine samples, and even more so for what we can influence. Regards, Fred Coe
Thank you for publishing this article. I have been plagued by large uric acid stones for about 10 years, and was proscribed a potassium supplement about 2 years ago – not sure what is next recent blood test has my gfr at 30. Have you prepared any studies on the impact of a daily dose of sodium bicarb ? Apparently baking soda was a go to remedy for my grandmother, who lived into her late 80’s.
Hi Tony, sodium bicarbonate is an alternative but may raise blood pressure. With an eGFR of 30 your physician will want to monitor your potassium carefully as you take the medication. The stones will stop as urine pH rises about 6. Here is the right uric acid article. Regards, Fred Coe
Hello Doctor. My endocrinologist put me on 30 meg Ic Potassium Citrate ER daily because my citrate level in my 24 hour urine is 230. I was just diagnosed with osteoporosis so I am on Fosamax, 1 aspirin a day, 1000 units of calcium citrate a day and 2000 units of Vitamin D. My question is should I take all three 10meq pill togehter, and do they inter act with any of the other medicines.
Thank you, kim
Hi Kim, the calcium citrate is best taken with your main meals, the potassium citrate whenever easiest, the fosamax is always fasting, and the aspirin is anytime as is the vitamin D. Regards, Fred Coe
Great article Dr. Coe. As a fellow physician, I admire your blend of content and style in your writing. About a decade ago I suffered from recurrent oxalate stones requiring multiple medical interventions. Since then I have taken K citrate and Mg and have been symptom-free. I also altered my diet a bit and try to push fluids. My question for you is how to know if and when I would remain stone free without taking K citrate? In other words, how do I interpret the “negative stone state” I am in vis-a-vis continuing ongoing K citrate/Mg therapy? Thanks!
Thanks, Dr Flaherty, A great question. You did four things: K citrate, Mg, altered diet, higher fluids. No attacks for 10 years, do you need the citrate and magnesium? I would get a CT and see if there has been new stones or growth of old stones. If not, a sanguine person might just stop and continue the diet and fluids. A more nervous type – like me – might stop the citrate but raise the fluids and or make the diet as close as possible to the kidney stone diet which is, after all, the recommended one for all of us in the US. If any new stones, or growth, I guess you and I would both say – do something more, even. Warm Regards, Fred
I had a ruptured bladder repaired last year and 3 months later a bladder stone formed on internal bladder wall near repair site (brushite stone) and was removed through cystoscope. Had a 3rd surgery to remove more calcification that had formed on the bladder wall 3 months later. I had my 4th surgery last month in which the doctor went back and removed the damaged bladder tissue that did not heal properly and also more calcification. I have always had small kidney stones that did not require treatment. My doctor gave me script for 20mg Potassium Citrate but I am hesitant to take them. Any suggestions? I am drinking 3 liters water a day.
Hi Gretchen, I believe I answered you several times, and hope you could see what I wrote. I believe I suggested 24 hour urine testing to discover what is producing the crystals. They were brushite, I believe, and I suspect a high urine calcium level that needs treatment. Take a look at my prior comments to you. Regards, Fred Coe
Hello, do you have an email that I could contact you with?
I have calcium oxalate stones.I would like to send you the results of my 24 hr urinalysis but I dont want to post it on a public blog.
I had high calcium level, but my nephrologist said she wasn’t worried about lowering it. She wanted to raise my citrate level. Also, will potassium citrate and hydrochlorothorizide cause kidney damage if I dont drink enough water?
Hi Eve, I hesitate to interpret results privately in that I would be practicing medicine without a real working medical knowledge of your case. A lack of complete knowledge could lead to misunderstandings and poor care for you. In general a high urine calcium is from idiopathic hypercalciuria provided your serum calcium is normal and you have no systemic disease. Reduced diet sodium is ideal as a first step. Potassium citrate and OHCTZ are not known to damage kidneys except in unusual circumstances and will lower urine calcium provided diet sodium is low. Enough water is always important for stone prevention. Regards, Fred Coe
Congratulations on the article. This is a very interesting subject, and the article is very well written. I often use this type of illustration (application) when teaching chemistry. This particular case of kidney stone is a typical example of chemical equilibrium. My urologist commented to me that people who have kidney stones usually have a citrate / oxalate ratio in the urine, different from people who do not have the problem. Chemically, both oxalate and citrate have affinity for Ca2 +, but citrate forms a complex with calcium, which has a charge and is water soluble, whereas oxalate is insoluble. This way, in the presence of citrate the formation of stones becomes difficult. The pH determines whether the citrate is free or not: at low pH it is bound to H + and therefore is not free to complex Calcium and therefore does not work, so it works at higher pH. His article is good, too, as it shows how the organism controls pH to optimize the drug’s functioning, looks like (and is) a certain “intelligence.” Well, I’ll stop here. A hug. Caja
Hello, thank you for an interesting article. Between late 2016 and the summer of 2017, I passed 9 stones in as many months. I finally went to a nephrologist, who prescribed 60 meg (4 pills) of potassium citrate a day. The good news: I have not had a stone in just over a year. The bad news: The pills cause severe muscle weakness and fatigue, bloating, and ‘restless’ legs, which make it hard to fall asleep at night. This was bearable during the winter when I was fairly sedentary, but since I started to workout in the spring, the problems listed above have returned/intensified. I have gone from 4 pills a day to 1 a week, trying to find a balance between preventing stones and these problems. Recently, my kidneys have started to feel sore (actually as I write this, they seem on fire), which is one of the symptoms I get when I am getting ready to pass a stone. Besides going back to the doctor (which I am), any suggestions/recommendations on how to better tolerate the citrate (I take on full stomach and drink lots of water) or something else I should be doing ? Thanks.
Hi Mike, I presume the citrate pills were to raise urine citrate. ALternatives abound – fruits and veggies, for example, some beverages like Crystal Light (20 mEq/liter) so perhaps you and your physician can fashion a workaround. But frankly, a good diet has 110 mEq/d of potassium, mostly as anions like citrate, so I cannot imagine why 60 mEq of potassium citrate is such a problem. Maybe it is because food anions are not all citrate. Discuss this with your physician and see what s/he things. Regards, Fred Coe
I wonder if Mike’s problems are because of too much potassium. If so, he could substitute some magnesium citrate for some of the potassium citrate and still retain the benefits of the citrate.
Hello Dr Coe. I take Pottasium Citrate 1080mg Potrate 10. I have been taking it for 3 years. I was recently diagnosed with having lot of renal cysts and nephrologist said I have PKD. However, no one in my family had PKD including my grandparents on both sides. I am wondering if Pittasium Citrate pills could have caused that. Is 1080mg is very high dose?
