Unlike Zeus, or Athene, Janus did not come to Rome from Greece but from myths about a person living early in Roman history and later deified. Janus – deity – presides over beginnings and endings, gateways and doors, invariably dual in nature.
For the Full Video – 19 Minutes
For the Shorter Version – 11 Minutes
What is dual here?
Calcium stone formers are dual. A minority arise from systemic diseases we must screen for. Each systemic disease has its own universe of causes and treatment decisions. A majority are “idiopathic”, systemic causes have been excluded.
Idiopathic calcium stone formers are dual. They have no systemic cause of calcium stones. Most form calcium oxalate stones. A minority, more women than men, form calcium phosphate (CaP) stones.
Idiopathic calcium phosphate stone formers are dual. Most have hydroxyapatite (HA, like bone mineral) as their stone calcium phosphate. Some have brushite (Br, calcium monohydrogen phosphate) in their stones. These latter have more kidney damage than HA CaP stone formers, and are a special high risk group of patients.
Both kinds of CaP stone formers need special attention. That is why I have written this article for them.
Basic Facts about Phosphate Stone Formers
Phosphate Stones Damage Kidneys
Phosphate stones, HA or Br, can grow faster and larger than calcium oxalate ones. Calcium phosphate crystals invade kidney tissue – so called tubule plugs. Tissue damage is common, as is Nephrocalcinosis from plugging – often misdiagnosed as medullary sponge kidney. Kidney tissue damage is worse with Br than HA stones. Potassium citrate, a common stone prevention, may not be appropriate as a treatment because it raises urine pH.
Alkaline Urine Causes Phosphate Stones
Stone phosphate replaces oxalate when urine is too alkaline. Kidney and GI tract physiology raise urine pH, especially in women. Diet is not the cause of higher urine pH. Diet will not reliably lower the pH, and we have no specific drugs to do it, either. So although treatment uses the same tactics as for the more common calcium oxalate patient, it must follow a different strategy.
How Stone Analysis Distinguishes CaP from CaOx Stone Formers
Only CaOx and HA Present
If the average stone mineral composition of all available stones for a given patient is above 50% calcium oxalate, the patient is considered a calcium oxalate (CaOx) stone former. If the average calcium phosphate content is above 50% the patient is considered a calcium phosphate stone former.
The average must be computed using 0 – for example, given CaOx/CaP percentages of 100/0, 0/100, 40/60, the correct classification is 140/3 vs 160/3 or 46% CaOx vs 53%, and so a CaP stone former.
Uric Acid, Struvite, Cystine Also Present
If uric acid, struvite, or cystine are present we name the patient for that constituent. A patient who forms mixed stones – for example, 60% calcium phosphate/20% struvite 20% CaOx is called a struvite stone former. The reason is that these stone types have special causes and treatments.
Any Brushite Present
Brushite is very uncommon in human kidney stones, and associates with large tubule plugs and more severe tissue damage. So when any stone contains brushite we classify the patient as a brushite stone former even though brushite is a minority of stone mineral.
Sex and Age
Single Clinic Experience
Percentages of Cases
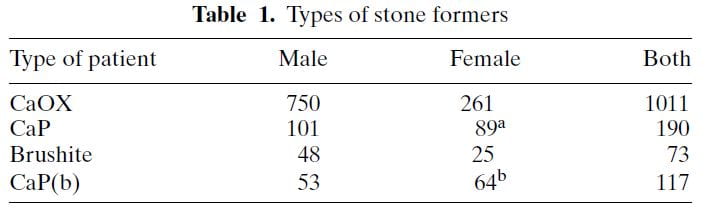 The table shows ‘CaP’ as cases where HA or brushite was the stone phosphate crystal (in early years we did not distinguish). CaP(b) are CaP stone formers with only HA, no brushite in any stone.
The table shows ‘CaP’ as cases where HA or brushite was the stone phosphate crystal (in early years we did not distinguish). CaP(b) are CaP stone formers with only HA, no brushite in any stone.
CaP predominate among females (a and b superscripts denote outsize high frequencies). Brushite does not show this difference a statistical level of significance. CaOx stone formers predominate among the total of all cases. Brushite patients are least common.
