Kidney stone types
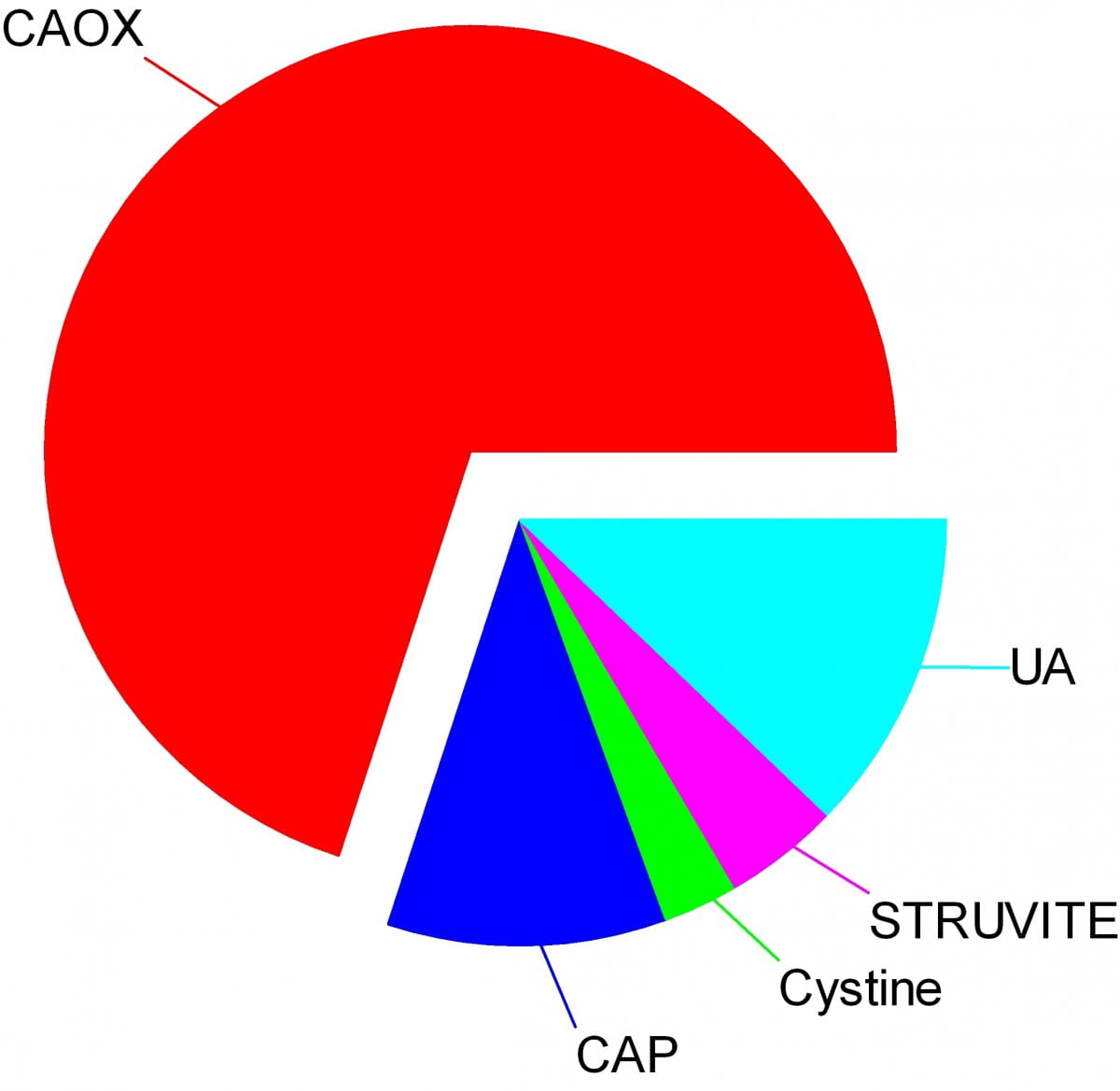
Crystals make stones and their names signify the kidney stone types. Here are the names of the crystals that make the stones: CAOX, Calcium Oxalate; CAP, Calcium phosphate; UA, Uric Acid; Cystine; Struvite.
The wedges on my pie chart show the relative abundances of stone types in our large population of stone forming patients. Calcium oxalate stones predominate by a wide margin in our clinic and in all others I know of.
The names, matter because the whole science of stone prevention focuses upon stone crystals. Each kidney stone crystal creates its own unique illness and requires specific treatment. That is why we name stones by the names of their crystals and why when stones are analysed the results are reported by these very same names.
Being a bold and rather large graphic, the featured picture does what I intended, brings the main facts into view as, at a circus, the great animals and the small animals circle the ring by way of an introduction. Come. I will show you all the common stones, like at a fashion show, or a circus parade. You can watch as they go by and remind yourself, or wonder, which ones might have been yours.
Here they are.
Which type do you have?
You might think your doctors know what stones you have formed, but don’t rely on it. People move, doctors move, health records are far from ‘all electronic’. That stone report from 4 years ago could lie in a dusty filing cabinet, your new doctors unaware it exists. Worse, it could hide in a dresser drawer and you forgot it you put it there. Perhaps even more worse, the stones might stay in that drawer, never analysed at all. Find the stones, find missing reports, urge analysis by your physicians. They can help you most if they know your stone analysis.
When they do not know, physicians can still mount prevention efforts but with less focus and probably less effect than when guided by a knowledge of the crystals. So always seek treatment. If a stone comes along the way, make every effort to get it analysed.
Why should you care to know all this?
Because you will conduct much of your own treatment, and over many years.
Since stones tend to recur, prevention requires treatment over long periods. These treatments work by altering urine chemistry in a direction that minimizes the risk of forming crystals. Such altering of urine chemistry requires control of fluid intake, lifestyle, and diet, and sometimes additional use of medications.
Just as the sailor who aims along a chosen track against the random, misdirecting, confusing sea and air maintains a constant way in proportion to that skill which comes from knowing the way of the boat, patients who aim to keep a certain kind of condition in their urine despite the demands and temptations of the world do so, I believe, in proportion to skills that come from knowing how their work and lives and foods affect their bodies, and how those crystals form which they so much desire to prevent.
Put another way, knowledge is power.
Why is this article so long?
I wanted to put all five main types of kidney stones. That makes a long story. But probably you will care to read about only your own type.
I should mention here, to save a lot of confusion, that stones often contain mixtures of crystals.
The pie chart refers to the most common crystals in a stone, for which the stone is usually named. Much of the time, minor crystal components are not crucial, but sometimes – to jump forward a bit – they are. Even a trace of struvite or cystine, for example, can have great diagnostic importance.
Calcium stones
Calcium Oxalate Crystals
In the great circle atop this page article, the calcium oxalate stone, being most common, occupies a lion’s share of the space. 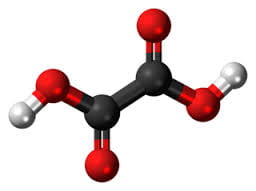
The calcium oxalate crystal forms when calcium combines with oxalic acid. Oxalic acid (at left), a dead end waste product that the kidneys remove, contains two carbon atoms (the large black spheres), four oxygen atoms, and two hydrogen atoms (silver).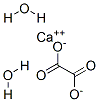
At the acidity of urine, the positively charged hydrogens leave their negatively charged oxygens. As a result the oxalate molecule carries two negative charges. In the figure at right one negatively charged oxygen attracts the hydrogen of a nearby water molecule (H – O -H) while another attracts a positively charged calcium atom.
You can imagine how another oxalate ion (the name for a charged molecule in water) could attract the same calcium, or another calcium atom attract the bottom oxygen on the oxalate molecule so the chain extends and makes a crystal. You can see more about this in a video I made. Broadly speaking – though my more expert colleagues may bridle at such a simplification – the calcium atoms and oxalate molecules combine by the attraction of their opposite charges.
The calcium oxalate kidney stone comes in two varieties, calcium oxalate monohydrate and calcium oxalate dihydrate. The former are harder and therefore more resistant to fragmentation by lithotripsy. Likewise, the former appear more often when elevated levels of urine oxalate are present.