Thank you and looking forward to your response.
Hi Nila, The diagnosis of PKD is not easy, but there are recessive forms that do not show up in families. Potassium citrate will not cause cysts, and 1080 mg is about 1/3 of the usual daily dose. I would suggest your physicians be sure about the diagnosis, and that your subsequent care is suitable if you indeed have PKD. Ideally, given the odd circumstances they may wish to help you obtain a second opinion from a PKD expert. Regards, Fred Coe
Hello again, Dr. Coe!
So appreciative of you!
I just did a recheck 24 h urine after nephrology advising me to stop the Potassium Citrate (and urology advising me to increase dose of Potassium Citrate) 6 months ago. I ended up stopping it and hoping to see how things looked, since both doctors disagreed on treatment. Nepehrology’s reasoning for stopping it was that my pH was 7.3, and my Supersaturation levels otherwise looked extremely low risk for stone formation. Urology’s reaosning for recommending an increase was that my oxalate was a bit high risk at 49.
My urine pH now with no Potassium Citrate is 7.1. Citrate is 335. Supersaturations all under risk factor range. Calcium is 100.
My citrate was I was on the pot cit was 482 and pH 7.3, calcium 42, and supersaturations even lower.
My question is if you think the small increase of citrate is worth the increase in pH. Am I ok without the pot cit? My preference is to not take it, but don’t want to hurt anything.
Furthermore, as my citrate isn’t dangerously low indiciating some sort of metabolic problem, but just somewhat lower end, I’m wondering what can be done. Can I affect this with my diet? I would live to lower the pH if possible, as that’s now (coupled with the citrate) my only real risk factor.
Hi Joyce, Of course your urine oxalate has little to do with citrate – it has to do with too much diet oxalate or too little diet calcium or poor time matching between diet oxalate and calcium – they need to come together in the same meals so calcium blocks oxalate. Given the very low urine calcium I suspect very low diet calcium. The high pH is seemingly a fixed thing, and there even without the k citrate. As for adding citrate, why? Fruits and veggies are indeed a good way to get citrate, as you suggest. Regards, Fred Coe
Thank you to Dr. Coe and UChicago for making this resource possible. It is nice to see something intelligent and witty on the Internet. I am a 59yo male Type 2 diabetic of 18 years also hypertensive. My BP medicine is not K sparing. I bought some K citrate capsules at a vitamin store recently. 99mg. I take two a day. I bought this as a K source because I know I don’t get enough. My diabetes is in pretty good control. I urinate a little too much but not so bad as one in poor control. I know the symptoms of high K. Now this 200mg is a small dose in normal people, but I am not normal. Three questions 1) is k citrate good for the kidneys in general, might it help prolong the health of the kidneys 2) would K chloride be better for me, 3) should I call my doctor and tell him I’m doing this or wait to next appt in three months to tell him of this new supplement. I realize intent of this site is mainly stones. If my question is inappropriate I apologize. Thank you.
Hi BillC, The dose of potassium is so low as to be trivial. A proper diabetic diet has five servings of fruits and veggies that provide about 120 mEq/day of potassium, or about 4,500 mg. So yes, potassium is good, and no you do not need the potassium pills given a good diet. Potassium from food lowers blood pressure and so is desirable. Unless you have significant diabetic kidney disease, there are no special limits. The ideal drug for a diabetic is an ARB or ACE, both reduce potassium excretion efficiency so I am surprised your blood pressure drug does not affect potassium. Regards, Fred Coe
Hello! Thanks for the very informative articles. Can you comment on the new OTC product Litholyte as substitute for Potassium citrate pills? I know you have recommended crystal light in past and was curious what you think of this product (safety, efficacy, etc). Thanks in advance!
Hi Brian, I looked on the web. On page one I found a lot of commercial junk with no real substance. ON page 2 – Google – I found an AUA review in PDF that somehow was odd and did not open. Right now I cannot get anything worthwhile, just hype. Fred
This was a very informative and enjoyable article, even if a bit confusing for me as a patient. Does this theory apply to people who get sones due to medullary sponge Kinsey disease as well? I ask because I’ve had one urologist tell me I needed to take potassium citrate, one not, while one said to avoid calcium, and the other disagrees. I’ve also been told to skip the potassium citrate and to just drink lemonade. I had interstitial cystitis as well which makes that somewhat painful. Any advice or insight? Thanks-April
Hi April, Use of potassium citrate is just one part of a program of prevention. Here is a reasonable version of the whole story, that includes serious diet efforts and meds as needed. MSK is usually not the right diagnosis, in that CT scans do not show the sponges. Here is a good article about the problem. One never avoids diet calcium; not effective and bad for bones. Maybe the best overall is this one that shows why no one measure is ever right. Regards, Fred Coe
Dr Coe,
You often suggest Crystal Light Lemonade as a way to increase citrate a little with a tolerable taste. Can I point you toward True Lemon? Crystal Light uses aspartame to mask the bitterness. That’s a particularly nasty artificial sweetener. It has been definitively shown to increase appetite. True Lemon uses stevia (which has its own set of issues, but is a far lesser evil).
It can be found next to Crystal Light in the supermarket.
Patients seem to love the stuff. You tear a pixie stick and add to Poland Spring water bottle.
Hi Doctor B, We have never measured the citrate content of this beverage, so I cannot be sure if it is comparable to Crystal Light. If you have used it, did you find a definite increase in urine citrate?? Best, Fred
Dr. Coe,
Excellent work,
Wouldn’t have been better to use tri-potassium instead of mono-potassium?
3x times meq of citrates with the same mg of citrates
Do you know which cytochrome metabolises citrates?
Thanks,
Vasileios
Hi, Potassium citrate includes 3 potassium atoms per citrate molecule. Citrate is taken up as citric acid in the Citric Acid cycle. I believe citrate lyase is the first enzyme. Regards, Fred Coe
Hi Dr. Coe. I have a reading of 644 mg/d for 24h Urine Citric Acid with ref interval (320-1240). I understand that citrate is the ion form of the citric acid molecule. Is my citric acid result numerically comparable to a citrate result ?
Hi John, The measurement is citrate in mg/d, a common and regrettable US clinical convention. It is this value that has been associated with stone risk in epidemiological studies, and from that point of view your value would be ample and require no medical intervention. To get it into equivalents one would have to divide by the MW in mg/mmol, then multiply by the valence – which varies between 2 and 3 depending on the urine pH. Regards, Fred Coe
Hello Dr. Coe,
Excellent article. I’m a scientist and potential kidney donor. My goal is to reduce my urine calcium in time for my next urine test in 3 weeks and I have the following question: In your opinion, will taking potassium citrate (presumably as a urine calcium sink) possibly increase my urine citrate to a level the kidney folks would find alarming? In my initial test, the urine calcium was quite high (394) and the SS Caox was a bit in the red (6.32), but not too bad. I know this is a very specific question, but I’m hoping that I can balance out the two reads at the next test. I appreciate your input.