Sex vs. Percent CaP in Stones
The same study furnished this nice graph showing the sexes as the percent of stone CaP increases. The bulk of patients have very little CaP in stones (tall bars at the left of the graph). These are the common CaOx stone formers, mainly men (% female, dots, right axis about 25%). But when CaP percent is 20 – 50% in stones, women and men are nearly equal.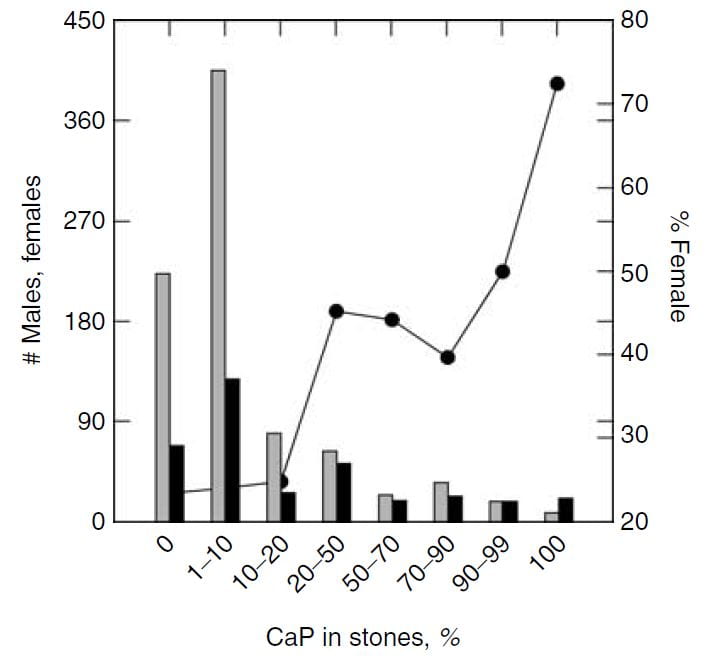
This graph blurs the sex distinction because we used stone CaP% from both brushite and hydroxyapatite. Today, I would have left the brushite to one side, which would have made the female preponderance among those with high stone CaP% more marked – because the sex ratio for brushite stone formers is closer to 1.
National Laboratory Findings
The Mayo Clinic kidney stone analysis laboratory analyzed 48,446 stones in 2010, and of these 43,545 were the first submitted to the lab for that person. From these stones, they report the distribution of stone type by sex and age. I have made a graph from Table 2 of their publication.
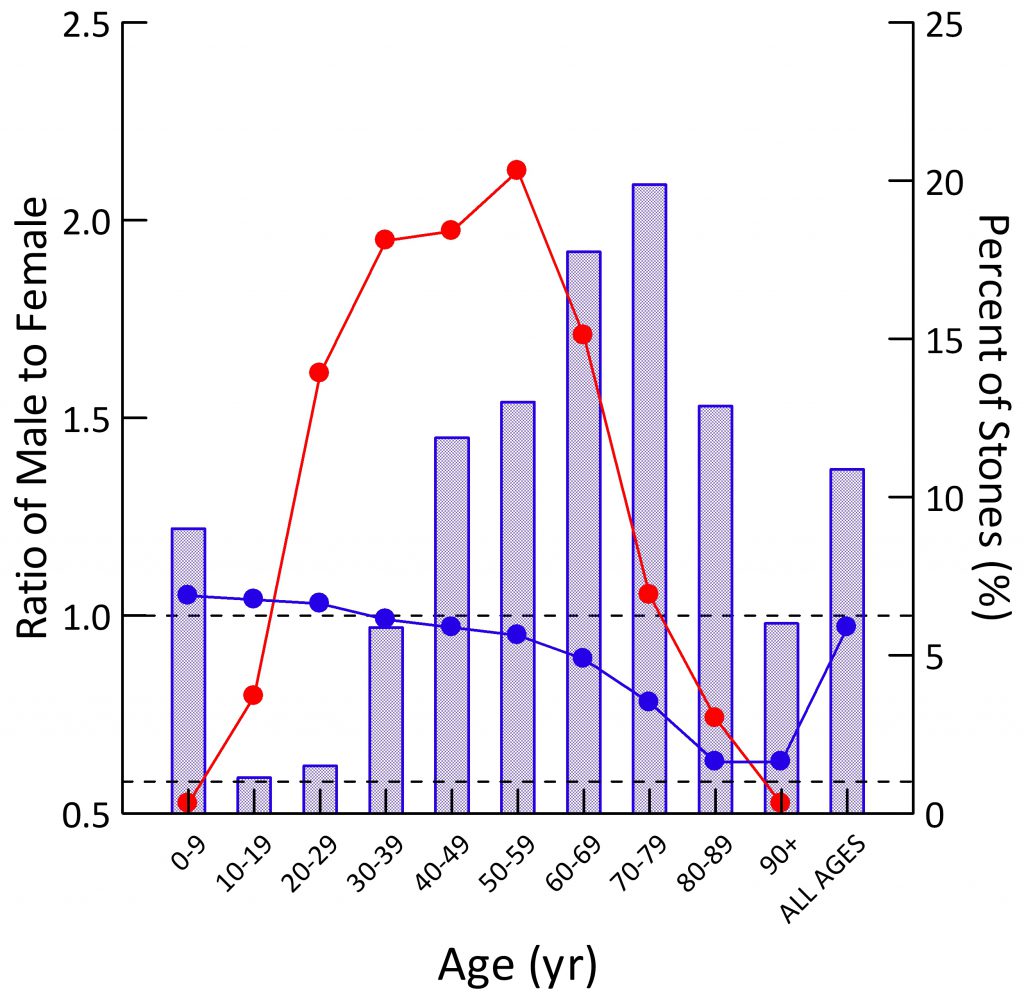
Population Sex Ratio
The general population contains more males than females at younger ages (blue dots). By age 30-39 the two sexes are present in equal numbers. Thereafter, as men predecease women, their blue dots slump downward.