Calcium oxalate stone formers
From Systemic Diseases
Sometimes this kidney stone arises from a systemic cause, like bowel disease, primary hyperparathyroidism, or primary hyperoxaluria. Only physicians can establish that a known disease – like bowel disease – is the cause of stones. Only physicians can discover underlying primary hyperparathyroidism as a cause of stones. Patients cannot do much for themselves except provide as complete a medical record as possible.
Idiopathic
Most of the time this kidney stone arises simply from the interplay between inheritance, diet, and aspects of daily living. We call such patients idiopathic calcium oxalate stone formers, from Greek ἴδιος idios “one’s own” and πάθος pathos “suffering”.
Even though physicians discover the links between daily living and stone production, and select those changes that can prevent new stones, patients themselves must create and maintain those changes. I believe patients can so this in proportion to how well they understand what is needed, and why. When changes in daily life are not enough, physicians add medications, so even then patients remain active therapists for their own disease.
Stones usually form on kidney surfaces
About one million nephron units make up a normal adult kidney. The calcium oxalate kidney stone type does not grow in the tubules of the nephrons but ‘outside’ them, on the surfaces of the renal pelvis where final urine collects and drains through the ureter to the bladder. Here is a video that shows how they can form.
Calcium phosphate crystals
Phosphate ion and urine pH
Calcium phosphate stone crystals form when calcium atoms combine with phosphoric instead of oxalic acid and produce the calcium phosphate kidney stone.
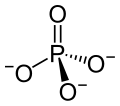 Phosphoric acid is simply a phosphorus atom (shown as the ‘P’ in the line drawing to the left) with 4 oxygen atoms bonded to it. One oxygen atom has two lines for its bond to phosphorus; this oxygen cannot provide any charge with which to bond calcium atoms to make a crystal. The other three have ordinary bonds that are shown by a line, and a dashed and solid arrow. These two arrows mean simply that the oxygens lie above and below the plane of the paper – so if you built the molecule with sticks and balls it would have a three dimensional shape.
Phosphoric acid is simply a phosphorus atom (shown as the ‘P’ in the line drawing to the left) with 4 oxygen atoms bonded to it. One oxygen atom has two lines for its bond to phosphorus; this oxygen cannot provide any charge with which to bond calcium atoms to make a crystal. The other three have ordinary bonds that are shown by a line, and a dashed and solid arrow. These two arrows mean simply that the oxygens lie above and below the plane of the paper – so if you built the molecule with sticks and balls it would have a three dimensional shape.
One of the three negatively charged oxygens never has a hydrogen on it in urine but only in exceedingly acidic solutions. A second charged oxygen is always occupied by a hydrogen atom in urine.
This makes the third oxygen, variably occupied by a hydrogen in urine, a tie breaker.
In a urine of average normal acidity (pH around 6), most of the tie breaker oxygens have their hydrogen leaving the phosphate ion only one negative charge. Not enough to make a crystal.
When the urine is abnormally alkaline (pH above 6.3 or 6.5), the variable oxygen becomes charged so the ion has two negative charges that can combine with calcium to make crystals. For this reason the calcium phosphate kidney stone tends to occur in people who produce a more alkaline urine than those who produce calcium oxalate kidney stones.
Brushite vs. hydroxyapatite
Much like calcium oxalate, calcium phosphate crystals begin simply as one to one pairings of doubly negative phosphate ions with doubly positive calcium atoms. This initial crystal is named brushite. Brushite, which is an equal mixture of calcium and phosphate ions, can convert to hydroxyapatite (HA), which has a more unbalanced proportion of calcium to phosphate. Hydroxyapatite crystals make bones hard.
Because less soluble than brushite, hydroxyapatite cannibalizes brushite. The organic molecules in urine modify this process.
Calcium Phosphate stone formers
From Systemic diseases
Primary hyperparathyroidism and renal tubular acidosis raise average urine alkalinity (higher urine pH) and foster calcium phosphate kidney stones. Many uncommon genetic diseases do the same.
Idiopathic
Idiopathic calcium phosphate stone formers share a common set of traits. Perhaps because urine contains far more phosphate than oxalate, they form more frequent and larger stones than idiopathic calcium oxalate stone formers. Often the stones originate as crystal plugs at the terminal ends of the kidney tubules. More crystals deposit over the end of the plug open to the urine, to make the final stone. Crystal plugs damage the cells that line the tubules and cause local scarring.
Uric acid stones
Uric acid crystals
Structure and charged sites
A breakdown product of DNA and RNA, uric acid forms crystals in abnormally acidic (low pH) urine. Obese and diabetic people, those with gout or kidney disease typically produce abnormally acid urine. I know how the urine becomes acid, but leave it for elsewhere on the site.
Uric acid, the molecule we are interested in here (shown to the far right), has two linked rings made of carbon atoms (they are at the angles where lines join), with 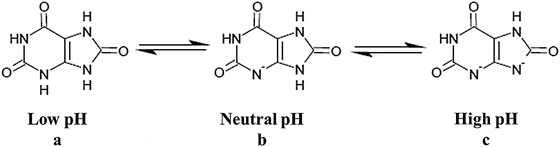 interposed nitrogen (N), oxygen (O), and hydrogen (H) atoms.
interposed nitrogen (N), oxygen (O), and hydrogen (H) atoms.
This molecule has only two charged sites, the nitrogen atoms at the bottoms of the rings. In urine of pH 6 or so, one nitrogen lacks its hydrogen and therefore carries a single negative charge. In more alkaline solutions both nitrogens lack hydrogens, but urine does not normally achieve such alkalinity (pH>8).
When urine pH is low (<5.5) and both nitrogens have their hydrogens, the molecule lacks any charged site, so water can no longer find a hold on the molecule. It crystallizes. It simply leaves the water as water droplets themselves form from the high and vaporous late afternoon clouds and fall from the air as the warm rains of springtime.
Relation to water
Water molecules are each a single oxygen atom (large ball) bonded with two hydrogen atoms (small balls) as in this picture from Wikipedia. The hydrogen side has a positive, the bare side of the oxygen a negative charge. So water molecules link to each other, 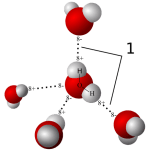 positives to negative surfaces, to make up the clear and seemingly continuous fluid we drink, swim in, and hold up umbrellas to keep off of us when it rains. They link by charge at angles, shown by the number ‘1’ so as to make up a three dimensional macrame.
positives to negative surfaces, to make up the clear and seemingly continuous fluid we drink, swim in, and hold up umbrellas to keep off of us when it rains. They link by charge at angles, shown by the number ‘1’ so as to make up a three dimensional macrame.
To be ‘in solution’ means to have some charge to which water molecules can link up with by attraction. Calcium atoms are positive and become surrounded by a shell of water molecules facing it with their bare negative surfaces. Oxalic and phosphoric acids have negative charges and are surrounded by water molecules pointing their positive or hydrogen sides to them.
Uric acid at neutral pH has its one negatively charged nitrogen water can grasp. But when pH falls, and neither nitrogen has any extra charge for water to bind with, how can the molecule remain among the water molecules? It cannot. The molecules stack into solid crystals and fall from solution.
Uric acid stone formers
The stones can be orange – red, large, and numerous
The stones can be red or orange because uric acid crystals absorb hemoglobin breakdown products that are red – orange pigments in urine. Sometimes uric acid crystals pass in urine as a red orange gravel.
Uric acid does not have to connect itself to some other atom or molecule to make a crystal, in the way that calcium must bond with oxalate or phosphate ions to make calcium oxalate or calcium phosphate crystals. When pH is low enough to extinguish its charge, uric acid can crystallize very fast, in seconds, and pass as an orange gravel in the urine. If retained, such crystals can grow rapidly into large stones. Because there is much more uric acid in urine than there is oxalic acid, uric acid stones can grow very large and rapidly. Some fill up the entire collecting system of the kidney.
Urine pH controls stone formation
But because the whole process depends almost completely on the acidity of the urine, uric acid stones are very easy to treat. Just a modest amount of supplemental alkali will make the urine of almost any patient alkaline enough that the hydrogen atoms are removed from the one crucial charged nitrogen. Water can bond there so uric acid remains in solution. Because so simple, treatment prevents stones with certainty. Relapse need never occur.