Susan
Hi Susan, I imagine you have idiopathic hypercalciuria but do not form stones. Potassium citrate lowers urine calcium, probably via activation of distal tubule TRPv5 (very pH sensitive on its apical side) and reduced diet sodium to 50 – 60 mEq/day is synergistic with it. But… nasty word!… I am not a fan of donating by you because of stone risk in a single kidney. If you do, low sodium and lots of veggies and fruits (give K citrate the old fashioned way) or pills would be a good idea. Fred
Thank you Dr. Coe,
I’ll give the potassium citrate a shot and, in case you’re interested, check back into this board with the resulting urine citrate level in 3 weeks. I know I”m not the best donor, but it’s a big deal to my partner who is very sick. I’m willing to make the sacrifice and be as healthy as possible post donation. Wish me luck!
Hi Susan, You are loving and virtuous. Good luck. Fred
Dr Coe, I am not in the medical field, but I am a chronic stone maker. Over the last 30 years, I have passed or had procedures on over 100 stones. I am concerned about the radiation I’ve received over the years and this has left me at a point where I don’t seek medical help nearly as often as I should. Recently, myself, my children and other family members were diagnosed with a rare, Connective Tissue disorder. Only 2 other families in the world have the same diagnosis. They have noted in their published findings, that they have renal stones as well. I am not sure if my stones are part of my DNA and something I will never be free from. My 7yo has kidney disease and a neurogenic bladder that, I believe, may be from this mutation as well.
I don’t know if Potassium citrate is my answer, but I am so thankful to know that there are medical professionals out there who are actively trying to find answers to this very painful condition. Thank you for your work.
Hi Jill, You do not mention the gene disorder. Many cause stones. Treatment depends on how the genetic disease causes stones. Potassium citrate is just one remedy, but in your family one needs to know the details. I gather you are under the care of skilled medical genetics experts; if not, you should be. They live in universities. Regards, Fred Coe
Dr Coe
I had a ureteroscopy just over three years ago to remove a 5mm Calcium Oxalate stone and I am now almost certain
I have another stone. I am going for an ultrasound soon. I have substantially increased water intake and made some dietary changes since the first stone, but if I’m perfectly honest I probably haven’t done enough. If this does turn out to be another stone, then I will make further dietary changes. What is the success rate of potassium citrate tablets at preventing further recurrences of kidney stones? I am going to request these tablets if I do in fact have a second stone, to hopefully prevent any more. Regards, Paul.
Hi Paul, Sorry if it is another stone. Prevention is not so hard as it is a bit technical. Here is my favorite article on prevention of the common calcium oxalate stones. As you can tell, K Cit is one alternative but never just by itself. Diet is so powerful, one should always use it and add meds to it. Regards, Fred Coe
Hi Dr Coe. I have history of kidney stones. Based on previous 24 hr Urine analysis the doctor has prescribed Chlorthalidone 25 MG tablet. After taking the tablet for 3 months, recent 24 hr urine analysis shows below results. Will Potassium Citrate help me? If yes, what dose?
Urine Volume 3.17 L/d
pH 6.678
Supersaturation CaOx 3.22
Supersaturation CaP 0.80
Supersaturation Uric Acid 0.06
24 hr Calcium 219 mg/d
24 hr Oxalate 37 mg/d
24 hr Oxalate 37 mg/d
24 hr Potassium 92 mmol/d
Hi R, I am not sure about the question. Your SS values are low, as is urine calcium. It is urine oxalate that poses some mild stone risk, and I suspect inadequate diet calcium. You do not show urine sodium. Urine potassium is rather high, which is good. So I see no basis for potassium citrate. Here is a good review of how all this can fit together, but frankly you seem to be doing well. Regards, Fred Coe
Hai…
Im one of the kidney stone patien…now im take the urocit k 10 pillls…but..when i defecate, the pill still in original shape (not soluble)…this is normal or something wrong with me???
Tq Dr.
Hi Rusydi, the wax matrix is not absorbed, but the medication is. Not a problem. Regards, Fred Coe
Hi dr.coe.
Thank you for writing this article.
I underwent a radical nephrectomy for RCC last year in August.
I’m living on one kidney which has 3 stones.
Is it safe for me to take potassium citrate given that I have been advised to decrease my potassium intake?
Thank you
Hi Sapna, loss of one kidney usually leaves one much like a kidney donor, and at no risk from potassium, nor in need of reduced potassium intake. But your physician needs to determine all this because perhaps your other kidney has been found to have less than normal function. If it is functioning normally – and your physician can tell, potassium restriction is not usually an issue. As for the stones in your one kidney, you need to pursue comprehensive evaluation as to the cause of the stones, and prevention aimed at those causes. Potassium citrate may have no role, or may, depending on the results. Regards, Fred Coe
Hi Dr. Coe,
Thank you for this very thorough explanation. I am a stone sufferer and currently taking potassium citrate. I have been having a very difficult time with dry eyes (and mouth) to the point I have been having eye complications. This is only noticeable at night, obviously because I stay well hydrated during the day. I was put on this and allopurinol at the same time, but I’m nearly convinced it is the potassium citrate causing the eye issues. I am not sure the reason, but have always been very sensitive to salt (sodium) consumption in foods, to the point I feel the urge to drink lots of water after eating anything with significant sodium content. Given that, I am assuming the potassium citrate (also a salt) is causing me issues. Is it possible to get potassium supplementation through regular OTC supplement pills or other way, perhaps a combination? I was thinking I could also increase my allopurinol (currently only at 50mg/day) to help with the acidity. I am also taking 50mg HCTZ. I am 37 yo male, otherwise very healthy. My nephrologist is willing to try different combinations to find what works for me, but was hoping you had experience with patients that had similar struggles.
Thanks for all you do!