For all ages combined, the ratio of men to women is just under 1 (last blue dot at right).
Stone Former Sex Ratio
The blue bars show male to female ratios among stone formers. Remember this is counted from the sex of the person whose stone was analyzed. A survey based on symptomatic rates of stone passage, by contrast, might give different results altogether.
In childhood, men have slightly more stones than women (blue bar is above 1.0). In the teen years and up to age 39, women predominate over men (blue bars are below 1.0). After age 40 men predominate, until at age 90 and more, in this and perhaps most things, the sexes come into a near perfect alignment. Averaged over all of life, men have more stones, which appears to be because of their midlife excesses (Height of the ‘ALL AGES’ bar above 1.0).
The fraction of all stones formed (red dots; scale along the right axis) for both sexes combined is highest from age 20-69, with only a small fraction in childhood or old age.
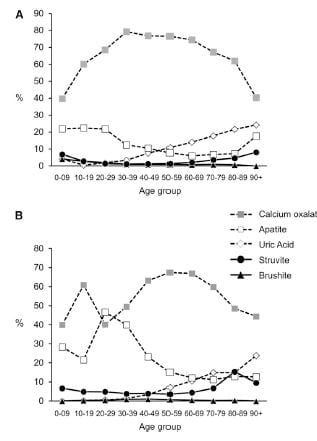
Types of Stones
The men are on top, women on the bottom of the picture to the left.
Stones were classified using the system I have used on this site. Uric acid in any amount meant the stones were classified as uric acid stones, and likewise for any struvite or cystine.
CaOx stones preponderate among both sexes over all ages, except in women between ages 20 – 39 stones were about half CaOx and HA. With age, HA stone frequency fell in both sexes, so that most men, and most older women (over 40) have CaOx stones.
Brushite stones, in both sexes, are very uncommon. You can see them as triangles along the bottom of both graphs.
Over age 50, uric acid stones become a significant concern in both sexes.
Struvite stones, which always arise from infection with bacteria that possess urease, are more common in women than men, a fact known for ages.
The Mystery of Brushite
Brushite stones are rare but should be rarer still. I have written a whole article on brushite because it is so important and yet so evanescent. It forms first of all crystals in human urine. If pH is not too high, oxalate steals away its calcium atoms so it vanishes. If pH is high, HA does the same, and brushite vanishes.
Why, then, are there any brushite stone formers?
I do not know nor does anyone I know of. It is an open question that seems obscure but whose answer might well lead to some new understanding of how stones form.
The Importance of Brushite
Being the first crystal to form, brushite supersaturation is crucial for stone prevention, a fact not intuitive but worthy of special emphasis. Rare in stones, vanishing in most urine, yet brushite supersaturation is foremost in importance for clinicians and patients. The goal is a supersaturation below 1, so brushite cannot form. For those who want to know more about why, please look at the parent article.
Time and Shock Wave Lithotripsy
We (left hand figure below) and others have noted an increasing percent of CaP in stones over the past 30 years. In women (black dots) CaP percent is always higher than in men, but it has risen in both. For those of a quantitative bent, the time trend of stone CaP tested by ANOVA with post hoc contrasts was significant for both sexes, and women were higher than men throughout.
In the publication, we found a relationship between CaP 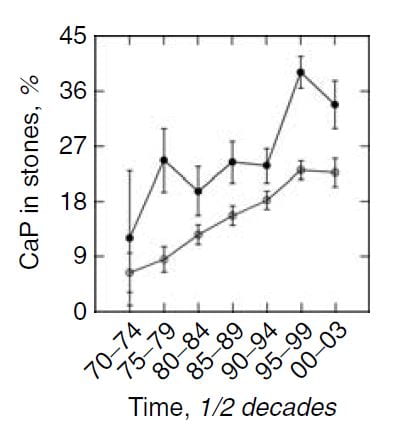 percentage and numbers of shock wave lithotripsy procedures. Use of potassium citrate, however, did not seem to increase stone CaP.
percentage and numbers of shock wave lithotripsy procedures. Use of potassium citrate, however, did not seem to increase stone CaP.
The number of shock wave procedures per patient adjusted for the number of stones and the years of stone disease rose with the percent of CaP in stones (Panel A of the figure below to the right) and the percent of CaP likewise adjusted for number of stones and duration of stones and sex rose progressively with the number of shock wave procedures (Panel B of figure to the lower right).
Not shown here, but of interest, the number of shock wave treatments was higher among BRSF than HASF suggesting a link between shock wave treatment and brushite stones.
