 The term ‘infection stones’ means stones caused by the action of bacteria.
The term ‘infection stones’ means stones caused by the action of bacteria.
At the present time only struvite stones belong in this category. Struvite is a composite of magnesium, ammonium ion, and phosphate ion. Certain bacteria can decompose urea in urine to ammonia creating conditions that promote crystallization of ammonium ion with the phosphate and magnesium normally present in urine.
That is the big picture. I mean to put in the details. I have already placed struvite stones within the pantheon of stone disease.
The beautiful photograph of a struvite stone is by Susumu Nishinage (from the remarkable photographic collection ‘Science Photo Library Art’, part of finartamerica.
The Broad Picture of Struvite Stones
This straightforward and useful clinical review tells us that struvite stones comprise about 10 – 15% of urinary (kidney and bladder) stones, and are more common in women. They are often large, and by nature infected. Not rarely they enlarge so as to fill the collecting system causing so called stag horn stones. Surgery is usually percutaneous nephrolithotomy.
Unlike the common calcium and uric acid stones, struvite stones do not so often pass as grow in the kidney to cause flank pain and, being infected, fever, along with bleeding and slowly developing obstruction. The combination of obstruction, infection, and large masses of crystal themselves not rarely injure kidneys and reduce kidney function. As one might presuppose, factors that raise the risk of infection stones include congenital or acquired urinary tract malformations, obstruction, catheters left in for any significant amount of time, and urological procedures themselves.
Chronic indwelling urinary catheters are an ideal locale for bacteria to lodge and form crystals. Of the crystals, struvite is most common and arises as bacterial lodge on catheter walls and on urothelium whose defenses are reduced by the catheterization.
Mechanism of Struvite Crystallization
This will seem foreign and technical, but I advise interested patients to read it because once one understands just what is happening prevention and treatment will be immediately clear.
Urea Hydrolysis Reaction
Utterly unlike the calcium and uric acid stones, or less common cystine stones or even drug stones, struvite stones do not rely at all on kidney water conservation to produce supersaturation sufficient to nucleate crystals. The bacteria themselves provide the free energy for crystallization through their multiplication, and unleash a saturation latent in normal urine.
This latent saturation arises from hydrolysis of urea. Hydrolysis means lysis (taking apart) by water (hydro). Water spontaneously combines with urea to take it apart, releasing ammonia.
All mammals excrete urea as an end product of nitrogen metabolism. It is concentrated in urine by kidneys 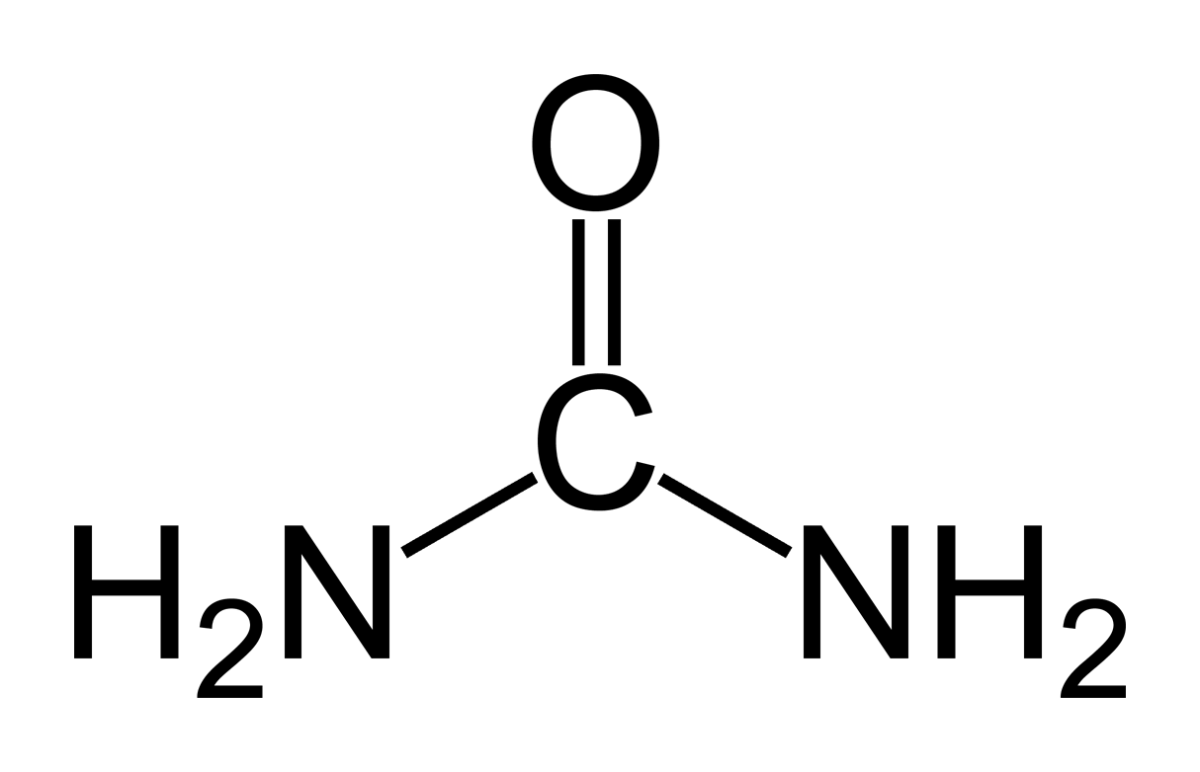 as they conserve water, and specifically because urea is part of the machinery kidneys use for that water conservation.
as they conserve water, and specifically because urea is part of the machinery kidneys use for that water conservation.
Here it is as a stick figure – a single carbon atom (C) double bonded to a single oxygen (O) and two nitrogens.
In water, urea has the chemical potential to decompose by hydrolysis. By this I mean, it is like supersaturation and crystals. If water combines with urea so this structure comes apart, the energy that was used to make the chemical bonds is released, so the reaction of ‘hydrolysis’, bonds lysed (broken) by water combining, is energetically favored. It will occur by itself.