-Tad
Hi Tad, sodium and potassium are utterly different. In fact potassium causes modest losses of sodium from the body. So I do not believe potassium is acting like sodium did. Allopurinol is of no value for uric acid stones, and is used for calcium stones only as a last resort. I think the diuretic may be causing dry eyes, as that is not a rare situation. But my main issue is why your physician has chosen these meds; since I am not in possession of your real data and S/He is, I do not want to offer what might be improper comments, and surely cannot offer medical advice. Perhaps it is best for you to bring my very general comments to your physician who probably will find them obvious but perhaps might see some use for them. Regards, Fred Coe
Hi dr. Coe, I’m a chronic uric acid Stone former. I watch my diet. My doctor has me on potassium citrate 30 meq a day. my blood potassium is a little on the high normal side. Problem is my pH in my urine started at 5.5 and after adding the potassium citrate it is still 5.5. My uric acid in my blood is normal since I am on Allopurinol. Uric acid in my urine however is high. Since being put on potassium citrate I am constantly peeing .worried that this is a serious side effect. I can’t take bicarbonate because I have heart problems . Don’t want to raise sodium in my body . Is there anything else I can do?
Thank you
Hi Mary, Here are a few things to do. Since the urine pH did not rise with 30 mEq of K citrate, let’s be sure it was absorbed. Check, or ask your physician to check if the urine potassium rose, if the urine ammonia fell; both should have occurred. Potassium salts can act like a diuretic in someone with heart failure, and that is not a bad thing. Be sure your blood tests for potassium are always fasting; after taking the potassium we all would get a surge in blood levels. If you need more K citrate and blood potassium is limiting, your physician can add a tiny dose of chlorthalidone – 12.5 mg daily or every other day, to get rid of the potassium. Regards, Fred Coe
Hi Dr. Coe. Thank you for the informative article. My story in a brief is. 2.5 years ago I had My 4th baby and my only c section, 3 days after I was Released or 5 days post partumn I woke Up with a terrible head ache and my bp was sky rocketed. I never Had bp issues in my life. Anyway I stayed 5 days in the hospital after that. Now fast forward. While i was breast feeding I had Very foul smelling urine but no pain or visible blood. I decided To go to the urologist and he did a CT and an X-ray and found a large 20mm stone on my right and an 8mm on my left. I had A PCNL surgery for the right and he said we could watch the left or lithotripsy the left. And I decided To wait and see if it grew. 9 months later I go In for a recheck and the left 8mm stone has now grown to 17mm. And I am Forming 2 small stones on my right side where I had The first surgery. Now I have To have surgery on the left and will lithotripsy the right. The dr put me on Potassium citrate ER 10meq 2 once a day. They did a 24 hour grind test before the first surgery and it only showed a slight increase in calcium which he believed was because I had A calcium stone. My blood work is normal no thyroid problems. I guess My question is, will I have To be on this medication forever? Will i always form stones now? And is all of this a precursor for kidney disease? Thank you for your time.
Hi Kate, You do not say what the stones are made of. Are they possibly struvite?? If so, it is infection that causes them, and treatment is very different. You say the stone was calcium, but was it just that? Was it possibly calcium phosphate? Whatever they are they are very aggressive and treatment needs to be precise. If your urine calcium was high, how high? Risk begins at 200 mg/d. PERC is a big surgery and not completely benign in terms of kidney tissue, one does not want another if possible. Of course your physicians are entirely responsible for your care, but you might want to bring these questions forward. Regards, Fred Coe
Hi Dr. Coe. The first stones were calcium oxalate. When they did a 24 hour urine test a year ago he said my calcium was “slightly” elevated and he thought it was because it was a calcium oxalate stone. He did not offer to do another 24 hour urine test this time. I just Don’t understand why they are growing so fast and why this is all happening. Before I had My baby I was Completely healthy no issues. And then I had High blood pressure 5 days after my c-section and was in the hospital for 5 days. They put me on 2 high blood pressures medications. But when i returned home my bp started to bottom out so they weaned me off. I currently do not have any problem with my bp. In fact my bp at the drs the other day was 98/75. How long will I have To be on this medication for? And is it effective with keeping stones at bay. I don’t Want to have another surgery, and I really Don’t want this to be my life of having to deal with these stones forever. The small ones on my right I will Have blasted after the surgery on the left. And I hope To be stone free and can stay that way.
Hi Kate, I know you are frustrated with how things are going, and concerned about what will happen, but I can only do a little bit with what you have provided. I suspect your urine calcium is too high, urine volume to low, diet sodium too high, but I do not have the information. I shared with you a link to my favorite article about all this. If you could come as a patient, I could do more. If you want to post the actual 24 hour urine volume, likewise. But as it is I am rather at a loss to be helpful more. Regards, Fred
Thank you for responding Dr. Coe. When they analyzed the stone it was a calcium oxalate stone and it was 20mm. When the 24hr urine test was done it was done before the initial stone removal and he said that my calcium was “slightly” elevated. But he did not tell me the number. And he said at the time it suspected it was from a calcium oxalate stone so at that time he did not put me on medication. But, after my last appointment when he found 2 small ones in my right kidney where I had The previous surgery and the 8mm grew to 17 mm in my left kidney he decided to start me on potassium citrate. He did not order another 24 hour grime teat or fasting bloodwork. Which i am going to bring up to him about that. He initially did blood work. Not fasting. But my bloodwork was normal. I would Prefer not to keep going through these surgeries. And I honestly Think after my bout of high bp post c section and being placed on bp meds messed my body up. I has Zero problems before all of that. I had 3 previous babies and had no issues and right after the last baby my body has not been the same. I am Not currently on any bp meds since my bp started to lower once I returned Home from the hospital. And they weaned me off bp medication. Again, thank you. I just want some answers as to why! I feel Like he put me on meds but doesn’t really know why I am Making these stones. He doesn’t have the answers.
Hi Kate, I think I have done what I can to help, and perhaps you might be able to provide some of the actual findings from your 24 hour urine studies which would help a lot. Regards, Fred Coe
Hi Dr Coe, Thank you very much for such a clear article on how potassium citrate works. Beginning in my twenties, I had some renal colics associated with nephrolithiasis. About ten years ago (now I am 62 years old) I had to go through lithotripsy but, unfortunately, the composition of the kidney calculi was never determined. Since then, I was prescribed Potassium Citrate ER 15 M pills that I take twice a day. About one month ago I had hematuria that lasted one day that might be related to the passing of a kidney stone, according to my urologist. After that episode, I had a CT scan that confirmed the presence of many small calculi in both kidneys (this was shown several times in follow-up CT scans that I went through due to a pre-existing clear cell renal cell carcinoma that was successfully treated three years ago through cryoablation). In my last visit to my urologist, he prescribed Sodium Citrate and Citric Acid oral solution (500 mg/334 mg per 5 ml) instead of Potassium Citrate pills since he believes that the absorption is better when taken as solution. Once I noticed that the solution contains Sodium Citrate rather than Potassium Citrate, I asked my urologist whether the new treatment will be efficient to prevent the formation of stones that “might” be made of calcium oxalate rather than urate, and he answered that they should work the same since both will alkalinize my urine. My I have your opinion on this? Thanking you in advance, Sincerely, Daniel.