One might infer from this set of graphs that the advent of shock wave lithotripsy caused the increase in phosphate stones, and there is nothing to contradict the idea. In fact, the very physiology of phosphate stone formation and the effects of shock waves on kidney 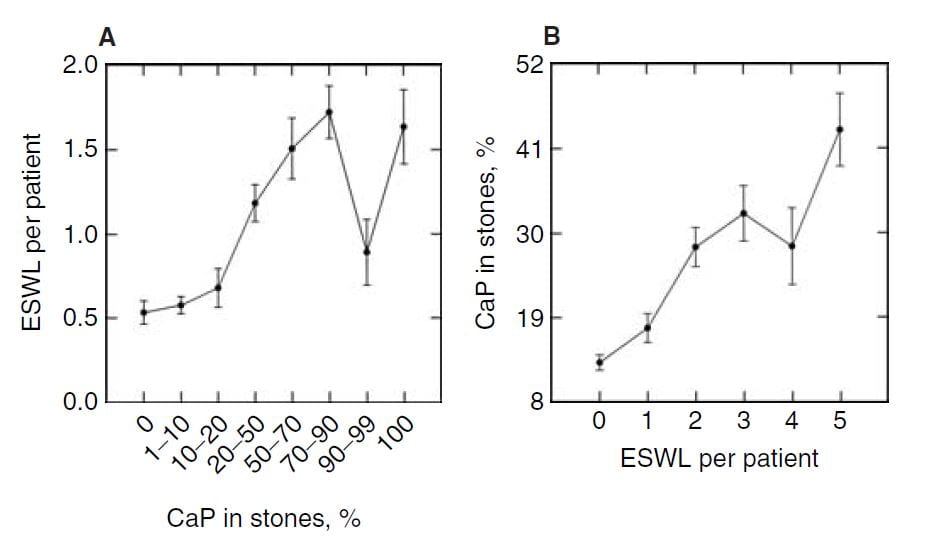 function strongly support that idea as I shall show you.
function strongly support that idea as I shall show you.
Mechanisms of Phosphate Stone Formation
High Urine pH
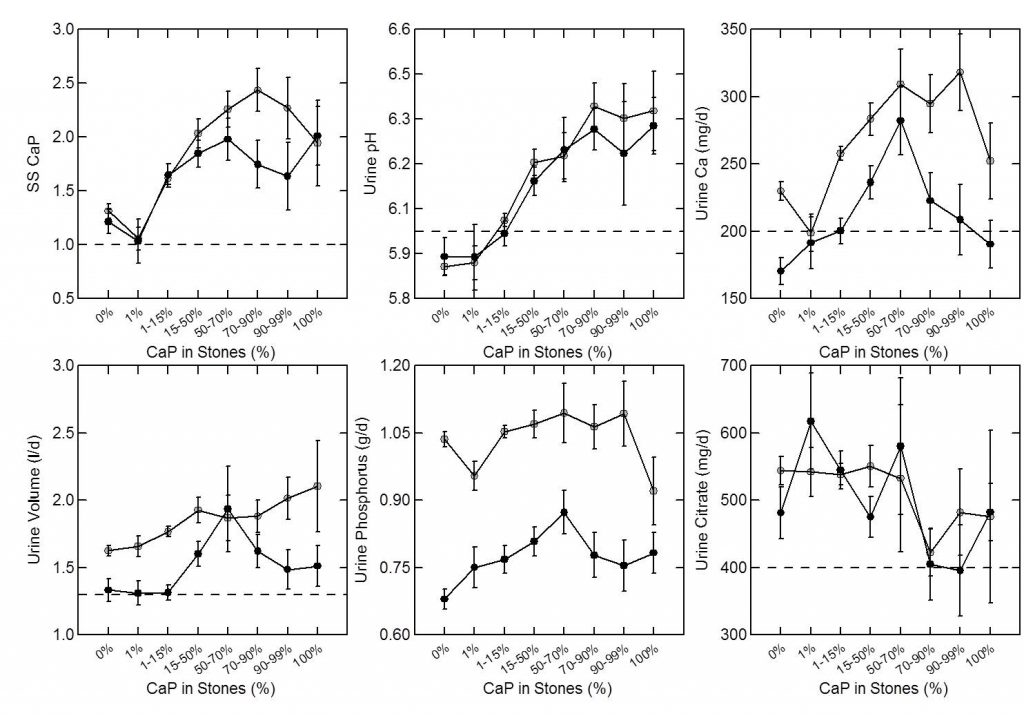
As expected, percent CaP in stones (upper left panel of the figure below) rises with CaP SS. I have shown elsewhere on this site that stone crystals parallel urine supersaturations.
Because CaP SS depends powerfully on urine pH one expects and finds (upper middle panel) that urine pH tracks very closely with stone CaP percent. Urine calcium, volume, phosphate, and citrate excretions (remaining panels) had no important relationship to stone CaP percent.
But take a look at the urine calcium excretions (Upper right panel). They are very high on average. This is because a high fraction of all calcium stone formers have genetic (idiopathic) hypercalciuria. Risk for stone forming begins at a urine calcium of 200 mg/d in both sexes.
So you can think of CaP stones as a two hit model.