Mixed stones require special care
Unfortunately, however, stones commonly contain uric acid mixed with calcium oxalate. In this case, one needs to track down the cause of the calcium oxalate stones as well as make the urine alkaline enough to stop uric acid stones from forming. Calcium phosphate crystals mix with uric acid only rarely, because it takes a rather alkaline urine to remove the hydrogen atoms from phosphate so it has two negative charges and can bind efficiently with calcium atoms. At that higher pH, uric acid will have its charge site and remain in solution.
Struvite stones
Urea and the planet
Kidneys cannot make struvite. Bacteria make it. Not all bacteria, either. It takes bacteria that normally thrive in the soil, and they do it for ancient and compelling reasons. These bacteria produce the kidney stone named Struvite after Heinrich Christian Gottfried von Struve (1772–1851).
Animals deposit urea (at left) all over the planet when they urinate. Plants cannot use it.
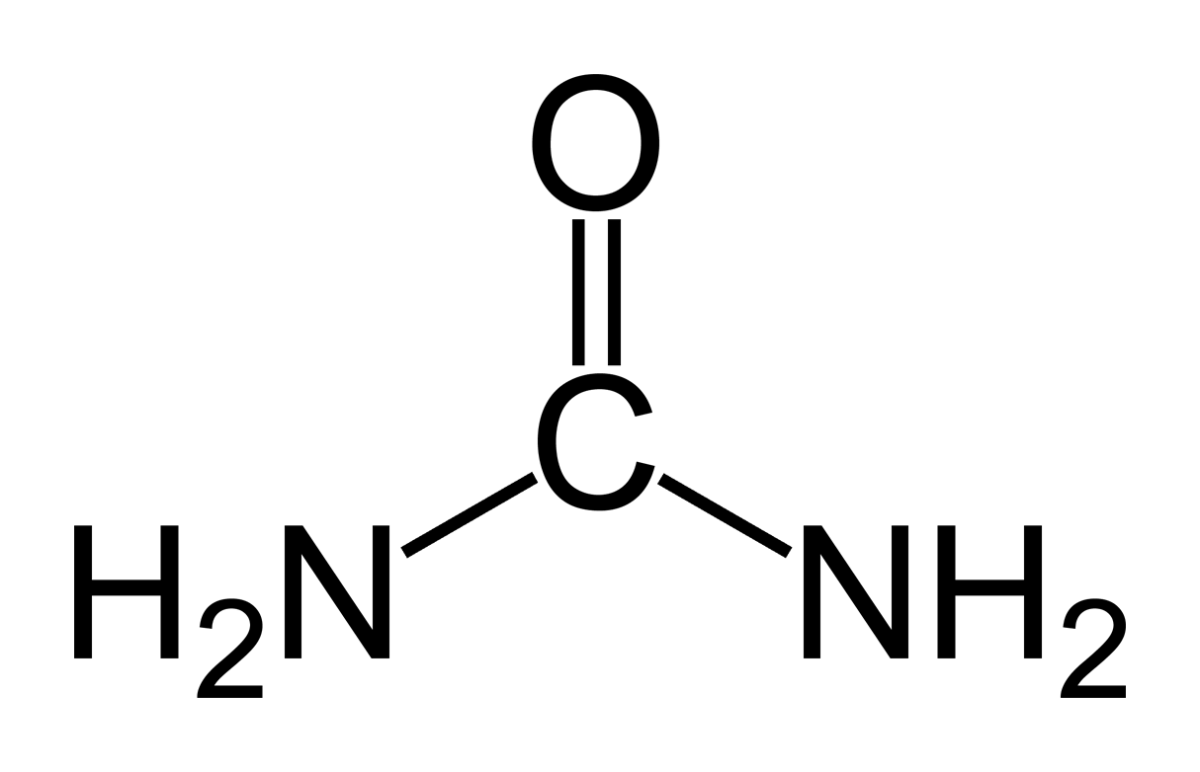 Like oxygen, nitrogen is an essential for life yet dangerous. It is integral to proteins, DNA and RNA. As these molecules are broken down and remade, some of their nitrogen slips by and can form poisonous compounds unless caught up in safe waste products. Of these, the main one, urea, contains 2 nitrogen atoms bound to a single carbon atom (‘C’ in the picture to your left).
Like oxygen, nitrogen is an essential for life yet dangerous. It is integral to proteins, DNA and RNA. As these molecules are broken down and remade, some of their nitrogen slips by and can form poisonous compounds unless caught up in safe waste products. Of these, the main one, urea, contains 2 nitrogen atoms bound to a single carbon atom (‘C’ in the picture to your left).
Uric acid contains 4 nitrogen atoms (look back at the picture of it). Birds and reptiles excrete most of their nitrogen as uric acid; mammals like us excrete nitrogen mainly as urea.
As the animals of the world urinate on the soil, their urea brings nitrogen to plant roots, but the plants cannot use it. They cannot release the nitrogens from the carbon atom that holds them. Those soil bacteria that make struvite crystals have an enzyme, called urease, that can release the nitrogen for plants to use as their nitrogen supply.
So, soil bacteria with urease maintain the nitrogen cycle of the earth.
Struvite crystals
As they release nitrogen from its carbon in urea, the nitrogen takes up a proton making ammonia (NH3). Ammonia is a powerful alkali and takes up another proton.
As it does so, the working bacteria surround themselves with spheres of very alkaline fluid enriched with ammonium ion (NH4) that carries one positive charge. Soil magnesium ( an atom with two positive charges) and phosphate sans all of its protons (an ion with three negative charges) spontaneously form their triple salt: three negative phosphate charges, two positive from magnesium, one positive from NH4).
The crystals anchor the bacteria and help create a porous nitrogen rich soul good for plants to grow in
The struvite kidney stone
Why they start
Because urine is filled with urea, soil bacteria that get into the urinary tract can break it down to ammonia and create struvite from the magnesium and phosphate urine always contains.
You might wonder how soil bacteria get into the urinary system.
Because we eat them, with foods that are not cooked, and they become part of the intestinal bacterial population from an early age. In us and around us, they find a way into the urinary system, especially in women whose shorter urethra makes entry easier. No matter how skillfully used, any instrument put into the bladder can carry our personal soil bacteria with it.
What they do
Because they live among molds and fungi, soil bacteria easily mount resistances to antibiotics, so antibiotics given for a urinary tract infection will tend to kill sensitive bacteria and select out those that can resist them.
Soil bacteria can produce struvite stones de novo, or infect calcium stones to produce a mixed stone. Either way, struvite stones are infected by their very nature. They can become huge. Their bacteria can injure the kidneys, even enter the bloodstream and cause sepsis.
Treatment is a mix of thoughtful surgery and selection of antibiotics after such surgery to kill bacteria that remain. If the stones are a mixture of struvite and calcium crystals, new calcium stones need to be prevented.
Cystine stones
Inherited kidney abnormality
 Lemon yellow with a sugary coating these form only in people who have an inherited kidney disorder called cystinuria.
Lemon yellow with a sugary coating these form only in people who have an inherited kidney disorder called cystinuria.
Although the kidneys function well, they permit abnormal amounts of four amino acids to enter the urine. Three do not matter that we know of. The fourth makes crystals and the cystine kidney stone type.