Hi Daniel, I guess my first question is whether you need potassium citrate in the first place. Take a look here and see if you would fit into that group. Do your 24 hour urines indicate a reason to take the material? Exactly what is causing your stones. As for sodium, the trials – in the link – were for potassium, so I would favor one uses that form, if one needs to use it altogether. Regards, Fred Coe
Hi Dr. Coe,
Thank you very much for your answer. I haven’t had a 24 hour urine study for many years, and have always been treated as if my kidney stones were made of calcium oxalate. Based on your comment, I will ask my urologist to prescribe me an order for that type of study.
Thanks again for your help! Best regards,
Daniel
My husband had his first kidney stone in 2011. Afterward he would get one every few years. Now he literally gets one every one to two months. They are quite large, orangish in color, and cause him much bleeding and pain. We have an HMO and though he has been to see the urologist and has had 24 hour urine collection tests in 2017 and 2018, getting anyone to advise him beyond drinking enough water is impossible. HIs latest test showed his Citrate Rate was low at 103/mg/day, Uric Acid Rate was 615/mg/day, Magnesium Rate was 37/mg/day, Uric Acid Relative Supersaturation was 2.88. His other values fell within normal limits. I have been reading your articles to try to understand what can be done to try to prevent these all too regular kidney stone episodes. I thought of purchasing some Potassium Citrate in the hope that might help? He drinks plenty of water and lemon water as we had heard that might be helpful. Can you give me any direction on how I might help him figure this out? He’s 65 and still working full time through these episodes. I don’t know how he does it.
Hi Kathleen, from the color and your report of the urine I am sure these are uric acid stones, and can be totally prevented by raising urine pH with potassium citrate. Water is never enough to get rid of them, and lemons just do not have even a fraction of the citrate he needs. Please mention this to his personal physician before you do anything yourself, as his physician is totally responsible for your husband’s care. People with uric acid stones can have problems that affect tolerance for potassium. I would presume his orange stones have been analysed. If not, and they are frequent, have that done to be certain. Regards, Fred Coe
Hello, after my last stone (I’ve had too many to count) I called my doctor and asked if he’d be willing to prescribe Potassium Citrate. They said they were well aware of the benefits and my prescription is written for 1,080 mg 3x a day. No one I know takes anything close to that. I’ve not done a urine collection test in years so I assume this is their standard dose or it was prescribed wrong. Is that dose ok?
Hi Jill, Your physician prescribed a usual dose of this agent and is correct. But be sure it is the right thing for you. I presume you have calcium based stones without systemic disease. Here is one of my favorite articles on their prevention and use of K citrate. Regards, Fred Coe
Dr. Coe,
Have you come across potassium citrate in supplement products? Currently I take 275 mg potassium citrate capsules once per day for the benefit of alkalizing my urine. My question is, do you think this amount is enough to cause a significant change in urine pH?
Here are the inactive ingredients of the supplement as I know it can be different from the prescription version.
Rice Powder, Gelatin Capsule, Vegetable Magnesium Stearate.
Hi Scott, You are right that supplements have potassium citrate, but the amounts are modest vs a single K Citrate pill of 1080 mg of the compound. What you describe will not raise urine citrate or pH reliably, and if you have uric acid stones it is an inadequate treatment. Regards, Fred Coe
Hi Dr Coe,
I was prescribed Urocit K 10 twice daily and was relocating from UAE to US my doctor said on leaving that I could start to cut back in a couple weeks so if I forget a tablet one day no problem. He wasn’t very clear. Anyway I decided when I got to the US to just take one a day. I haven’t had a 24 hour urine test before and am not on US medical insurance as of yet, so would it be okay to continue 1 a day or is that just a waste of the medication and not enough. I have some left to continue 2 a day but with no tests was a bit concerned. Oh I only have a small stone in right and left large in meat of kidney. Left had procedure to remove another which was just in meat and visible no others and lots of Randall’s plaque. Thank you
Hi Denny, If you need this med or not depends on what causes your stones. Here is a good article on how one finds out. When you get some insurance, get evaluated and then your physician and you can determine if it is this material, or diet, or something else that will be most effective. Regards, Fred Coe
Hi Dr. Coe,
I’m struggling to understand acid base balance and the role of the kidneys. If you are up for it, I’d be most grateful for any help.
As rare as this may be, assume a scenario of metabolic alkalosis with respiratory compensation (or some mixed acid-base disorder), but as a lifelong condition of genetic origin.
With 24 hr urine pH around 7, and often higher. And with mixed CaP/CaOx stones, but without elevated blood Ca or PTH.
And accompanied by low bone formation, resulting in low bone density despite low bone resorption near the bottom of the standard range.
If I’m thinking about this right, the kidneys seem to be reacting to correct the high blood pH, not causing it. But I’m baffled about where so much excess base could come from in both blood and urine. And I wonder if the skeleton is somehow being deprived.
My main question is, within the context described above, do you have any suggestions on what systems or processes gone awry could potentially explain blood pH, blood bicarbonate, and urine pH all elevated simultaneously, decade after decade, in a generally healthy and fit person eating a healthy balanced omnivorous diet? Thanks!
Al
Hi Al, I gather you have increased blood pH and bicarbonate and urine pH. As you mention respiratory compensation, I gather pCO2 of the blood is increased. Men with calcium phosphate stones have high urine pH, and produce excess ammonia, which leads to higher acid excretion and possibly increased bicarbonate production. Alternatively, bone mineral loss is a source of alkali as bone mineral phosphate is trivalent negative and will take up protons, so bone loss is alkali gain. In any one case, yours if you are describing yourself, it is hard to know what is happening without more detail, but in general idiopathic hypercalciuria produced low bone production and bone loss and, in those men who make more ammonia, calcium phosphate stones. I hope this is helpful. Regards, Fred Coe
Hi Dr. Coe,
Thank you very much for your fresh set of eyes and ideas! Yes, (mildly) elevated blood pH, bicarbonate, and pCO2. And urine pH averaging ~7.25, highest at night, and peaking at 9.0 or higher as measured by the lab. Compensation is working – I’m basically asymptomatic other than bones and stones. I was trying to keep my question general and educational, but f there are any numbers that would help, please ask. I probably have them. The VBG was one of the most eye-opening.
Ammonia is an interesting idea, however it doesn’t seem to fit because NH4 averages only 48 in my 24 hour urines for PCR averaging 1.1. Cl 24 is more variable, and averages 128.