Despite this potential, urea is very stable in urine, with a 1/2 life of over 3 years. The reaction with water is slow. But if the enzyme urease is added hydrolysis rate rises 10,000 fold meaning it occurs in minutes. Because urea is a molecule with one carbon atom, one oxygen atom, and two nitrogen atoms, hydrolysis means that water reacts this way with the urea:
(1) NH2(CO)NH2 + H2O → 2NH4+ + HCO3– +OH–
The result is two ammonium ions a bicarbonate ion, and a hydroxyl ion (OH– the alkaline radical, as protons (H+) are the acid radical). The latter two, bicarbonate and OH– raise the pH of the solution (urine in our case). The ammonium ions crystallize with Mg and Phosphate to make struvite. The two drivers of the crystallization are high ammonium ion from the hydrolysis, and high pH which opens two negative charges on the phosphate so it can crystallize with ammonium and magnesium ions that carry a positive charge.
Urease in Human Urine
 This very fine work (Environ. Sci.: Water Res. Technol., 2018, 4, 87) illustrates what happens when urease is introduced into human urine or a solution made up like human urine.
This very fine work (Environ. Sci.: Water Res. Technol., 2018, 4, 87) illustrates what happens when urease is introduced into human urine or a solution made up like human urine.
Within the first 15 minutes (upper panel to left) ammonia (mg/l) has risen quite a lot both in human urine (filled boxes) and a synthetic urine. The pH rises with ammonia. Unlike ammonia which rises steadily for 4 hours, pH jumped from 6 to 8 within 15 minutes and then rose very slowly thereafter to 9, which is what one finds in the urine of people with infection stones.
The reason ammonia rises over hours and pH over minutes is that ammonia is a buffer. As it is released from urea it provides alkali as bicarbonate and hydroxyl ions (protons from water are taken up into the NH4+ structure). As more is made, the ammonium ion begins to lose protons into the solution to combine with the new hydroxyls because it has a dissociation constant of pH 9.5. The loss of protons produces ammonia, and patients often smell it in their own urine.
So a fancy chemistry leads to conditions that make struvite and to the humble scent of ammonia in urine that tips people off to what is happening.
Does this mean that a strong ammonia smell in urine suggests one should test for bacteria that possess urease?
Yes.
Struvite Crystals
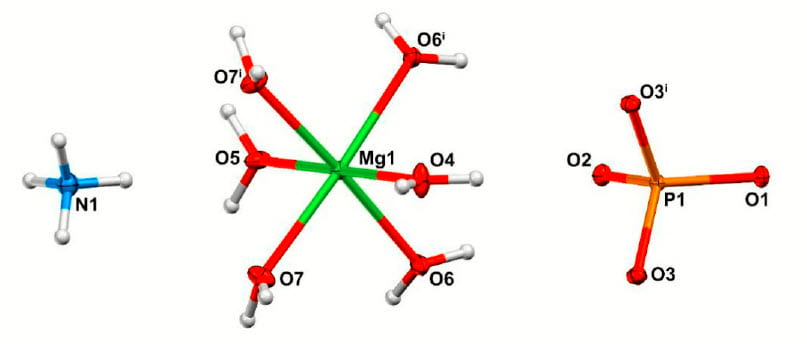 Without losing ourselves in the precision of modern crystal structural analysis, I might say struvite is a loosely woven crystal of a rather charming structure I would like to share with you.
Without losing ourselves in the precision of modern crystal structural analysis, I might say struvite is a loosely woven crystal of a rather charming structure I would like to share with you.
The main components are like ‘Tinker Toys’ or Lego, that fit together to make up the crystal. At left is the NH4+, the blue nitrogen (N, with its 4 protons (white spheres) each positively charged. At the right, a phosphorus atom with its oxygen atoms that have a negative charge. In the middle is a magnesium atom that is surrounded by 6 water molecules. Their oxygen atoms face the positively charged magnesium atom, their two (H2O means one oxygen with two protons) positively charged protons stick outward (white tiny balls).
You can just guess how this will make up a structure. The negative oxygen on the phosphate will bridge between the protons on the nitrogen and the water molecules on the magnesium atom. If you had an atomic modeling kit you could make it yourself if you knew how everything fit. The people who wrote this paper 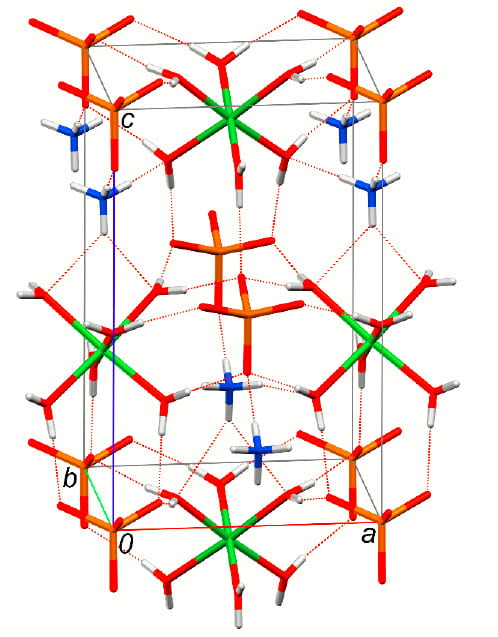 determined the fitting in ways too arcane for me to describe, but this is the shape you would make.
determined the fitting in ways too arcane for me to describe, but this is the shape you would make.
Is it not a beautiful shape?
The huge magnesium hexahydrate is held in place by charge bonding (negative attracts positive) from phosphate and ammonium ions (lines show the charge bonding) to make a repeating structure (magnesiums inside at the top and bottom, outside in the middle) that is the crystal. The whole thing is self assembling, like all crystals. This ‘unit cell’ architecture shown here is repeated endlessly to make up the crystals one can see through a microscope or in a stone.
What They Look Like
In this lovely picture (below right) from the same research article, the crystals are seen under light microscopy. The web has hundreds of similar photos, incidentally. The individual crystals are often compared to ‘coffin lids’ as they are rectangular and their tops mount to an apex like a peaked roof. The one near the upper left shows this.
Physicians can see this rather specific shape in urine from patients, and make a provisional diagnosis. Laboratory technicians are trained to do that and report it as such.