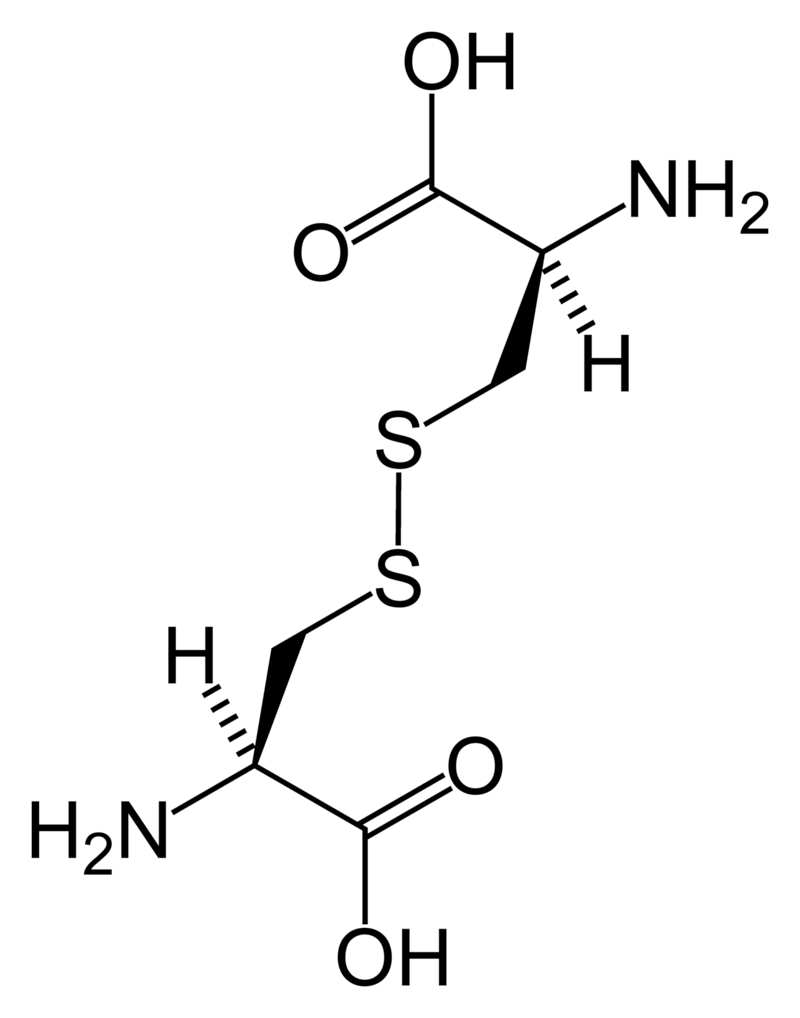
Cystine
Cystine (left) forms through the coupling of two identical amino acids – called cysteine – through their sulfur atoms (‘S’ in the line drawing).
Each cysteine contains two carbon atoms – not shown except as corners – bonded together (shown by the single long line that connects the two corners) as in oxalic acid.
One carbon atom has 2 oxygens bonded to it; the other has one nitrogen (which makes it an amino – nitrogen containing – acid), a hydrogen atom, and a sulfur atom. As for phosphate, the dashed and solid arrows simply mean the hydrogens and sulfurs lie above and below the plane of the page and a stick model would have a three dimensional shape.
Cystine Crystals
Cysteine itself is very soluble because the sulfur atom has an appreciable negative charge.
But the big, long cystine molecule has very little charge because the sulfurs bind to each other. So, like uric acid, cystine loses intimacy with water molecules and simply leaves the solution as crystals. Also like uric acid, the process is fast.
Cystine stones
Because people with cystinuria lose large amounts of cystine in their urine stones readily grow large, and fast.
Stones probably form in the urine itself. But cystine crystals can plug the ends of kidney tubules, as calcium phosphate crystals do, causing cell damage.
Since cystinuria is an inherited disease, stones may begin in childhood.
Effective treatment always requires very large amounts of fluids to dilute the urine. The few effective drugs resemble cysteine. Their sulfur groups bond with cysteine to form a ‘mixed disulfide’ more soluble than cystine. But their side effects can limit use.
Rare stones
Here and there we find patients who make uncommon crystals and require very special care.
Uric acid, as an example, can form odd crystals such sodium or ammonium acid urate, especially in people with bowel disease and chronic diarrhea.
Anti-viral drugs can crystallize in urine and form stones only recognized for what they are through stone analysis.
Very rare disorders of metabolism can produce molecules which crystallize in the urine, for example 2-8 dihydroxyadenine.
Although it can take a while before the right answer emerges, stone analyses will put physicians on the right track for these special cases.
The end of a very long post
That’s my parade.
The common animals and the rarer animals have gone by, and you have glimpsed the main ones, big and small.
The one point is what it was at the beginning. Each kind of kidney stone has its own ways, and treatment requires we know which one you have.
Likewise, for whatever that one may be, it is good to know as much about it as you can know. For long term prevention of stones is hard to come by and ultimately what the patience and and consistency of patients themselves matters most.
If you don’t know which stones you have made, find out.
Track down old reports and pull them together.
Keep copies and send everything to the doctors who care for you.
Fred Coe MD


Dear Mr. Coe,
thank you for your detailled presentation of the pathogenesis of the different kidney stones. What I still do not understand is why different types of acids (oxalat vs. phosphorus e.g.) are either charged in acidic environments whereas others are in more alkaline environments. Could you give me a brief explanation? Thank you and kind regards JW
Hi Jonas, oxalic acid has a pKa below 4, so it is always fully ionized. Phosphate has a second proton dissociation constant in the range of 6.8 (varies with ionic strength of course) and it is the divalent phosphate that combines with calcium to form stones. So from pH 6- the mean for human 24 hour samples – to 6.8 the abundance of available phosphate rises steeply. Uric acid has a pKa of about 5.3 for its proton and when protonated has a very low solubility in water so it crystallizes in acid urine. Regards, Fred Coe
Hi Dr. Coe,
There is a plethora of information in this website, much of which I’ve read. I appreciate your work on this website. Do you have any articles that you could point me to that have an emphasis on ulcerative colitis and calcium oxalate monohydrate stones? Most of what I have seen on this website are resources for those that have had idiopathic stones. I’ve had 3 stones in the past four months and am being seen by a Urology specialist. I think the stones are a symptom of my ulcerative colitis, however, my provider won’t know specifically until they get my 24 hour urine results back. I think I may have to go on a low sodium/low oxalate diet and would like to get ahead of the game with some knowledge. It is quite overwhelming at the moment.
Sam
Thanks,
Sam
Hi Sam, You are right, I did not do an article on this kind of stone former even though I have published an extensive paper on the subject. In UC dehydration and consequent low urine volume and pH are the main issues. UC does not raise urine oxalate as the small bowel is not involved. Your stone panel will tell you. A lot of patients in my paper formed stones before their UC diagnosis, which worsened with lower urine volumes. I will put up an article on this. Regards, Fred Coe
Hi Dr. Coe,
There is a plethora of information in this website, much of which I’ve read. I appreciate your work on this website. Do you have any articles that you could point me to that have an emphasis on ulcerative colitis and calcium oxalate monohydrate stones? Most of what I have seen on this website are resources for those that have had idiopathic stones. I’ve had 3 stones in the past four months and am being seen by a Urology specialist. I think the stones are a symptom of my ulcerative colitis, however, my provider won’t know specifically until they get my 24 hour urine results back. I think I may have to go on a low sodium/low oxalate diet and would like to get ahead of the game with some knowledge. It is quite overwhelming at the moment.
Sam
Thanks,
Sam
Hi Sam, You are right, I did not do an article on this kind of stone former even though I have published an extensive paper on the subject. In UC dehydration and consequent low urine volume and pH are the main issues. UC does not raise urine oxalate as the small bowel is not involved. Your stone panel will tell you. A lot of patients in my paper formed stones before their UC diagnosis, which worsened with lower urine volumes. I will put up an article on this. Regards, Fred Coe
Hello Dr Coe,
My name is Andrea and I am 43. My father is from Thailand so I was wondering if the last stone I had could be because of where my dad is from. I got my first stone when I was 25 when I finished my obligated service in the USN. After my first stone I would get more around 18 months later and each would be in some way worse than the previous. I passed them all until around 7 years ago when that stone popped a whole in the back of my kidney, (that is what the doctor told me) luckily with that stone I didn’t follow my normal procedure of denying it for a few days even though I knew I would have to go to the doctor because I started vomiting so much that I just went, which I guess was lucky because I ended up with a bacterial infection. The following stone was to big to pass on my own. At the beginning of this month I woke up in pain an knew it was yet another stone. I went in and had a ct scan where they found a 10 millimeters stone and another that was almost the same size. I had them removed in two different surgeries and they sent one in. The lab report came back and said that it was made up of 90 percent ammonium acid urate kidney stones 10 percent ca oxalate. I’ve been trying to see if I could find out more about it but haven’t been having much luck. Could you please explain it a little to me. I go back for a follow up in a couple weeks and just want to be prepared. Either way thank you very much.