I hope you have more thoughts on what systems or processes might be responsible for so much excess base in blood and urine, and kidneys needing to purge base all night.
Here’s one I haven’t heard…What might be the effect on blood pH of the “leftover” alkali that “should” have been used to build and/or mineralize bone on a daily basis, but which was never actually was used because although resorption is low, bone formation appears even lower? Best regards, Al.
Hi Al, An ammonia level of 48 with a pH of 7 is remarkably high and consistent with what we have found in our newest research. There is no leftover alkali. Warm regards, Fred
Hi Dr. Coe,
That’s very helpful. Thank you. May I please confirm one thing…?
The high NH4-24 of 48 was *after* a 78% increase that coincided with starting Chlorthalidone 25 mg.
Was NH4-24 of 27 (with PCR of 1.5 and pH-24 of 6.8) from before CHD unexpectedly high too?
And you mentioned new research – anything published that you can easily point me to? Best regards, Al
Hi Al, Thanks for the extra information, but I have no more to add. We have not published the new work yet, but soon. Fred
Hello Dr. Coe,
is there a conversion factor between Potassium Citrate and sodium bicarbonate? I’m working on a retrospective study on urinary alkaliztion, in which patients received different combination of these medication, and I’d like to standardize them
thank you
Nevo
Hi Dr Nevo, Both are alike per mEq, but citrate must be metabolized to bicarbonate and bicarbonate is bicarbonate. Regards, Fred Coe
I have 5 stones as of current Cat Scan. Had on and off for 25 years. 3 years ago had one removed due to size. Was a calcium oxcilate Stone. Utica acid levels are fine, never had gout. Would potassium citrate be of any value and or straight calcium citrate?
Hi Richard, Given the CaOx stone, I would urge full evaluation with 24 hour urines and matching serums, and treatment based on what is wrong. Here is a good article on this. Take a look. Regards, Fred Coe
I have had GI issues but unknown cause or treatment yet. 3 years ago had similar issues, lots of tests and zero findings. 2 days ago and ahead of a scheduled colonoscopy they had met get X-rays for my upper and lower abdomen. No issues with GI tract seen on X-ray but did note a 4MM stone in the lower section of one kidney.
I have gout and have been on allopurinol for years. My uric acid levels have always been on the high side but just barely. Still, have tried to go off of meds several times but could not as the gout would show up like a clock. I have gout and was confirmed with a fluid extraction from an affected joint.
I obviously need to see a urologist and going to try to get a referral today. Colonoscopy is tomorrow but feel like someone told me I have an explosive inside of me and flushing my system for the procedure is scaring the heck out of me.
I guess I’m here because I have been reading up on the use of citrate to dissolve uric acid based kidney stones. This has inspired hope as the electric shock treatement seems to not be as effective / selected if in the lower quadrant of the kidney and the other methods sound painful and risky. What sounds even more painful is 4MM as that appears to be slightly larger than the diameter of ureter (which is 3-4MM).
I’m hoping they can tell the stone type based on the shape and density from an ultrasound and suspect that will be what the urologist suggests.?
Hi Brian, You can ask your physician to try to measure the radiographic density of the stone; uric acid has low HU in the 200-400 range compared to 1000 and higher for calcium stones. You should get 24 hour urine testing, as it will probably show low urine pH, and if so you would then be wise to take potassium alkali. Be sure and re-measure during treatment to assure the pH has risen to 6 or so. Allopurinol will have no effect on uric acid stones. Uric acid stones fragment poorly with SWL, lower pole stones often do not clear, and ureteroscopy is a procedure, though the best of the lot. Regards, Fred Coe
hi….am a 44 year old female with a creatinine level of 90. I see a nephrologist but am not due for my next appointment until Jan 2020.
I am not overweight but have just started to take Duromine (phentermine) 40mg for a few pesky little kgs that won’t go away. My doctor prescribed it as he knows I am responsible and my nephrologist also is aware.
My nephrologist wanted me to take a tablet for my kidneys/creatinine, which would possibly raise my blood pressure, and this tablet, i would have to take forever. I did not want to do this, and also, as I knew i was going to start the Duromine, I did not want to take them both at the same time.
So he told me to take Urocit-K, as it would not interact at the phentermine, and I am going to get this soon.
I am only on my first day of taking duromine and have been doing some research and saw that it can cause increased serum creatinine.
My question is, will the Urocit-K help my creatinine levels remain where it’s at, or it’s only treating any stones i might have? I am hoping it will assist in keeping my creatinine levels at bay, especially while i am taking Duromine.
I am desperate to be super careful about not raising my creatinine levels while taking Duromine, so would appreciate any info you are able to disperse.
Thank so much!
Hi Paula, I presume the creatinine level is in blood and in micromole/liter, showing slight reduction of kidney function. Likewise you have stones. I suspect the drug from your nephrologist is to lower your blood pressure and is an angiotensin converting enzyme inhibitor (ACE, names terminate in ‘pril’) or angiotensin receptor blocker (ARB, names terminate in ‘sartan’). The urocit K is used to prevent stones and affects renal function only so far as stones and their passage and surgeries injure kidneys. On PubMed I found no references to its effects on kidney disease. Since I have no real detailed information about your care, I hope these few observations are helpful. Regards, Fred Coe
Hello Doctor!
First off, I must thank you for your research on crystal light being a good replacement for k-cit. I have stones made of both Uric Acid and Calcium Phosphate. I have been drinking around 2.8 litres daily since January of this year. I had ureteroscopy this year of a 7mm stone with an HU of 354. My stone risk profile(24-hour urine collection) as of 2 months ago gave me the following results:
STONE RISK PROFILE –
Total Urine Volume
Your Value
2.82 L/day
Standard Range
>2.00 L/day
Ph Urine
Your Value
7.2
Standard Range
5.5 – 7.0
Flag
H
Calcium Urine
Your Value
214 mg/day
Standard Range
<250 mg/day
Oxalate Urine
Your Value
25 mg/day
Standard Range
<45 mg/day
Uric Acid Urine
Your Value
1215 mg/day
Standard Range
320 mg/day
Sodium Urine
Your Value
235 mEq/day
Standard Range
<200 mEq/day
Flag
H
Sulfate Urine
Your Value
12 mmol/day
Standard Range
<30 mmol/day
Phosphorus Urine
Your Value
1198 mg/day
Standard Range
60 mg/day
Ammonium Urine
Your Value
30 mEq/day
Standard Range
14 – 62 mEq/day
Potassium Urine
Your Value
71 mEq/day
Standard Range
19 – 135 mEq/day
Creatinine Urine
Your Value
1747 mg/day
Standard Range
800 – 2000 mg/day
Calcium Oxalate
Your Value
0.48
Standard Range
<2.00
Brushite
Your Value
2.02
Standard Range
<2.00
Flag
H
Sodium Urate
Your Value
3.15
Standard Range
<2.00
Flag
H
Struvite
Your Value
7.75
Standard Range
<75.00
Uric Acid
Your Value
0.14
Standard Range
<2.00
The Patient Has
Your Value
See Below
Hyperuricosuria
High urinary pH
High urinary sodium
Supersaturation Index With Respect To
Your Value
See Below
Brushite (Ca phosphate)
Monosodium urate
Suspected Problem Is
Your Value
Hyperuricosuric Nephrolithiasis
Last year's 24-hour test suspected I had CaP stones; as well as another collection that was done in 2015.