The Matrix
In urine, this pristine crystallinity is complicated by proteins and complex sugar molecules made by the bacteria, as well as native to urine. So one does not get pretty prisms in stones but a mixture more like concrete. The stones can grow very fast because urine magnesium and urea are limitless, and bacteria are alive and furnish enzyme. Their own bodies add to the stone mass, which is also alive, in a way, and thoroughly infected.
Crystals Grown in Vitro
Many labs have added urease possessing bacteria to human urine or solutions closely simulating it. This well done and recent study gives a dramatic picture of the bacteria on the crystal surface (Below left). 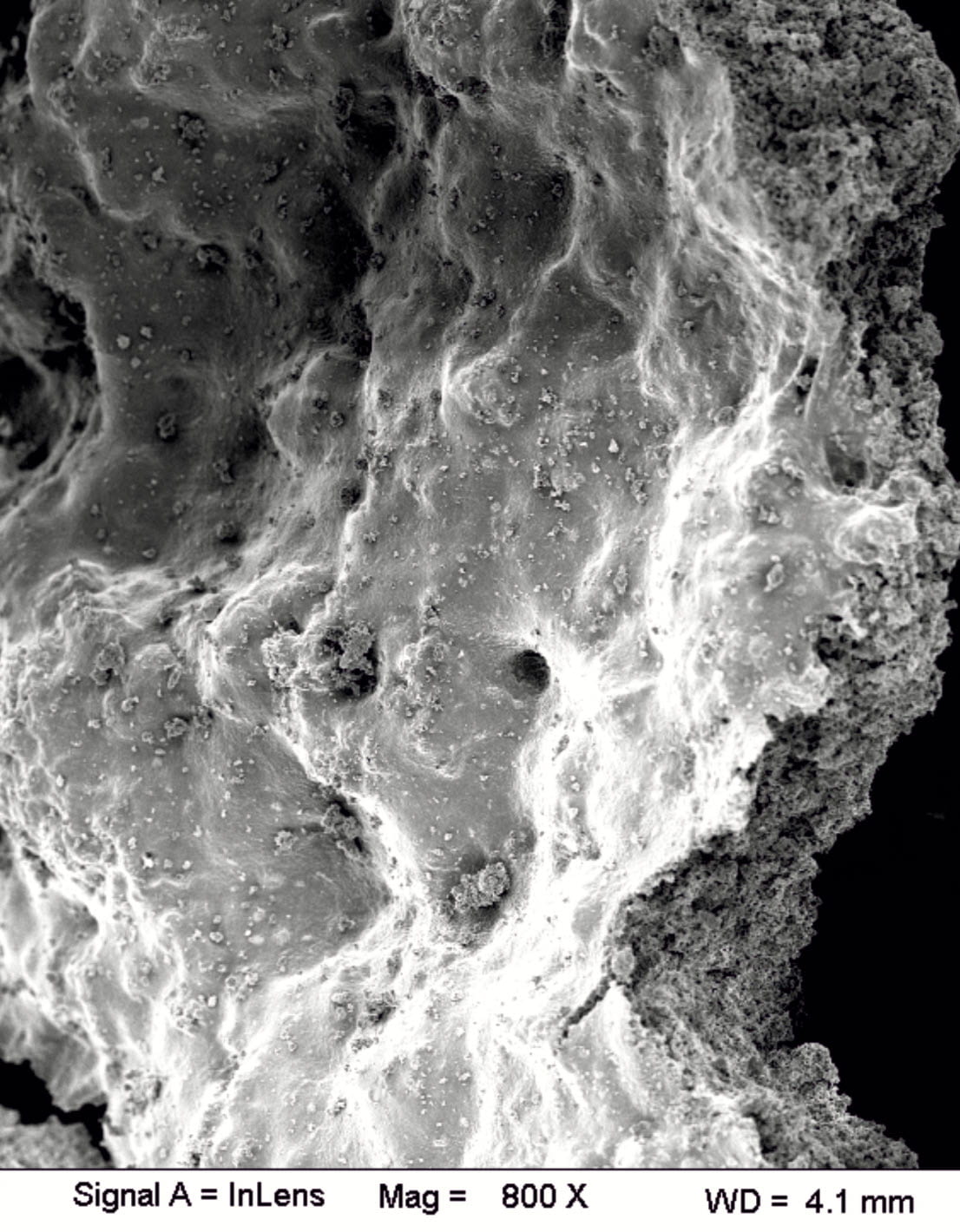
The crystal mass fills the frame, like a mountain range. The tiny dots are colonies of bacteria and also – tiniest – individual bacteria, the founders of this relatively massive solid phase. We are looking at an open face at 800 fold magnification, for reference. If one broke open another layer one would find the same picture so the mass of bacteria is quite large.
An ancient study like this one but with techniques of far lesser power is a worthwhile read just for the illustrations and a sense of history. It described crystals and bacteria in 6 patients and shows what the crystals look like under a common light microscope and with the (then) cutting edge electron microscope. It shows bacteria and crystals clumped together, the bacteria ‘gluing’ crystals together to make the large stone structures. My copy has grainy images that I do not want to show. The Journal of Urology does not seem to offer high quality images from 1985.
Secondary Crystals
No surprise that high pH and new formation of bicarbonate will promote crystallization of calcium crystals. At a pH of 8 or 9, urine phosphate will be exclusively divalent negative and prone to crystallize with calcium. In addition, carbonate species will be prevalent because of the high pH. So calcium phosphate crystals will form, and gradually mature into apatite but in the presence of considerable carbonate ions the apatite will commonly include carbonate in its lattice (carbonate apatite is a common shorthand).
This means that struvite stones not rarely include calcium phosphate crystals, or calcium carbonate or – more usually – carbonate apatite – hydroxyapatite as in calcium phosphate stones with a rich addition of carbonate ions. This seeming arcana matters because of confusion. Calcium phosphate or carbonate crystals do not automatically imply urine abnormalities of metabolic origin as in routine calcium phosphate stones. They can be produced simply by the actions or urease in urine. On the other hand, struvite does not exclude the possibility of an underlying metabolic stone origin, either. So as in all of stone management clinical evaluation and common sense must always dictate action.
Does this mean that I do a full evaluation for cause in patients with struvite stones? It depends. If infection has an obvious cause timed well with the struvite stone and no prior calcium stones ever former, perhaps not. Otherwise, indeed yes.