Genetic hypercalciuria promotes calcium stones, and urine pH controls the fraction of phosphate in the stones. High CaP SS and CaP stones require a urine pH significantly above 6 as shown in the upper middle panel.
Kidney Tissue
Plaque and Plugs
CaOx stones can be produced as overgrowths in interstitial HA deposits, called plaque.
Idiopathic CaP stone formers, and patients with stones from bowel disease, ileostomy, renal tubular acidosis, and primary hyperparathyroidism, form stones on plaque but also on plugs of HA that fill and damage the last millimeter or so of the nephron, the inner medullary collecting ducts and ducts of Bellini.
Although we are not certain, I think it is fair to say that the plugging of CaP stone formers is because more CaP crystals form in urine and can produce plugging. In a recent article I trace out how calcium phosphate actually forms, how it is a rapid process compared to calcium oxalate, and therefore more able to make plugs during the short times it takes for urine to pass out of tubules into the renal pelvis.
Distinctions Among the Three Idiopathic Calcium Stone Formers
We have 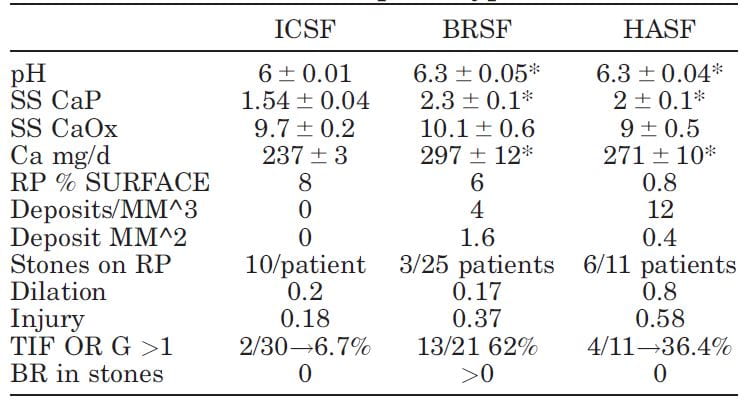 published selected laboratory and tissue findings of CaOx, brushite and HA stone formers, in an attempt to clarify differences in how stones form, and amounts of tissue injury.
published selected laboratory and tissue findings of CaOx, brushite and HA stone formers, in an attempt to clarify differences in how stones form, and amounts of tissue injury.
Numbers are small because each patient had been studied with intra-operative imaging of the renal papillae and papillary biopsy: 11 CaP (HASF), 25 BR (BRSF), and 30 CaOx (ICSF) stone formers.
As expected, urine pH was higher in the BRSF and HASF than in the ICSF, as was supersaturation (SS) for CaP. Incidentally, urine calcium (Ca) was also higher in both CaP groups than in the ICSF.
Mainly CaOx and BR stone formers formed plaque, and mainly CaOx SF form stones on it. About 8% and 6% of papillary surfaces were covered with plaque in ICSF and BRSF but only 0.8% among the HASF. CaOx stone formers had an average of 10 stones/patient attached to plaque, vs. only 3 plaque stones in 25 BRSF and 6 in 11 HASF stone patients: 10/ CaOx patient vs 0.12/brushite patient and 0.54/HA patient. These are 80 and 18 fold differences, respectively!
Plugging (‘deposits’ in the table) was absent in ICSF, but common in BRSF and HASF. Plug size averaged 1.6 mm2 in BSRF but only 0.4 mm2 in HASF – a 4 fold difference. The number of plugs was 3 times higher in HA vs. Br patients: 12 vs. 3/mm3 of tissue volume. BRSF formed fewer but much bigger tubule plugs.
Calyx dilation (a abnormal finding) estimated during surgery was higher in HASF than in the other two groups, and papillary injury (papillae are the parts of kidneys inside calyces) higher in both phosphate groups than in ICSF.
In the kidney cortex, far from where stones form, many CaP stone formers had scarring (TIF, tubular interstitial fibrosis) vs. very few CaOx patients. Brushite patients had most cortical damage.
So phosphate stone formers have injury involving the papillae and cortex, whereas CaOx stone formers have almost none.
Why is Urine pH High?
Being Female
I wrote a whole article on how women raise their urine pH. They do it by absorbing from their food a higher fraction of its alkali content. No sense copying all that here, it is better to read the article. High GI alkali absorption is not easy to treat. Those alkali are nutrient – anions that cells metabolize to get energy.