I know that K-Cit is an effective treatment for Uric Acid kidney stones, but I am finding conflicting opinions on whether it's an effective treatment for CaP stones. My dosage has been 40 mEq daily, mostly in completely crystal light form. Is this regimen beneficial for CaP stones? If not, what other options are there? I have been diagnosed with hyperparathyroidism, the endocrinologist I saw dismissed my 10 years of kidney stone episodes with a simple "it's simply low Vitamin D". I was prescribed ergocalciferol. I intend to get a second opinion on that from my nephrologist. In the meantime, is my consumption of K-Cit helping or hurting with regards to the nucleation of CaP?
Hi, Christopher, This confusing to me. I gather you have made uric acid stones but all I have is the HU of a CT of a stone removed by URS. Was the stone uric acid? Then, is it you passed calcium phosphate stones or that the 24 hour urine points out that you have supersaturations for calcium phosphate? I gather it is the latter. What matters is the stones, and if uric acid and if you are raising the pH for prevention it is quite possible to raise the pH high enough to also raise SS for calcium phosphate. If you want to reduce the latter, perhaps you might just reduce the amount of extra alkali you are taking in the form of crystal light. Then there is the issue of primary hyperparathyroidism. That requires your serum calcium, fasting and in the morning, be high and PTH not suppressed. Low vitamin D can raise PTH but not serum calcium. Can you clarify what your stones really are, and if serum calcium levels are indeed high? Of course, your physicians are responsible for all of this and perhaps they already know exactly what is the case – I am far away. Regards, Fred Coe
Hi Dr Coe
I have low citrate and have been taking potassium citrate , it’s not raising my urine citrate Levels by much , ironically my highest ever 24h urine citrate level was when I ate a lot of veggies and lower than usual protein. My average over 4 collections is 190mg /24h.
Are there any other viable alternative options I can look into? Bicarbonate etc…? My oxalate is lower now I’ve increased calcium in my diet but calcium excretion did go up too.
Many thanks
Best
Ray
Hi Ray, I presume your stones are calcium oxalate, not calcium phosphate, as increasing urine pH from potassium citrate may make the latter more frequent – no trials have been performed for this situation. As for the lack of response, if the pills do not work but veggies do work, why not use those foods? The mechanisms we know about for citrate all concern alkali, from metabolism of citrate of of the huge variety of similar anions in vegetable foods. As for urine calcium, if it went up with higher calcium intake, reducing diet sodium to 1500 mg or so will lower it, as will a low dose of chlorthalidone. Regards, Fred Coe
It has been suggested that I begin taking potassium citrate after multiple issues from uric stones, and a high 24-hr uric level. However, I have developed much more serious problems with my GERD issues recently (partly due to a bout of sepsis from a uric stone – multiple antibiotics). My gastroenterologist is against taking these pills. at least until the GERD improves a bit – if it does. Are there any recommendations for those who can’t take potassium citrate?
Thanks so much for all you do to help everyone.
Hi Kerry, if you are forming uric acid stones your urine is too acid and you do need alkali. But if you cannot take the potassium citrate pills sodium bicarbonate pills – 10 grain OTC – 2, 4 times a day should work, as would sodium citrate liquid products – your physician can look up products, or prescribe the requisite amount of potassium or sodium citrate and your pharmacist can find a product you can tolerate. Crystal Light lemonade has 20 mEq of potassium alkali in a liter so one liter will take care of 1 of your daily doses. Be sure and measure 24 hour urine pH and uric acid supersaturation when you are on treatment to be sure treatment is adequate. Regards, Fred Coe
Thank you so much for your suggestions. I will ask about using other than potassium citrate. There seems to be some discussion as to what to do; my stones came back in Sept. and April from the lab as 100% uric (the dr was convinced after the latest lith that they were calcium-based due to appearance, as they were not the usual color of reddish/orange). Stones continue to show up on x-ray radiopaque as if they aren’t 100% uric, but that is what I’ve been passing. Kind of frustrating. Maybe a trial of something to see if the stones reduce will help figure out what’s what. Thank you again and I will be asking my dr about the options you suggest if I can’t take the potassium citrate due to GERD.
Hi Kerry, Uric acid stones show up on CT scans, but not on routine x-rays. If the latter, they may be mixed uric acid/calcium oxalate stones. One help us urine pH. If it is above 6 potassium citrate is not appropriate and stones are not likely uric acid. I suspect is closer to 5 given uric acid in stones analyzed. Be sure and check on this. Put more clearly, uric acid stones require an acid urine (pH <5.5) to persist, calcium oxalate stones are indifferent to pH, calcium phosphate stones form and grow when pH is above 6. SO pH and stone composition matter a lot! Regards, Fred Coe
Thank you, Dr. Coe, for your insightful essays. I enjoy them and benefit greatly in knowledge and understanding as I am an oxalate stone former. I truly appreciate your expertise and wisdom.
All the best,
Neil
Thanks, Neil, I hope they help with prevention. Fred
Thank you for this article, it was very instructive. I have been suffering with calcium oxalate kidney stones for 7 months now. I have passed 16 stones on my own and had one lithotripsy for 1 that was blocking the ureter. I still have 7 more stones in both kidneys (around 6-7mm each). I’m in pain all the time, I thought that the pain was only while they were passing, not when they were attached to the kidney. That is my first question, why the pain? I’m on potassium citrate 40 mEQ a day. I started 3 weeks ago on this dose since the low dose didn’t elevate the citrate in my urine, Hipocitraturia was my diagnosis from the 24 hrs collection analysis. My second question is, can the potassium citrate really dissolve the stones? It may prevent future formation, but what about the stones I already have? Thank you Doctor Coe.