The Bacteria
Associations with Struvite
Using DNA as opposed to routine culture methods and advanced crystallography to document stone crystals, this study has correlated bacterial species with stone crystals.
The work is not large from an epidemiological standpoint, using only 83 stone samples, but the quality of the measurements is so high one can be confident of associations. Proteus species (vulgaris, rettgeri and mirabilis) predominated in struvite stones. The common e coli bacteria were inversely associated – not found with regularity.
Of passing interest, e coli were associated with calcium oxalate stones, but that may be more because of surface adsorption than that they play a role in their cause. Of greater interest was negative associations between one bacterial type and another. Proteus infections in stones were not accompanied by e coli.
My own view, for what it is worth, is that any bacterial species that can grow vigorously in urine and possesses urease can produce struvite stones, so although Proteus is a leading example one should look for all urease possessing organisms in struvite stones. Modern labs all search for urease in bacteria and report its presence.
Proteus Mirabilis
As a prime example of a struvite forming organism, this one is perhaps the best studied and the most attractive. The miraculous 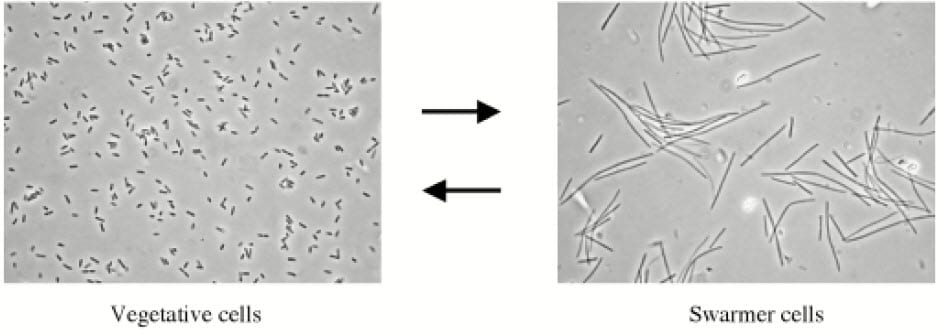 character that evoked is naming is from its behavior. It exists in two forms, vegetative and swarming. The latter are huge and have multiple cilia, the elongated hair like projections that move them along as swimmers.
character that evoked is naming is from its behavior. It exists in two forms, vegetative and swarming. The latter are huge and have multiple cilia, the elongated hair like projections that move them along as swimmers.
Curiously, both forms cause stones and infection, although the large swarming forms have more urease.
Urease is called a virulence factor in this article and others because it is one. If you were a bacterium and could make microcrystals form in urine, how effective that would be for your long term occupancy! Indifferent to water flow through the calyces and down the ureter you are anchored in a place filled with nutrient. Your progeny, for bacteria reproduce by dividing, will have from you a similar legacy and together produce stones of clinical significance.
This review article mentions the fact that ammonia may increase human cell permeability so blood and cell nutrient become more available to bacteria. To us, this is injury.
Clinical Meaning
Stone Forming and Urease
Infection stones are at once a massive complexity and utter simplicity side by side, or perhaps as in a Chinese (or Russian) Box of decreasing sizes fitted into one another. The stones form in the most direct and comprehensible manner, via hydrolysis of urine urea to ammonium ions and their counterparts bicarbonate and hydroxyls – the fragments of the original molecule. The crystal assembles itself, as all crystals must, and gathers about it the matrix material available from urine and the bacteria themselves.
The resulting stones are not so much infected on their surfaces but entirely and from within as well. The bacteria make the crystals around themselves, so to speak, and every morsel of stone can contain live organisms with the power to produce more crystal as they grow and divide.
It is this engine of performance that makes struvite stones such clinical problems.
Although the miraculous Proteus stands out as prime among peers, any microorganisms that produce urease can in principle produce struvite in human urine. Therefore given failure to culture any seemingly appropriate organisms, and struvite crystals in a urine or stone, or the signal of high pH (>8) and high ammonia means one must culture more until the causative organism is found.
Treatments that Inhibit Urease
Acetohydroxamic acid can be used in patients and inhibits urease thus stopping the process of struvite production. A trial of 94 patients showed stone growth in 17% of treated vs 46% of controls, a significant difference. However side effects were intolerable in 10 of the 45 treated patients.
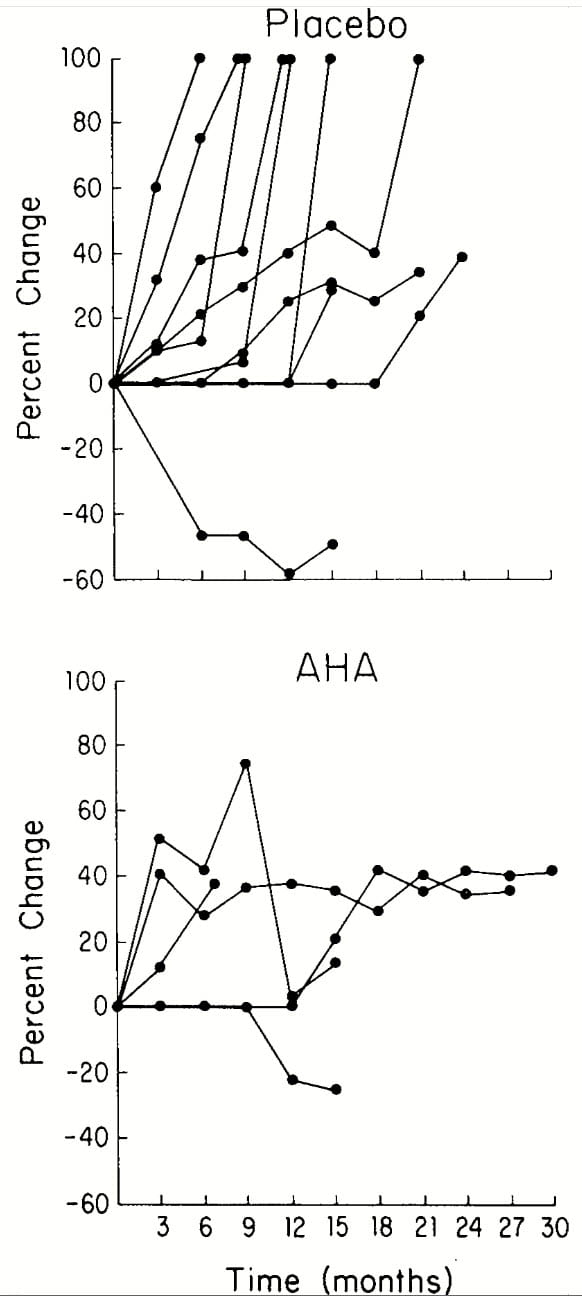 A smaller trial of superior quality compared 20 treated to 19 untreated struvite stone formers in a randomized double blind design over about 16 months. A change of 100% means new stones formed. All values are stone surface area determined by planimetry from plain abdominal radiographs. Changes below 20% are not shown, meaning the AHA group had few episodes of stone growth and no new stones.