Being Young
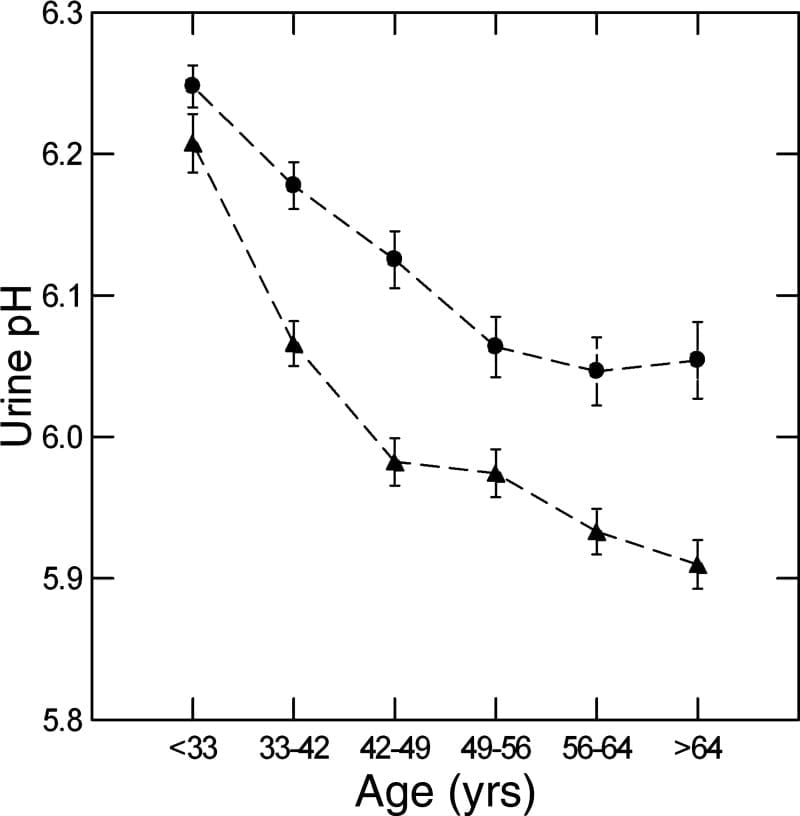
We used a massive database of kidney stone formers to ask what happened to urine pH in men and women with age. The answer is it falls, in both sexes (women are circles, men triangles).
Why is a long story. We could exclude gain in BMI, loss of kidney function, and GI alkali as reasons, but could not find the reason itself. In fact, GI anion absorption rose with age, as if to offset the falling pH.
Here the important fact is on the graph – higher pH in women and in youth are an obvious cause of more CaP stones.
Shock Wave Lithotripsy (SWL)
No practical experiments permit us to measure effects of SWL on urine pH in humans.
For these reasons we turned to an animal model: The farm pig whose kidney is much like that of a human, and likewise is similar in size.
In these animals we could shock one kidney, and then compare the treated to control side at time intervals after the treatment, the untreated side being a perfect control as both kidneys are bathed by the same blood.
SWL Raised Urine pH.
Urine pH from the treated kidneys was 0.18 pH units higher than the control, meaning SWL had increased urine pH (first line of table under ‘Basal’).
SWL Damaged Kidney Tubule Function
There was a lot more.
Urine flow, and excretion of bicarbonate, potassium, chloride, sulfate, calcium, magnesium, sodium and oxalate all were higher from the treated side (bolded). This means that shock wave treatment affects tubule handling of multiple molecules, presumably because of injury.
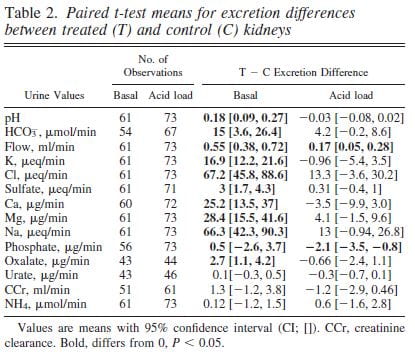 We could find these abnormalities up to 90 days after shock wave. The control kidney reduced its losses in compensation so blood remained entirely normal.
We could find these abnormalities up to 90 days after shock wave. The control kidney reduced its losses in compensation so blood remained entirely normal.
Bicarbonate Losing Raised the pH
The higher urine pH could have been due to damage of final urine acidification in the collecting ducts or to high delivery of bicarbonate from higher up in the nephron so that acid secreted lower down was neutralized by a flood of bicarbonate.
To tell these apart we gave the pigs an acid load that lowered their blood bicarbonate and therefore filtration and downstream delivery (‘Acid load’ columns). Acid load brought urine pH and almost all other measurements to equality between the shocked and control kidneys (loss of bolding).
The tissues from the pigs showed widespread injury to the thick ascending limbs, and you can read the paper for details.
SWL Can Raise Urine pH by Damaging Kidney Tubules
The meaning of the work is clear.
After shock wave treatments the treated kidney may excrete excess calcium and produce a urine of higher pH than it would otherwise do. The effects are precisely those needed to produce calcium phosphate crystals. From the bladder urine, which mixes urine from both kidneys, one could never know this was happening.
It is possible that the advent of shockwave lithotripsy has contributed to the rise in CaP stones, and I hope that further science sorts out whether this hypothesis is false or true.
High Kidney Ammonia Production
 Ammonia Production Regulates Urine pH
Ammonia Production Regulates Urine pH
Kidneys excrete acid by making ammonia that can carry acid (protons) into urine without lowering urine pH. They also excrete acid by titrating urine phosphate, which does lower urine pH. If ammonia production goes down, from kidney disease, for example, urine pH has to fall so acid can be lost on phosphate.