Hi Leo, Pain from non obstructing stones is common and whether or not surgical removal helps untested. Potassium citrate is a treatment when urine citrate is low, but it cannot dissolve calcium oxalate stones. If your urine citrate is not going up, the drug is futile ane perhaps your physicians might want to consider alternatives. Here is a good article on treatment strategies. Regards, Fred Coe
I’m a 38 yo male that has had 3 kidney stone events (2007, 2 in 2018 – calcium oxalate). A CT scan in Sept 2019 showed bilateral nonobstructing stones, right greater than left, measuring up to 4mm. It also showed a circumaortic left renal vein and subcentimeter calcifications in the spleen of unknown origin. I have an irregularly shaped colon, diverticulosis and chronic constipation. I have struggled with high blood pressure and bad cholesterol since my mid 20s. To attempt to right that, I have gone from 240lbs to currently 160lbs. I have been experiencing strange pinching near my pubic symphysis since August 2019 which brought me to the CT scan that has no mechanical cause and I have basically lost the sense of urgency when I should urinate.
Blood work shows I have borderline low vitamin D (31 ng/mL); borderline high calcium (10.4 mg/dL), protein (7.7 g/dL), CO2 (30 mmol/L); normal intact PTH (30.5pg/mL)
A urine supersaturation from Litholink in October showed borderline high calcium (220 mg/day), oxalate (42 mg/day), magnesium (148); high pH (6.827) and low citrate (374 mg/day). My urologist prescribed potassium citrate 10meq 3x/day and I’ve been on HCTZ 25mg 1x/day since 2018 stones.
After 2 months on potassium citrate and dieting (low salt, low added sugar, low oxalate, DV calcium, ~120oz water/day), an updated urine saturation shows that my oxalates decreased to 30, calcium increased to 231, pH increased to 7.178 and my citrates actually decreased to 306.
I have an appointment scheduled with my urologist but I’m not optimistic about the effectiveness of his recommendations. I’m at a loss on how I’m not absorbing any of the potassium citrate; how is my protein high but my urine pH is so alkaline? In general, I’m not getting any coordination of care or much attention from my PCP, urologist & dietician & I don’t understand the root cause of my electrolyte imbalance and how to address it.
Hi Matt, I presume the slight rise in serum calcium is because you are taking hydrochlorothiazide or perhaps the blood was not fasting. The low citrate may well reflect potassium depletion from your thiazide – serum potassium can be surprisingly ordinary looking despite potassium depletion as most of the potassium is in your cells. Potassium citrate has raised urine pH, and perhaps has not had a chance to replete all of your potassium losses. I would ask your doctor if potassium chloride might be a better bet, to see if some months of potassium sans citrate might raise your urine citrate without raising pH. You say your stones are calcium oxalate, but is that conjecture? Your baseline urine pH is very high and I am suspicious the stones may be calcium phosphate – your physicians will know for sure as stones were analysed. You do not mention urine sodium, but it is very important to keep it low for control of blood pressure and to allow an adequate diet calcium while controlling urine calcium – the high oxalate is a major stone risk and may reflect inadequate diet calcium. These are just ideas for your physicians to ponder. Regards, Fred Coe
Hi Dr. Coe,
You mention urine sulfate excretion as a “decent index” for net acid production. What would be the optimal level/range for this?
Hi David, It is ‘decent’ because only about 1/2 of actual acid production. The other half requires testing far beyond present clinical methods. A value between 30 and 50 is commonplace, and reasonable. Regards, Fred Coe
Hello Dr Coe,
Thank you so much for all this insight! How long would you reckon the effects on these tablets last on the body, how long would it take (ceteris paribus) for the blood test to show similar results to pre-medication levels?
Best wishes, Ela
Hi Ela, I think the rise in urine citrate lasts a few hours – not exactly sure. Blood will show nothing unless you measure citrate – not a clinical test – as kidneys regulate it. So dosing should be at least twice a day and three times if possible. Regards, Fred Coe
Hello Dr. Coe,
Do you have any information regarding the efficacy between OTC potassium citrate supplements versus pharmaceutical preparations such as Urocit-K for hypocitraturia and alkalinization therapy? I have a number of patients who cannot afford the rx (roughly $350/ 3 months with GoodRx coupons) and cannot financially appreciate the long term benefits of stone prevention such as fewer ER/OR and imaging costs due to more immediate concerns such as fixed incomes etc. I’m sure my patients would be agreeable to more frequent dosing as long as the options are affordable.
Thank you,
Justin
Hi Doctor, The OTC pills are small, mostly 100 mg of the potassium citrate salt, meaning 10 of them equals one standard 1080 mg Rx pill. Food grade K citrate – Amazon sells it – can be weighed out into 1 gm portions using readily available home scale devices, and taken in juice, or even put into capsules. I have notes on this site from many patients who have done this. I warn all the patients who write in to be sure their physicians have reviewed what they are doing. If one of my patients wanted to try, I would surely do the same, so I presume you would as well. The price for this option is nearly nill. Crystal Light lemonade is cheap and one liter has 20 mEq of potassium citrate, at least when we made measurements; another option. Regards, Fred
When weighing food grade citrate into 1 gm portions, wouldn’t you need to account for the weight of the citrate? One common combination I’ve seen is 275mg dose with 99 being K, which means you would actually have to weight out 2.75 grams if using this formulation, to get about a gram of K. Am I missing something? Also, can you give a clear formula for converting mg (in this formulation) to mEq?
Hi, No. 1080 mg of potassium citrate crystals is 10 mEq of potassium and citrate. You are missing how things get quantified. Potassium citrate has three potassium atoms on one citrate, and that citrate has three valances that can take up protons on metabolism to generate bicarbonate. Given 1080 mg – 1.080 gm are 10 mEq, the formula is 1080/10 or 108.0 mg of potassium citrate crystals per mEq. Regards, Fred Coe
Hello Dr. Coe,
I am taking Potassium Citrate because I have been diagnosed with Medullary Sponge Kidney associated with hypokalemia periodic paralysis. Eversince I have been compliant with taking potassium citrate I have never experienced paralysis anymore. I just want to know how long does potassium citrate will take effect after I take it. Thank you!
Hi Rachelle, The combination of periodic paralysis and many stones suggests renal tubular acidosis, and I would suggest your physicians might want to see if you have it. It is hereditary and diagnosis very important. Regards, Fred Coe
I have been diagnosed with DRTA and nephrocalcinosis. My citrate level is 24 and my PH is 6.
What diet should I follow and would Potassium Citrate work for me?
Hi Shannon, Here is my best article on this condition. Most important, why do you have it. Is it a drug? Inherited? Part of another disease? Stone prevention depends on that. Regards, Fred Coe