A smaller trial of superior quality compared 20 treated to 19 untreated struvite stone formers in a randomized double blind design over about 16 months. A change of 100% means new stones formed. All values are stone surface area determined by planimetry from plain abdominal radiographs. Changes below 20% are not shown, meaning the AHA group had few episodes of stone growth and no new stones.
A third RCC trial in 210 patients with indwelling catheters because of spinal cord injury stones that AHA increased time from catheterization to 15 vs. 9 months in controls. At 12 months stone growth occurred in 33% of AHA treated vs. 60% of controls. Patient loss was high in both groups (62 vs. 31%).
Whole new classes of compounds are emerging to inhibit urease in Helicobacter species – that promote peptic ulcer, and gastric inflammation and cancer. The market for drugs here is far greater than kidney stones, but stone formers may benefit. Here is a recent paper about a candidate molecular class, and below the abstract a group of related papers – of interest more to physicians and scientists. For those few with detailed knowledge and interest, here is a recent paper concerning strategies for producing novel inhibitors.
My own experience with AHA is unpublished because scattered and modest. I have used it with success but found as many have that patients disliked its side effects. They include headaches as a special problem. Likewise there is some increased risk of venous thrombosis and pulmonary embolism – not common. Even so, I favor using the drug in patients with new growth of stones after surgery, patients with small stones and signs of urease activity, and after surgery. Within these general classes use should be tailored to the specific situation.
Urease as a General Virulence Factor
Many organisms can cause struvite stones because they possess urease. Some are hard to culture. Struvite stones have possible relationships fo autoimmune diseases.
Wide swathes of microorganisms produce urease that confers survival advantages on them and on ourselves pain and sorrow. It is found in MRSA, Helicobacter species (that use the ammonia to neutralize gastric acid), M. tuberculosis and bovids, and Clostridium perfringers. Although the common E Coli usually do not express urease, it is found in pathogenetic O111, O157:H7, O145, and O26 strains that often produce severe disease. Apart from Proteus, urease can be expressed by urine pathogens that include Klebsiella, Pseudomonas, Providentia, and Morganella strains.
The urease enzyme itself is a chemo-attractant and has been linked to auto antibody diseases such as RA, in association with P Mirabilis infection.
I mention all this in passing to provide a perspective view of infection stones. They seem one corner of a much wider landscape in which micro organisms play complex roles in human disease simply though their desire to persist and multiply on the same planet and in the same places as ourselves.
Struvite Stones May Produce Unusual Symptoms
Unlike common stones, infection stones themselves usually do not cause renal colic because of stone passage.
More commonly, they cause vague flank pains, symptoms of urinary tract infection, malaise, fevers of unexplained cause, hematuria. One needs to be aware and look for these stones. Prior stone passage is a risk factor, and especially if surgery was needed. Not rarely, struvite stones may be the first kind of stone for a patient, so suspicion of stones is low.
A strong ammonia smell is a so important, if not most common clue. Although urine can normally contain considerable ammonia, it is as the ammonium ion, that has no odor.
Another clue is pH above 8. Kidneys do not produce urine above pH 8 because their bicarbonate buffer will not sustain it (bicarbonate recombines with itself to produce carbonic acid).
Prevention and Surgery
Common sense in medical and urological practice perhaps most matters. Indwelling catheters are a prime hazard so their use is limited in time by all fine clinicians. Antibiotics for urinary tract infections need to be as in all clinical use – time limited and focused on proven infections because many urease producing bacteria are excellent at producing antibiotic resistance factors. All this is routine.
Kidney stones of any kind are a powerful risk factor because they involve urological procedures that each offer some risk of colonization. Moreover, obstruction by a passing stone can promote infection via stasis of urine in the renal pelvis and ureter. So stone prevention, calcium or uric acid or cystine or any other kind is a prime defense against the more complex struvite stone.
Treatment of struvite stones usually requires percutaneous nephrolithotomy. The most crucial element is to remove as much of the infected material as possible, because such stones are infected foreign bodies within the kidney. How to manage antibiotic use in the surgical situation is beyond the scope of this article, and requires one more detailed and specialized I hope to obtain from an interested urological colleague.

I have had the opportunity to find struvite stones in two patients who had no signs and symptoms of urinary infection. The stones analysis was done by infrared spectrophotometry. The patients were with hypocitraturia.
Unfortunately I had to prescribe citrate but I provided to acidify urine. Is medical therapy with urine acidification useful? Are there other therapeutic possibilities? Can stones slowly break down in an acidic urinary environment?