Ammonia production relates itself to body acid load – from food and metabolism – so that the average urine pH is just around 6. But what would happen if regulation were abnormal so more ammonia than normal was made for a given bodily acid load?
Urine pH would rise.
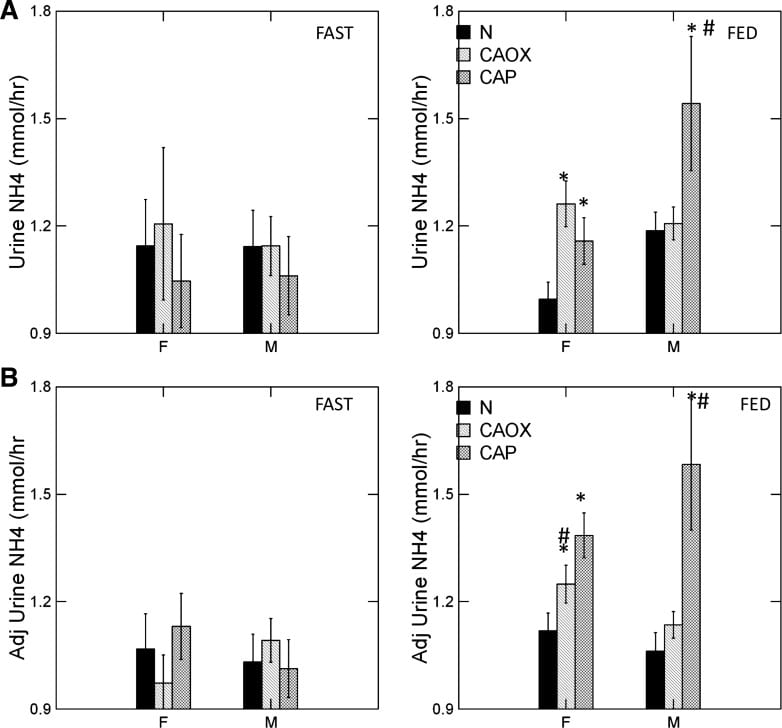
CaP Stone Formers Make More Ammonia
The graph shows urine ammonia excretion from normals, and CaOx and CaP stone formers studied eating the exact same diet in a research center.
Fasting, all three groups are the same (left panels). Food increased urine ammonia in male CaP patients (#). Fed, the female CaP stone formers produce more ammonia than female normals (*, top right panel). So do the female CaOx stone formers. Ammonia production is governed by body acid load, which we measure as GI anion and urine sulfate – a residue of metabolic acid production. When we adjust ammonia for acid load (lower right panel) CaP stone male and female stone formers remain high compared to same sex normals.
We suspect the high urine pH that causes CaP stones arises in part from high ammonia production, perhaps an inherited trait.
Low Urine Citrate
Many articles on this site explore the powerful effects of citrate to bind calcium and inhibit calcium crystal growth. In these closely
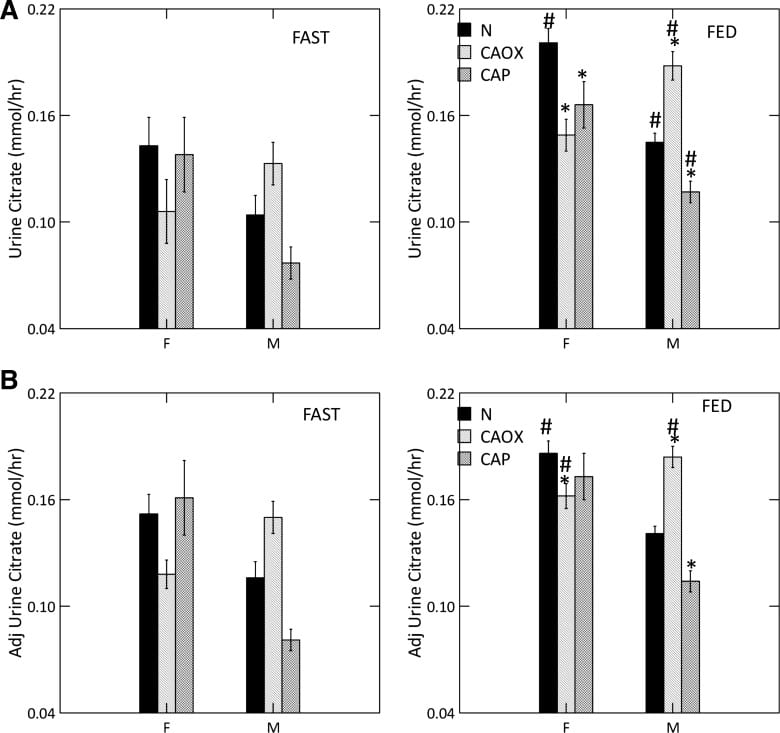
studied men and women we could document a uniquely low urine citrate of CaP stone formers vs. normal people.