Hi Andrea, I a answering all four of your queries here as they seem shortened versions of this one. Ammonium acid urate stones are unusual and arise typically from chronic diarrhea, laxative use, or some other GI problem. They require a very high urine ammonia level, yet a urine pH that is not unusually high. You do not mention any Gi illness now or in the past. Likewise use of laxatives. You need 24 hour urine testing to determine urine pH, ammonia, sulfate – all related to the cause of this problem. It needs to be done by a specialized lab that does all of the kidney stone measurements. Litholink is the best of the vendors I believe. If you have such data, please be willing to post them, or if you desire you can send them to me directly as this is so unusual a problem. Regards, Fred Coe
Hello Dr Coe,
My name is Andrea and I am 43. My father is from Thailand so I was wondering if the last stone I had could be because of where my dad is from. I got my first stone when I was 25 when I finished my obligated service in the USN. After my first stone I would get more around 18 months later and each would be in some way worse than the previous. I passed them all until around 7 years ago when that stone popped a whole in the back of my kidney, (that is what the doctor told me) luckily with that stone I didn’t follow my normal procedure of denying it for a few days even though I knew I would have to go to the doctor because I started vomiting so much that I just went, which I guess was lucky because I ended up with a bacterial infection. The following stone was to big to pass on my own. At the beginning of this month I woke up in pain an knew it was yet another stone. I went in and had a ct scan where they found a 10 millimeters stone and another that was almost the same size. I had them removed in two different surgeries and they sent one in. The lab report came back and said that it was made up of 90 percent ammonium acid urate kidney stones 10 percent ca oxalate. I’ve been trying to see if I could find out more about it but haven’t been having much luck. Could you please explain it a little to me. I go back for a follow up in a couple weeks and just want to be prepared. Either way thank you very much.
Hi Andrea, I a answering all four of your queries here as they seem shortened versions of this one. Ammonium acid urate stones are unusual and arise typically from chronic diarrhea, laxative use, or some other GI problem. They require a very high urine ammonia level, yet a urine pH that is not unusually high. You do not mention any Gi illness now or in the past. Likewise use of laxatives. You need 24 hour urine testing to determine urine pH, ammonia, sulfate – all related to the cause of this problem. It needs to be done by a specialized lab that does all of the kidney stone measurements. Litholink is the best of the vendors I believe. If you have such data, please be willing to post them, or if you desire you can send them to me directly as this is so unusual a problem. Regards, Fred Coe
Thanks so much for this elaborate explanation!!
Hopefully this will aid my husband and I on our journey of ridding him off of his kidney stones. It’s absolutely terrible to go through child labor pains every couple years and I made it my mission to help him stop it.
If anyone else has some insight on calcium oxalate monohydrate stones’ Ill be more than happy to take your advice.
wishing luck to everyone in taking care of their specific issue’ stay safe and healthy.
Hi hana, for stone prevention, here is a good article to get things started. Regards, Fred Coe
Thanks so much for this elaborate explanation!!
Hopefully this will aid my husband and I on our journey of ridding him off of his kidney stones. It’s absolutely terrible to go through child labor pains every couple years and I made it my mission to help him stop it.
If anyone else has some insight on calcium oxalate monohydrate stones’ Ill be more than happy to take your advice.
wishing luck to everyone in taking care of their specific issue’ stay safe and healthy.
Hi hana, for stone prevention, here is a good article to get things started. Regards, Fred Coe
Dear Dr. Coe,
Twice I have had stones which have caused significant renal colic and associated symptoms, but they did not appear on CT scans. I know for sure that one was a combination of Calcium Oxalate and Uric Acid, and this recent one visually appears to be two stones fused together. In my UA, the ph was 5.5. How can I work with ED physicians better who don’t believe an obstructing stone is there despite blood in urine, protein in urine and a CT scan that shows multiple stones in both kidneys?
Hi Beth, If uric acid, the stones can be prevented or even dissolved. Here is a good introduction which should help you orient yourself along into prevention of stones. Regards, Fred Coe
Dear Dr. Coe,
Twice I have had stones which have caused significant renal colic and associated symptoms, but they did not appear on CT scans. I know for sure that one was a combination of Calcium Oxalate and Uric Acid, and this recent one visually appears to be two stones fused together. In my UA, the ph was 5.5. How can I work with ED physicians better who don’t believe an obstructing stone is there despite blood in urine, protein in urine and a CT scan that shows multiple stones in both kidneys?
Hi Beth, If uric acid, the stones can be prevented or even dissolved. Here is a good introduction which should help you orient yourself along into prevention of stones. Regards, Fred Coe
Am a stone sufferer, however have never found out which kind.
After 2nd one while in Hawaii, I started researching and had what I thought was an epiphany as I was also involved in Saltwater Reef aquariums. Those require a delicate Ca/Mg balance.
So started researching and self-treating with supplements of Mag, and now PO Citrate.
Until I can catch a stone, I am assuming I fall into the normal CAOX bundle.
I only seem to have issues when I drink a coke or two, as I’m a low fluid intake person.
Really just wanted to say that Dr. Coe you’re got one of the very best sites going that I’ve run across. You DO spend a lot of time breaking down the med-speak to us laymen, and with excellent analogies. Please keep up the outstanding work, this is an area that most MD’s fail to take into account when they complain about why patients don’t seem to adhere to advice.
BTW, any suggestions on when one might supplement with say Mag-Citrate vs Potassium Citrate? Before I upped my h20 intake, I measure Urine pH at 5-5.5.
I know Citrate will alkanize pH, and Mag will raise supersaturation rate, correct?
Which would be preferred, or since most of us are Mag deficient anyways, should one consider both?
Thanks, Fred. Given your urine pH, potassium citrate is the better choice. But why not get 24 hour urine testing and find out why you make stones? If you have a CT scan, your physician can check the HU – low is uric acid, very high usually calcium phosphate. The 24 hour urine will tell you about your magnesium intake and also supersaturations. Regards, Fred
Am a stone sufferer, however have never found out which kind.
After 2nd one while in Hawaii, I started researching and had what I thought was an epiphany as I was also involved in Saltwater Reef aquariums. Those require a delicate Ca/Mg balance.
So started researching and self-treating with supplements of Mag, and now PO Citrate.
Until I can catch a stone, I am assuming I fall into the normal CAOX bundle.
I only seem to have issues when I drink a coke or two, as I’m a low fluid intake person.
Really just wanted to say that Dr. Coe you’re got one of the very best sites going that I’ve run across. You DO spend a lot of time breaking down the med-speak to us laymen, and with excellent analogies. Please keep up the outstanding work, this is an area that most MD’s fail to take into account when they complain about why patients don’t seem to adhere to advice.
BTW, any suggestions on when one might supplement with say Mag-Citrate vs Potassium Citrate? Before I upped my h20 intake, I measure Urine pH at 5-5.5.
I know Citrate will alkanize pH, and Mag will raise supersaturation rate, correct?
Which would be preferred, or since most of us are Mag deficient anyways, should one consider both?
Thanks, Fred. Given your urine pH, potassium citrate is the better choice. But why not get 24 hour urine testing and find out why you make stones? If you have a CT scan, your physician can check the HU – low is uric acid, very high usually calcium phosphate. The 24 hour urine will tell you about your magnesium intake and also supersaturations. Regards, Fred
Hello dr Coe
Just reading your article it has some good information for me, I have recently had operation left sided upper ureteric stone with left ureteroscopy and laser fragmentation, I have been long term keto dieting for past 5 years, I was drinking a lot of sugar free soda drinks and energy zero to replace sugar cravings, could this be the cause ? Also will it be enough to drink only water but maintain a low carb diet ? Or will I need to cut meat out to ? Also the analysis results for my stone is a mixture of calcium oxalate 48 % and calcium phosphate 52 %
Hi James, Your stone has a lot of phosphate in it, and that foretells more than usual problems with recurrence. I would be sure to get a full evaluation as to the cause of the stone – this link is my best for planning. Whatever the causes, treatment seeks to reverse them, but you need to know what they are. Regards, Fred Coe
Hello dr Coe
Just reading your article it has some good information for me, I have recently had operation left sided upper ureteric stone with left ureteroscopy and laser fragmentation, I have been long term keto dieting for past 5 years, I was drinking a lot of sugar free soda drinks and energy zero to replace sugar cravings, could this be the cause ? Also will it be enough to drink only water but maintain a low carb diet ? Or will I need to cut meat out to ? Also the analysis results for my stone is a mixture of calcium oxalate 48 % and calcium phosphate 52 %
I’ve had many calcium oxylate stones.
But this year I’ve had several bouts and just had stones analyzed.
Had 24 hr itinerary test awaiting results to discuss with new. Rohtologist this week.