Hello Dr Piergiorgio, Given the odd character of the struvite crystal it is not common for human kidneys to produce them – there is not enough ammonium ion available along with high pH. May I ask if the urine pH was high and the ammonia level as well? If so, the urease organism may be fastidious and very difficult to culture. Might I suggest measurement of pH and ammonia? Best, Fred
Thank you so much for publishing this very informative article. I live overseas. In September 2020, I went to the urologist and they found a staghorn stone in my left kidney from an ultrasound and CT scan. I took antibiotics for 10 days or so and then took low-dose antibiotics for a year. (I was supposed to have PCNL surgery, but I had asked if it could be postponed because of the COVID situation). In September of 2021, I had a PCNL; however, I lost blood and had to have a transfusion. The Doctor was not able to take out all of the stone due to this. The stone that was removed was analyzed and was a struvite. So in December of 2021, he did a ureteroscopy with laser lithrotripsy to try to get rid of the remaining stones. He said the stones were soft and they turned to powder from the laser. So the stone did reduce. However, evidently not all of the “powder” came out. It appeared to start growing again. I have been on antibiotics almost over 2 years, organisms were echoli or candida albicans. In January of 2022, I had to take 14 days of IV Invanz, because the culture sensitivity came back that I was resistant to many of the antibiotics. There was a break of not taking any antibiotics or Diflucan from February to July (about 5 months). In August of 2022, I was having symptoms and tested again and had echoli. SInce then I have tested 4 times and the organisms have been echoli and citrobacter freundil, and the last two times were kiebsiella pneumoniae. I am needing to have another surgery soon, because my understanding is that if the stones are struvite, the infections will probably not go away. I went to the ER a couple of days ago and my WBC in urine had increased from 80 to 1820 and the RBC from 10 to 34 in 6 days from the last culture (I had just started taking Furolin the morning before I went to the ER).I noticed on your article that you say usually for struvite you do PCNL. My doctor had recommended me to either have Shockwave Lithrotripsy or what I had last time (Ureteroscopy with laser lithrotripsy). The stone is not as big as before the first time I had the PCNL, when it was a staghorn. What do you recommend? Also, is there anything I can do to prevent this from happening again? Thank you.
Hi Kathy, It is a serious problem. Your physicians seem to be doing what they can. Do you think they might want you to be seen at a specialized kidney stone center? Perhaps it is time to consider that. Regards, Fred Coe
Thank you Dr. Coe. I’m not sure what a specialized kidney stone center is. I am currently in Jordan, but I am from Illinois (Near St. Louis). Currently, I am seeing a urologist, and an infectious disease doctor (who is taking the lead on the infection/antibiotic situation). I am also going to add a nephrologist. Do you or a urologist do internet telehealth sessions to get a second opinion?
Hi Kathy, If you are in Jordan or Illinois I can review things for you via telehealth, and try to get you what you need. My secretary is Banita WIlliams, US 773 703 1475
Regards, Fred Coe
Good afternoon-
I have a couple questions about management (and prevention) of struvite stones in children who perform CIC and have bacterial colonization, high urine pH, and form struvite stones.
First, do you feel that bladder irrigation is an effective management strategy to reduce stone recurrence in these cases?
Second, is complete stone removal necessary in order to fully clear potential urinary infection and prevent future recurring cycle of recurrent UTI/struvite stone formation? Or is it possible to clear bacteria fully with stones still present?
Third, is acetohydroxamic acid a promising treatment for these patients, or do the risks of side effects outweigh the potential benefit? I know there is limited experience in children, which is the population I care for specifically. Is treatment with this medication ‘indefinite’, given their chronic urinary risk factors, or is it just a short term treatment option?
Also, is this acetohydroxamic acid ever used solely to lower urine pH- such as in the case of similar patients who perform CIC, have high urine pH as a result of bacterial colonization, and form CaP stones (not struvite)? If not, are there any available medications to lower urine pH in children who catheterize?
These are the two subsets of patients I struggle with the most with regards to limited management options available to offer.
I appreciate your expertise and have found this database unbelievably helpful in caring for children with stones, so thank you for sharing your knowledge with us in this way.
Hi Dr Skilling, You have noticed my reticence about treatment options as trials are seriously wanting. I do not believe trials support bladder irrigation or complete stone removal but as these are surgical trials I perhaps have missed them. AHA inhibits urease, so if urease is raising urine pH the drug will indeed lower it. Absent urease as the mechanism of high urine pH AHA is not expected to be effective in so doing. In urease driven high pH and struvite stones AHA is often tolerable as it does help improve a very serious and otherwise relentless problem, so I have used it and will continue with it under those conditions. In children with high pH and urinary infection but without evidence of urease as a mechanism (pH is high and urine ammonia is low) I do not think the bacteria are the cause of the high pH and would consider possible renal acidification defects as possible cause. Regards, Fred
Hello Dr Coe, Thank you for such informative articles. I am female, 64 and live in France. A routine blood test on 17/04/2023 showed normal results for everything except eGFR at 50.6 and calcium slightly raised at 2.7 mmol/L. Previous eGFR in 2021 was 63.7. My general practitioner thinks I may simply need to drink more water but I am worried there may be more going on, given my history of struvite stones. I first had problems with my left kidney in 1962 when I was only 3 years old. Surgeons were apparently surprised to find a staghorn calculus – which broke during removal. In 1978 (when I was 19) that same kidney had to be removed because it was completely blocked by stones (presumably struvite again) and an abscess had developed on the outside. In 1991, I developed a psoas abscess on my left side, the cause of which was never established but doctors concluded might have been a legacy from the previous kidney trouble. I am not overweight, do not have hypertension, diabetes, high cholesterol or any other risk factors for CKD. I exercise daily, eat a balanced diet, average 1 glass of wine per day & drink about 1.5 – 2L fluids per day. A urine test has come back negative for infection, however albumin, ammonia & PH were not tested. In addition to another urine test, do you think it would be sensible to push for a CT scan asap to establish the presence or otherwise of stones that could be affecting kidney function? Although my GP has referred me to a nephrologist “for reassurance”, the earliest available appointment is end October and I feel it might be important to get some more information before then. While I don’t want to overreact, if my right kidney is heading in the same direction as the left one, I’d rather take action before it’s too late. Best regards, Lesley.
Hi Leslie, I do think a CT is important given your long history of infection stone. Likewise urine ammonia and pH are simple measurements and could help exclude urea hydrolysis. But your elevated serum calcium is important – potentially – as a sign of primary hyperparathyroidism. It could alter kidney function and is curable via surgery. Long standing secondary hyperparathyroidism from reduced kidney function could give rise to autonomous gland cells that no longer regulate correctly and are raising blood (possibly urine) calcium. Because serum PTH could be high simply from the prior loss of kidney tissue everything depends on serum calcium values – fasting and in the morning. Perhaps your regular physicians might think this is a worthwhile idea to pursue. Regards, Fred Coe
Hi Dr Coe, I’m sorry it has taken me until now to get to see your reply, for which many thanks indeed. I have arranged for a CT at the end of this month and will ask my doctor for the further tests you suggest. I really appreciate your time and advice. Best regards, Lesley
Hi Dr Coe,
Quick update: Since 5 June, a CT scan has shown no kidney stones or visible urinary tract blockage – which is a great relief. Urine: PH6 (ammonia not measured – not something they do here, apparently!). However, four, weekly, fasting & morning blood tests show hypercalcemia & PTH inappropriately normal: 1) Ca 2.67mmol/L – PTH 50.3ng/L; 2) CA 2.64 – PTH 52.9; 3) Ca 2.55 – PTH 44.0; 4) Ca 2.79 – PTH 52.4. Having read your great article and more material elsewhere, there seems, to me, no question that I have PHPT, which is most probably causing the low GFR. (If I’ve had longstanding secondary hyperparathyroidism, I think I would have known about it, and my kidney function would have been a lot worse to trigger it?) I shall discuss next steps with my doctor tomorrow. Assuming she agrees with the diagnosis, I hope to arrange a bone density scan and an operation asap. In the meantime, to try and help prevent calcium build-up in my kidney, I’ve been drinking around 2L of water this last month. I think you might normally recommend increasing that further (to 3L?), to avoid stones &/or calcification, but since my kidney is already struggling (eGFR currently averaging at 53.5), do you think that would help or hinder?
Dr Coe, I can’t thank you enough for pointing me towards PHTP. I don’t think my doctor would have bothered about the original high calcium level, had I not raised it with her following your reply on May 29. Best regards, Lesley
Hi Lesley, I am glad if I was helpful to you and your physicians. I am not clear about how or why the PHPT has lowered renal function apart from stones. In general your blood calcium values per se do not seem high enough to injure your kidneys so I imagine your physicians are thinking about the causes for reduced kidney function. High fluids while awaiting surgery is certainly prudent – about 3 liters /day should be safe and effective. Regards, Fred Coe
Many thanks once again. My doctor has managed to discuss my case with a nephrologist who has suggested some further blood and urine tests. At this point he thinks my reduced kidney function may simply be due to having only the one kidney, and that the parathyroid issue is separate. That would seem to gel with your thoughts above. I shall increase my water intake further. I shall leave you in peace now!
Best regards, Lesley
Hi Lesley, That seems fair. If there is any continuing hint of high serum calcium, pursue it relentlessly as PHPT is curable. Best, Fred
Hi doctor,
I have been trying to get in to your clinic for months but cannot get a call or reply back.
I would be a prime candidate. What started off as a calcium oxalate stone once or twice a year, turned into calcium phosphate stones (40 at one time).
Now I see the stones have changed again to struvite 20% and brushite 80%.
I need some one with your type of expertise as I am at a loss of hope.
My kidney function is rapidly depleting and everything the doctors try does not help.
How can I please get into your clinic.
I just completed multiple laser ureteroscopies as well as PCNLs in February, to ultimately be again riddled with huge stones, in the course of a 1 month period.
Hi Paul, Your email was sent to the people who schedule and I believe you will be getting an appointment with me or a colleague promptly. Sorry for the delay, Regards, Fred Coe
Hello Dr. Coe,
My sister came down with Transverse Myelitis a year ago. She is 71 and is paralyzed from the waste down.
She has to have an indwelling catheter, along with her bowel issues.
She is just getting over a sepsis episode, after at least 2 UTI’s over last 3 months. The Urologist did the
laser surgery and said all the bladder stones and those in the Ureta were soft [to his surprise] and easily
dissolved. What would you recommend her next steps to be for prevention of these infection and stone
tendencies for a neuroimmune illness person??
Hi Mike, As the article sadly reviews it is likely that struvite is forming from infection. Another cause in someone recently immobilized is rapid bone mineral loss causing calcium phosphate stones. That can be managed. I would analyse some of the material and find out what it is. Likewise her bones need looking after. Regards, Fred Coe
Possibly my condition seems similar to Paul Lechowitz than some of the other posts. My left kidney has stones, which are now two, I think, staghorn that are too large to pass. Thankfully, I’ve never had stones in my right kidney. Reading at this site has been so interesting, as I’ve always wanted to have a better understanding of this type of kidney condition. In addition to the staghorn stones in my left kidney, the tube close to where the kidney and tube meet is twisted, or a blockage. I asked if the tube is totally blocked, and I was told the blockage wasn’t total. At this time, I have a stent. Having never had health issues other than some stones in my left kidney, it was a change with blood pressure becoming very high that brought forth the kidney discovery. I’m assuming a connection, although I wasn’t told that was the reason for a change in blood pressure. I would like to have a better understanding of all aspects.
Hi Patricia, If you indeed have a staghorn type struvite stone – from infection – and a stent you have a serious problem. It is surgical and I am not a surgeon. But I presume your urologist is planning a definitive surgery to try to remove the large stone. Although I have every reason to believe your surgeon is excellent perhaps you might want to obtain a second surgical opinion from a kidney stone center. The usual surgery, a percutaneous nephrolithotomy is complex and often several opinions are obtained before it is undertaken. Your comment about the junction of your ureter with the renal pelvis suggests that an underlying problem led to the stones on that side. Likewise assuming you are infected with bacteria that are producing such large stones, there is risk of infecting your other kidney. As for blood pressure, it can rise with stone obstruction. All this is from a distance, I do not know the details of your case. The only thing I can be certain about is that it is a complex situation and in general experts consult with one another about the best solutions. Regards, Fred Coe
Bilateral staghorn
24 hr labcorp litholink results prior to surgery-
All normal except:
SS CaOx 2.54, Ph 7.64, Nh4 69
Already had 3 PCNl in the past month for left side, one more next week to try to get 90 % clearance. Right side PcNL in May should only require one.
1st Stone analysis: Carbonate Apatite (Dahllite) 100%
2nd and 3rd surgery stone analysis same results: 90% calcium phosphate and 10% minor components
Background: 52 year old female, Breast cancer 18 years ago, ovaries removed.