New stones looked very different from others and are 80% ammonium urate and 14 %uric acid with remainder hydroxyl apatite,protein ,and blood .
I’m 66,not overweight and have some bouts of irritable bowel
There was an incidental finding several years ago of a mobile cecum(?) that is not symptomatic except for occasional bouts of loose stools
Please advise…. appt is June 18
Hi Mimk, Diarrhea is famous for causing ammonium acid urate stones, and I am suspicious that even though seeming minimal from your note it plays a role here. Your 24 hour urine should – if I am right – show high ammonia, and a lowish pH of about 5.5 – 5.8. Prevention is not complex but depends on the urine findings. Regards, Fred Coe
I’ve had many calcium oxylate stones.
But this year I’ve had several bouts and just had stones analyzed.
Had 24 hr itinerary test awaiting results to discuss with new. Rohtologist this week.
New stones looked very different from others and are 80% ammonium urate and 14 %uric acid with remainder hydroxyl apatite,protein ,and blood .
I’m 66,not overweight and have some bouts of irritable bowel
There was an incidental finding several years ago of a mobile cecum(?) that is not symptomatic except for occasional bouts of loose stools
Please advise…. appt is June 18
Hi Mimk, Diarrhea is famous for causing ammonium acid urate stones, and I am suspicious that even though seeming minimal from your note it plays a role here. Your 24 hour urine should – if I am right – show high ammonia, and a lowish pH of about 5.5 – 5.8. Prevention is not complex but depends on the urine findings. Regards, Fred Coe
Hello Doctor,
I have 12mm stone and in test it shows calcium oxalate monohydrate – 60%
Calcium oxalate dihydrate – 20%
Carbonate appatite – 20%
Please suggest me foods to avoid and can I use whey protein because I do gym regularly.
Hi Sandeep, Diet may be right or not. You need to pursue a proper evaluation that will lead you to prevention. Here is my best on that subject. Take a look. Regards, Fred Coe
Hello Doctor,
I have 12mm stone and in test it shows calcium oxalate monohydrate – 60%
Calcium oxalate dihydrate – 20%
Carbonate appatite – 20%
Please suggest me foods to avoid and can I use whey protein because I do gym regularly.
Hi Sandeep, Diet may be right or not. You need to pursue a proper evaluation that will lead you to prevention. Here is my best on that subject. Take a look. Regards, Fred Coe
My right kidney stone of 7mm and 2.0 are 100% Carbonate Apatite. Meaning?
Hi Beth, It means you form calcium phosphate stones, a special problem area. Usually urine pH and urine calcium are both too high. Treatment is usually successful, but you need a proper evaluation. Regards, Fred Coe
My right kidney stone of 7mm and 2.0 are 100% Carbonate Apatite. Meaning?
Hi Beth, It means you form calcium phosphate stones, a special problem area. Usually urine pH and urine calcium are both too high. Treatment is usually successful, but you need a proper evaluation. Regards, Fred Coe
I often pass the red gravel like crystals and presume these are uric acid crystals. What I find strange is that when I have a bath instead of the normal shower I seem to pass a fairly large amount of these crystals when urinating straight after the bath. What could be the cause of that. Furthermore trying to solve the formation of these crystals I read that large amounts of vitamin c can cause it but at other sites some say eating citrus fruit could prevent the formation. I thought citrus fruit contained vitamin c.
Hi Evert, Uric acid stones arise from too acid a urine pH and are cured by raising pH. Here is the right article for you. Prevention is very important as these stones can grow rapidly. As for the bathing, I imagine the water is warm and your urine flow goes down – the crystals form in seconds. Regards, Fred Coe
I often pass the red gravel like crystals and presume these are uric acid crystals. What I find strange is that when I have a bath instead of the normal shower I seem to pass a fairly large amount of these crystals when urinating straight after the bath. What could be the cause of that. Furthermore trying to solve the formation of these crystals I read that large amounts of vitamin c can cause it but at other sites some say eating citrus fruit could prevent the formation. I thought citrus fruit contained vitamin c.
Hi Evert, Uric acid stones arise from too acid a urine pH and are cured by raising pH. Here is the right article for you. Prevention is very important as these stones can grow rapidly. As for the bathing, I imagine the water is warm and your urine flow goes down – the crystals form in seconds. Regards, Fred Coe
Hi Dr. Coe,
Wow! Best information about stones. Thanks. I’m a newbie to stones. I’ve passed 2 rice sized terracotta colored ones. My scan shows a small collection of little stones. I had the last one tested, but was not offered an explanation. What is carbonate apatite 90% and calcium oxalate dihydrate 10% kidney stone? Any help would be appreciated.
Hi Lori, Your stones are calcium phosphate and require some careful handling. Here is your article. Here is a general introduction to how to get evaluated properly. Be sure and read both and do the serum and 24 hour urine testing to find out the cause and then get treatment. Regards, Fred Coe
Just got results of my kidney stone back and it says Calcium Oxalate Dihydrate 10 percent . Calcium Oxalate Monohydrate 45 percent. and Carbonate Apatite 45 percent. I have had problems with kidney stones for about 10 years usually with pretty bad infection along with it every thing I’ve read though usually says that Oxalate kidney stones are a combination of two things. I’m confused.
Hi Pamela, The 45% is calcium phosphate, not calcium oxalate so you have both kinds of stones. Usually a lot of calcium phosphate means the urine is rather alkaline and urine calcium excretion is high. Be sure to get fully evaluated. After that, the abnormalities in the urine need to be reversed with diet and if needed medications. Regards, Fred Coe
Just got results of my kidney stone back and it says Calcium Oxalate Dihydrate 10 percent . Calcium Oxalate Monohydrate 45 percent. and Carbonate Apatite 45 percent. I have had problems with kidney stones for about 10 years usually with pretty bad infection along with it every thing I’ve read though usually says that Oxalate kidney stones are a combination of two things. I’m confused.
Hi Pamela, The 45% is calcium phosphate, not calcium oxalate so you have both kinds of stones. Usually a lot of calcium phosphate means the urine is rather alkaline and urine calcium excretion is high. Be sure to get fully evaluated. After that, the abnormalities in the urine need to be reversed with diet and if needed medications. Regards, Fred Coe
Just had my very first kidney stone (9mm) and had to have it removed over the weekend. It was made up of 50% Calcium Oxalate Monohydrate; 40% Calcium Oxalate Dihydrate; and 10% Calcium Phosphate (apatite). I haven’t had a follow up appointment yet but the urologist mentioned a lot of pre-packaged food can cause this. What else should be avoided? I do NOT want another stone.
Hi Gale, You have the common calcium oxalate stone, and this article is ideal for you in terms of evaluation and treatment. Regards, Fred Coe
Just had my very first kidney stone (9mm) and had to have it removed over the weekend. It was made up of 50% Calcium Oxalate Monohydrate; 40% Calcium Oxalate Dihydrate; and 10% Calcium Phosphate (apatite). I haven’t had a follow up appointment yet but the urologist mentioned a lot of pre-packaged food can cause this. What else should be avoided? I do NOT want another stone.
Hi Gale, You have the common calcium oxalate stone, and this article is ideal for you in terms of evaluation and treatment. Regards, Fred Coe
Hi! So my husband had his second bout with stones. He has horseshoe kidneys to add to the mix!
His stone analysis came back Uric acid dihydrate 80%
Calcium oxalate monohydrate (whewellite) 20%
This time he was prescribed potassium citrate but is very weary of taking meds if not absolutely necessary.
Also was told our water filter, which purifies and we’ve added an alkalinity filter, might be bad!?!
What are your thoughts? Taking a pill plus constant blood tests seems extreme.
Can we manage this naturally? What are your thoughts?
Hi! So my husband had his second bout with stones. He has horseshoe kidneys to add to the mix!
His stone analysis came back Uric acid dihydrate 80%
Calcium oxalate monohydrate (whewellite) 20%
This time he was prescribed potassium citrate but is very weary of taking meds if not absolutely necessary.
Also was told our water filter, which purifies and we’ve added an alkalinity filter, might be bad!?!
What are your thoughts? Taking a pill plus constant blood tests seems extreme.
Can we manage this naturally? What are your thoughts?