Increased UTI’s last 2 years… no estrogen. Did pass a stone in 2012, no issues until last six months with intermitted blood in urine
Any help would be appreciated
Hi Rose, These facts say it all: ‘ Ph 7.64, Nh4 69’. They mean that production of ammonia by bacteria is raising urine alkalinity, and you indeed in part form stones because of infection with urea splitting bacteria as in this chapter. The crystals noted form only at very high pH (alkalinity) and I suspect other stones will include struvite (magnesium ammonium phosphate). Calcium oxalate has no role in your condition. Essentially your stones arise from and perpetuate infection and treatment is a very complex medical surgical enterprise. From this distance I cannot say anything specific. But given the 3 PCNL procedures I imagine your physicians have already considered the idea of seeking consultation for you at a geographically accessible medical school stone program – perhaps you are already at one. Regards, Fred Coe
Thank you. We haven’t discussed treatment yet since the goal is removal. I had 4 PCNL on left side with 80% clearance and will have one surgery on right side in a few weeks. I’m being seen at Cleveland Clinic. Do you have any recommendations of who to see? Currently with urology for removal. I did start estrogen to address UTI’s the reoccurring UTI’s in the past 18 months. Could be a contributing factor. Any guidance of who to see for would be appreciated. Thank you
Hi Rose, I looked on their kidney stone page and saw mostly urologists. But if you call before hand and ask for a medical person who is interested in stone prevention I imagine that person can also see you during your stay. Best. Fred Coe
Hi Dr. Coe,
I’ve been dealing with a possible Proteus co-infection UTI issue for 2 weeks.
I am a physician by the way, so feel free to use medical jargon if needed.
Week 1: the UTI responded to nitrofurantoin initially for 3 days, but then I started having some burning in the urine around day 4 only in the mornings.
Week 2: I completed the nitrofurantoin course and woke up at 1am with burning pain with urination. I got tested and prescribed levofloxacin. I took it for 3 days while awaiting urine culture results.
My UA was ph 6.5 and no blood, no crystals. The urine culture came back positive for Proteus mirabilis. I was not tolerating levofloxacin well so it was switched to ampicillin. The drug was shown to be susceptible to both antibiotics.
Question:
How likely is it that I may have any struvite stone formation from this bacteria?
I have had recurrent UTIs, but they have all responded to nitrofurantoin in the past, so they likely weren’t Proteus? (which to my understanding does not respond to nitrofurantoin)?
I do not want to deal with recurrent UTIs from infection stones, so I’d like to know whether I should get checked. I haven’t had flank pain, hematuria, but I see in your article that since these stones don’t pass like other stones, you might not see these symptoms. Thank you so much! I look forward to your thoughts.
Hello Doctor, I would get a stone protocol CT to check for any fragments. Hopefully you are colonized but the bacteria are not active – the article details the amazing life cycle of Proteus. Given no stone, I would opt for an appropriate bactericidal drug in full dose for at least 6 weeks in hopes of ridding yourself of this organism before it begins to activate urease, swarm, and cause real trouble. If there is a stone, I would opt for the most skilled urologist around and URS aimed at stone free, then use the above. Best, Fred
Hi Dr. Coe,
One important detail I left out from my previous comment.
April 29, 2024 I had a UTI which responded to nitrofurantoin.
May 12, 2024 I got another UTI which initially responded to nitrofurantoin, then symptoms came back and the culture came back positive for Proteus mirabilis.
My question is the same, which is how likely do I have infection stones? Should I nudge for imaging?
I did not want to leave out my UTI that occurred two weeks prior to my current 2 week UTI in case it altered your thought process. Thank you.
Hi Doctor, I am overwhelmingly in favor of a stone protocol CT, no question about that. Hope it is negative! Fred
Dr. Coe, My 84 year old bed ridden sister in a nursing home had an undiagnosed UTI in November of last year which resulted in her going sepsis. In May of this year the same thing happened, but this time we were informed that she had kidney stones in both kidneys. The right one has 4 stones because of the make up (ESBL) there is too much risk to use ultra sound and a surgical procedure would be to hard on her. The left kidney had a stone blocking the ureter. A stent was put in. August 7th we arranged transport to go back to the hospital to have it changed, which is a difficult thing to do since she is so rigid. August 18th the stent came out. We then went back to the hospital to have it put back in. This time the doctor couldn’t even maneuver the scope to get it back in place. He suggested a nephrostomy, but he said he wouldn’t recommend it. Is there anything you could suggest for us to do, considering age, arthritis, dementia and aphasia. Thank you for your time Ann Bartosz
Hi Ann, I am so sorry to hear about this in your sister. I suspect the stones are from infection and perpetuating infection and believe her physicians are right in their opinions – from what I can tell from so far away. As a last resort they might consider acetohydroxamic acid which inhibits the urease enzyme and may palliate things for a time – but the drug is itself difficult. Fred
Hello Doctor
My son was already a teenager when I adopted him. Rescued him from a nightmare mother who adopted him when he was young. He has been paralyzed with a spinal cord injury since he was 3 yrs old and does get frequent UTI’s from constant catheter use- his last UTI the hospital doctors showed him one kidney stone (from imaging) very small and said it will pass sometime. The doctors also claimed he had very rare and multiple strains of bacteria and after a week or two of trying to figure out an antibiotic that could get rid of the bacteria sent him home with IV antibiotics. He did finally pass the kidney stone. Before It passed his urine had a light ammonia odor and disappeared as soon as it passed. Since the hospital in our area is known for killing ppl and NO ONE has anything nice to say – very low competent staffing…. My son is greatly hesitating to go back and he outgrew the Competent CHOP doctors… my real question is how much blood in urine is normal? It seems a lot of blood has been in his urine since passing a small dark colored pear shaped kidney stone. It has been 2 days of blood in his urine (also some blood clots) and I don’t know if it’s normal or urgent for him to get medical attention. And unfortunately I no longer have my microscope to look closer at the kidney stone to really know exactly which type it is. Because he is paralyzed he doesn’t feel pain but said he had discomfort on his right side before the stone passed. He was nauseous for about a day as well. He WANTS me to tell him he doesn’t have to go back to the nightmare hospital… but the internet says conflicting arguments about blood in urine is normal when passing a Stone and seek immediate medical attention if there I blood in urine when passing a stone.
Hi Megan, The blood is certainly from the stone. It is worrisome only if massive enough to cause systemic problems. CHOP is in Philadelphia. He could get his care at U Penn – they have a stone program. Regards, Fred Coe