Hi Joyce, uric acid stones cannot form when the urine is brought to the right alkalinity. They often dissolve. Weary or not K citrate is right for him. The water filter is nothing important by comparison. He needs 24 hour urine testing to be sure the dose is right. Regards, Fred Coe
I have passed a 7 mm stone through urine and know through stone analysis laboratory report as mentione
1 calcium oxalate monohydrate 20%
2.calcium oxalate dihydrate 70%
3.uric acid 10 %
Kindly need your guidance what should I do to stop formatting of stones and which madecine is best
Hi Ejaz, this is a common kind of stone. Here is a good introduction to what you can do. Regard, Fred Coe
I have passed a 7 mm stone through urine and know through stone analysis laboratory report as mentione
1 calcium oxalate monohydrate 20%
2.calcium oxalate dihydrate 70%
3.uric acid 10 %
Kindly need your guidance what should I do to stop formatting of stones and which madecine is best
Hi Ejaz, this is a common kind of stone. Here is a good introduction to what you can do. Regard, Fred Coe
My husband passed black, small rounds 3-4 attached to each other , 6 mm stone, what is the evaluation of type, cause and treatment or dietary advise for it? Suffering from 10 years
Hi Anjali,
You can read about the kidney stone diet here:https://kidneystones.uchicago.edu/the-kidney-stone-diet/#:~:text=The%20kidney%20stone%20diet%20is,and%20of%20course%20high%20fluids.
Best, Jill
I have Rare orphan genetic disease called MH Exostosis or M H Osteochrondromatosis . Have lost left kidney Dr. says from falling thru the cracks??? Now right kidney with cyst on it is flowing with black little what I guess are Calcium Oxalate stones. Last ER gall bladder surgery, Surgeon said she scooped these little tiny stones out by spoonfuls, quite a few spoonfuls and called them spiculated alculi and calculi fragments. I think she didn’t know what she was scooping spoonfuls of out of me and now, with no treatment I have it again. I am in agony. What is all this black small/tiny stuff? It just falls out of me! Thanks…
Hi Jill, From a brief PubMed search I cannot find a link between your syndrome and kidney stones. Likewise, I know of none relating gall bladder and kidney stones. The lost kidney is also unclear, did it ever form, was it obstructed and destroyed? The cyst in your right kidney sounds like a calyceal diverticulum with stones that formed via stasis. Since your physicians seem vague about your diagnoses, perhaps you might want to seek an second opinion. If so, they will be pleased to assist in that. I would be in favor of it if I were you. Regards, Fred Coe
I have Rare orphan genetic disease called MH Exostosis or M H Osteochrondromatosis . Have lost left kidney Dr. says from falling thru the cracks??? Now right kidney with cyst on it is flowing with black little what I guess are Calcium Oxalate stones. Last ER gall bladder surgery, Surgeon said she scooped these little tiny stones out by spoonfuls, quite a few spoonfuls and called them spiculated alculi and calculi fragments. I think she didn’t know what she was scooping spoonfuls of out of me and now, with no treatment I have it again. I am in agony. What is all this black small/tiny stuff? It just falls out of me! Thanks…
Hi Jill, From a brief PubMed search I cannot find a link between your syndrome and kidney stones. Likewise, I know of none relating gall bladder and kidney stones. The lost kidney is also unclear, did it ever form, was it obstructed and destroyed? The cyst in your right kidney sounds like a calyceal diverticulum with stones that formed via stasis. Since your physicians seem vague about your diagnoses, perhaps you might want to seek an second opinion. If so, they will be pleased to assist in that. I would be in favor of it if I were you. Regards, Fred Coe
Just got the stent out for my third round of Kidney stones. I was not offered much in terms of what to do to avoid future ones. Component 1
Reference range: SEE COMMENT
Calcium Oxalate Dihydrate (Weddellite) 30%
Calcium Oxalate Monohydrate (Whewellite) 35%
Carbonate Apatite (Dahllite) 35%
Hi Stephanie,
Here is the kidney stone diet. I am sad to hear that you have not been helped in as far as prevention. Most stones can be prevented.https://kidneystones.uchicago.edu/the-kidney-stone-diet/#:~:text=The%20kidney%20stone%20diet%20is,and%20of%20course%20high%20fluids.
Best, Jill
Just got the stent out for my third round of Kidney stones. I was not offered much in terms of what to do to avoid future ones. Component 1
Reference range: SEE COMMENT
Calcium Oxalate Dihydrate (Weddellite) 30%
Calcium Oxalate Monohydrate (Whewellite) 35%
Carbonate Apatite (Dahllite) 35%
Hi Stephanie,
Here is the kidney stone diet. I am sad to hear that you have not been helped in as far as prevention. Most stones can be prevented.https://kidneystones.uchicago.edu/the-kidney-stone-diet/#:~:text=The%20kidney%20stone%20diet%20is,and%20of%20course%20high%20fluids.
Best, Jill
Hello Dr. Coe,
I have only one functioning kidney on the left side.
2 small stones (3mm) in my left kidney were detected in Feb 2020. I passed one stone.
My stone analysis shows:
50% Calcium Oxalate Monohydrate
42% Calcium Oxalate Dihydrate
8% Carbonate apatite
I recently did 24 Urine Tests. Below are my results:
pH Urine 24 Hr 7.0
Potassium Urine 48.4 mmol/vol
Potassium Urine, random 26.9mmol/L
Calcium Urine 97.2mg/vol
Creatinine Urine 1.4 gm/vol
Magnesium, Ur 109.8 mg/vol
Sodium, Urine 124 mmol/vol
Urine Acid 480.6 mg/vol
Creatinine, Urine 24 Hr 1.3gm/24h
Citrate Urine 24 hours 295mg/day
24 Hr Urine Oxalate 44mg/day
24 Hr Total Urine volume 1800 ml
Can you please take a look and let me know what could be a reason for stone formation? Do I need to take any supplements? I drink 80-85 oz fluids every day. My BMI is 22.5. I don’t have any other health problems.
Thank you very much in advance.
Hi Jeh, Your urine has several abnormalities that could promote calcium oxalate stones: Urine oxalate is high, and urine citrate is low. Although you drink seemingly lots of fluids your urine volume is marginally low enough to raise stone risk. I cannot determine why your citrate and oxalate are abnormal, because the reason will be bound up in the details of your life and diet, and that requires a medical history of considerable complexity. Only your personal physicians are in a position to do that. But increased fluids and steps to reduce oxalate and perhaps raise citrate may be good for prevention. Of note, your urine is very alkaline so the low citrate may not respond to alkali supplements. Regards, Fred Coe
Hello Dr. Coe,
I have only one functioning kidney on the left side.
2 small stones (3mm) in my left kidney were detected in Feb 2020. I passed one stone.
My stone analysis shows:
50% Calcium Oxalate Monohydrate
42% Calcium Oxalate Dihydrate
8% Carbonate apatite
I recently did 24 Urine Tests. Below are my results:
pH Urine 24 Hr 7.0
Potassium Urine 48.4 mmol/vol
Potassium Urine, random 26.9mmol/L
Calcium Urine 97.2mg/vol
Creatinine Urine 1.4 gm/vol
Magnesium, Ur 109.8 mg/vol
Sodium, Urine 124 mmol/vol
Urine Acid 480.6 mg/vol
Creatinine, Urine 24 Hr 1.3gm/24h
Citrate Urine 24 hours 295mg/day
24 Hr Urine Oxalate 44mg/day
24 Hr Total Urine volume 1800 ml
Can you please take a look and let me know what could be a reason for stone formation? Do I need to take any supplements? I drink 80-85 oz fluids every day. My BMI is 22.5. I don’t have any other health problems.
Thank you very much in advance.
Hi Jeh, Your urine has several abnormalities that could promote calcium oxalate stones: Urine oxalate is high, and urine citrate is low. Although you drink seemingly lots of fluids your urine volume is marginally low enough to raise stone risk. I cannot determine why your citrate and oxalate are abnormal, because the reason will be bound up in the details of your life and diet, and that requires a medical history of considerable complexity. Only your personal physicians are in a position to do that. But increased fluids and steps to reduce oxalate and perhaps raise citrate may be good for prevention. Of note, your urine is very alkaline so the low citrate may not respond to alkali supplements. Regards, Fred Coe
Dr Coe,
I have stones that are 80% Uric acid Dihydrate and 20% calcium oxalate monohydrate. In the last two years I have had two surgeries to remove stones (10mm and 9mm) that we’re lodged in my ureters. I have been told that diet and water intake are the cause, but I have other factors at play. I had my colon removed (prevent colon cancer) and also have Crohn’s disease. I appreciate any advise you have to offer. Thank you
Hi John, Oh my – you have an ileostomy or its equivalent, and stones are occuring because of systemic alkali loss. Here is the article about you, and the treatment approaches. The article makes clear that alkali is the crucial missing treatment,m and that diet and fluids will be insufficient. Take a look, and ask your physician to consider alternatives for you that will actually prevent the uric acid stones. Regards, Fred Coe
Dr Coe,
I have stones that are 80% Uric acid Dihydrate and 20% calcium oxalate monohydrate. In the last two years I have had two surgeries to remove stones (10mm and 9mm) that we’re lodged in my ureters. I have been told that diet and water intake are the cause, but I have other factors at play. I had my colon removed (prevent colon cancer) and also have Crohn’s disease. I appreciate any advise you have to offer. Thank you
Hi John, Oh my – you have an ileostomy or its equivalent, and stones are occuring because of systemic alkali loss. Here is the article about you, and the treatment approaches. The article makes clear that alkali is the crucial missing treatment,m and that diet and fluids will be insufficient. Take a look, and ask your physician to consider alternatives for you that will actually prevent the uric acid stones. Regards, Fred Coe
Hi Dr. Coe, I have been dealing with stones for a couple of years now with no luck on what is causing them. The Components of my last stone was Calcium monohydrogen phosphate dihydrate (Brushite). Do you have any advise to how to prevent these stones from happing? My last 24 hour urine just showed that the Calcium Citrate was really low.
thanks Again
Nick
Hi Nick, Brushite stones are a special and nasty kind of calcium phosphate stone. A good approach is in this article. Surgery is complex as your urologist surely knows. Infection is a common problem. The low citrate may not go up with citrate supplements – we have found that men who form calcium phosphate stones are very abnormal in their acid handling. Usually urine calcium is high and that is the key to prevention. Your physicians need to manage all this, and if they find it a bit complex need to direct you to a convenient second opinion. Regards, Fred Coe
Hi Dr. Coe, I have been dealing with stones for a couple of years now with no luck on what is causing them. The Components of my last stone was Calcium monohydrogen phosphate dihydrate (Brushite). Do you have any advise to how to prevent these stones from happing? My last 24 hour urine just showed that the Calcium Citrate was really low.
thanks Again
Nick
Hi Nick, Brushite stones are a special and nasty kind of calcium phosphate stone. A good approach is in this article. Surgery is complex as your urologist surely knows. Infection is a common problem. The low citrate may not go up with citrate supplements – we have found that men who form calcium phosphate stones are very abnormal in their acid handling. Usually urine calcium is high and that is the key to prevention. Your physicians need to manage all this, and if they find it a bit complex need to direct you to a convenient second opinion. Regards, Fred Coe
Hi Dr. Coe,
Thank you for the detailed article.
As a 37 yr old male with a recently removed parathyroid adenoma ( very rare for my age and sex), which was causing inappropriately elevated Ca levels, I am interested in how this may interplay with stone formation. Post adenoma removal I suffered a suspected right sided stone but it was not seen on imaging nor could I find the little bugger despite urine filtering.
Are parathyroid patients with elevated Ca at greater risk for stone formation?
Thank you.
Hi Andrew, Hyperparathyroidism is a major cause of kidney stones. Here are several articles about the subject: Review, detailed. Even after cure PHPT patients may have risk from chronic high urine calcium – so stay with your physicians and get 24 hour urines to be sure. Very complex disease, lots of treatment subtleties. Regards, Fred Coe
Hi Dr. Coe,
Thank you for the detailed article.
As a 37 yr old male with a recently removed parathyroid adenoma ( very rare for my age and sex), which was causing inappropriately elevated Ca levels, I am interested in how this may interplay with stone formation. Post adenoma removal I suffered a suspected right sided stone but it was not seen on imaging nor could I find the little bugger despite urine filtering.
Are parathyroid patients with elevated Ca at greater risk for stone formation?
Thank you.
Hi Andrew, Hyperparathyroidism is a major cause of kidney stones. Here are several articles about the subject: Review, detailed. Even after cure PHPT patients may have risk from chronic high urine calcium – so stay with your physicians and get 24 hour urines to be sure. Very complex disease, lots of treatment subtleties. Regards, Fred Coe
You treated me quite a few years ago and as long as I am on K citrate, I am ok. My stones form because most of my colon is gone.
My sister suffers from kidney stones quite often and she tells me that she was told hers are protein based(if that makes any sense). Does this fall into any of the categories listed in your article?
You treated me quite a few years ago and as long as I am on K citrate, I am ok. My stones form because most of my colon is gone.
My sister suffers from kidney stones quite often and she tells me that she was told hers are protein based(if that makes any sense). Does this fall into any of the categories listed in your article?
Hi Tod, Loss of colon leads to acid urine pH and uric acid stones. If you ever stop the K citrate the stones will recur, often rapidly. I guess when I was your physician I failed to make this clear enough, so I am back telling you this. Never stop it. Protein stones do exist, and usually if you try hard enough tiny amounts of crystal will be found – almost a research level problem. Regards, Fred
My husband has ammonium hydrogen Urate 50%, magnesium mono hydrogen phosphate trihydrate (newberyite) 50% kidney stone. Weight is .026g. He has had extracorporeal lithotripsy, and is scheduled for a urethra laser lithotripsy. He has no pain. However he has had several urinary tract infections. How can he prevent the formation of the stones and is it likely that the laser surgery will be able to smash the stone so that he will be able to pass it through the stent?
Hi Arlene, Your husband has unusual stones of the kind seen with GI disease, laxative use or other diarrheic states, ileal loop diversions, possible infection. It is very complex and requires special attention. I would think his physicians might wish to suggest a second opinion concerning prevention and even urological management. If they cannot identify an ideal source, I can try given where you live. Regards, Fred Coe
My husband has ammonium hydrogen Urate 50%, magnesium mono hydrogen phosphate trihydrate (newberyite) 50% kidney stone. Weight is .026g. He has had extracorporeal lithotripsy, and is scheduled for a urethra laser lithotripsy. He has no pain. However he has had several urinary tract infections. How can he prevent the formation of the stones and is it likely that the laser surgery will be able to smash the stone so that he will be able to pass it through the stent?
Hi Arlene, Your husband has unusual stones of the kind seen with GI disease, laxative use or other diarrheic states, ileal loop diversions, possible infection. It is very complex and requires special attention. I would think his physicians might wish to suggest a second opinion concerning prevention and even urological management. If they cannot identify an ideal source, I can try given where you live. Regards, Fred Coe
When I deal with pregnancy I always deal with large rapid growing stones. This time was hydroxyapatite 68%, carbone apatite 24%, cal ox mono 6%, cal ox di 2%. What kind of stone is this and how can I help get rid of these? :-/
When I deal with pregnancy I always deal with large rapid growing stones. This time was hydroxyapatite 68%, carbone apatite 24%, cal ox mono 6%, cal ox di 2%. What kind of stone is this and how can I help get rid of these? :-/
Dear Christina, Pregnancy raises urine calcium a lot and usually does not cause stones In your case it does and poses some risk even for the pregnancy. The stones grow rapidly because urine becomes alkaline as well as high in calcium. Because pregnancy is so complex, I do not want to say much about what should be done except that perhaps you might benefit from getting care at a place that has physicians expert in complex pregnancy care, and consider reduced diet sodium as a way to keep urine calcium lower, as well as consider using lesser amounts of calcium supplements – pregnancy vitamins are very high in calcium, often. Thiazide like drugs lower urine calcium, but are not presently recommended during pregnancy – in this special case, perhaps pregnancy experts might consider their special use. Regards, Fred Coe
Recently i had a calcium oxalate stone 3*5 mm and the results of analysis was 85% monohydrate calcium oxalate and 15 dihydrate, after month i started to have pain again in both kidneys, honestly i am terrified and I don’t know what to do, medicine in my city is quite bad! And what kind of changes shall i do in my daily life?
Thanks a lot
Hi Ali, Here is my best on how to proceed toward treatment. I hope your city is not so bad as to preclude a proper evaluation. Regards, Fred Coe