Low Citrate in CaP Stone Formers
Food increased urine citrate is normal women and all three male groups (#). With food, CaP stone formers of both sexes have urine citrate excretions below their same sex normal counterparts (*, upper right panel) as did female CaOx stone formers.
As is well known, citrate is lower in normal men than women (compare black bars; we did not choose to compare the sexes statistically).
Adjusted for GI alkali and urine sulfate, (lower right panel) low citrate is concentrated among male CaP and female CaOx stone formers. Normal men remain below normal women.
Male CaOx stone formers have abnormally high urine citrate with and without adjustment for systemic acid balance.
Abnormal Kidney Cell Citrate Handling
Alkali loads, most famously potassium citrate, raise urine citrate and is an established stone prevention. Citrate also raises urine pH, because the alkali appears in urine as bicarbonate. That is why potassium citrate is not an ideal treatment against CaP stones, and why we have for decades needed a controlled trial to see if it works or makes things worse.
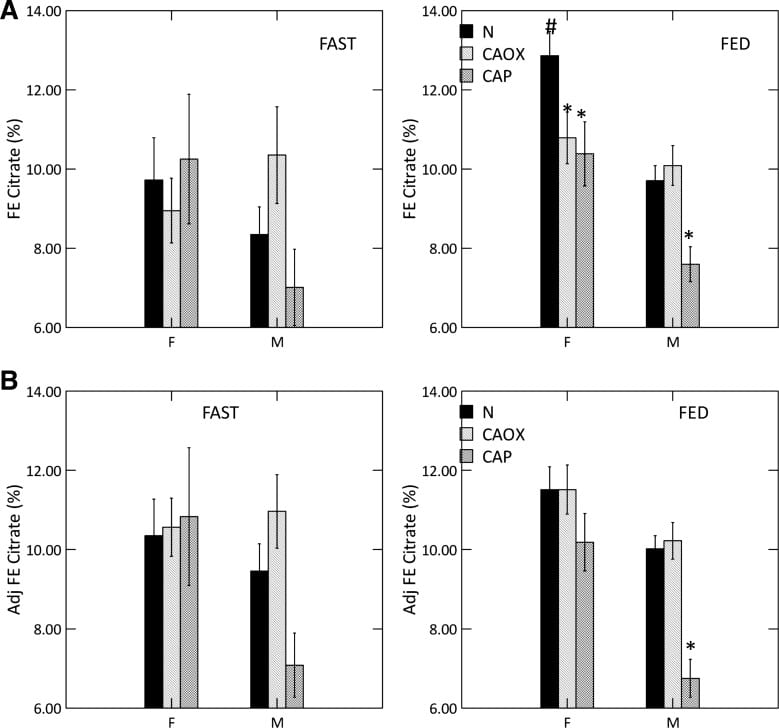
But here we have a high urine pH coupled with low urine citrate, in male CaP and female CaOx and CaP stone formers. That points to something wrong with kidney cell regulation.
We measured serum citrate and glomerular filtration so we could calculate the fraction of filtered citrate excreted (FE Citrate), shown in the upper right panel of the graph at left.
FE citrate is low in female CaOx and CaP stone formers and in males with CaP stones. This means that CaP stone formers are reabsorbing abnormal amounts of citrate back from the filtrate. It is used by kidney cells to produce metabolic energy.
Adjusting for GI alkali absorption (lower right panel) removes the female abnormalities but makes the male one even more prominent.
That male CaOx stone formers have abnormally high urine citrate excretion with normal FE citrate is because their serum citrate concentration is higher, a fact for which we had no explanation.
CaP Stone Formers Have Proximal Tubule Abnormalities
Citrate reabsorption and ammonia production are linked in the proximal tubules of the kidneys as part of overall kidney regulation of bodily acid base balance. In general alkali loads raise urine pH and urine citrate, and reduce ammonia production, whereas acid loads do the opposite.
Here we have high pH and high ammonia production coupled with low urine citrate, more marked in male CaP patients but detectable among the women as well.
It is as though the cells perceive a need to produce more acid excretion (ammonia) and conserve potential alkali (citrate is metabolized to bicarbonate), but there is no need. So urine pH rises and converts calcium stones to their phosphate forms. The cause(s) of these proximal tubule abnormalities are not known.
Incomplete Distal Renal Tubular Acidosis (dRTA)
A Questionable Disorder
Some have proposed that CaP stone formers have high urine pH and low citrate as part of “Incomplete renal tubular acidosis”. In proof, when given extra acid they may not reduce urine pH as low as normal people. In my primary article on dRTA, I present contemporary evidence that acid loading creates a continuous spectrum of urine pH responses, even among normal controls, so it is not a good basis for diagnosis. It seems better to say that CaP stone formers have abnormal proximal tubule functions, and make those the focus of new science.
Heterozygotes of Familial dRTA
With one exception, hereditary dRTA arises from gene disorders of the main proton transporters or of carbonic anhydrase itself, and these disorders are in general recessive. They are recessive because you need two defective genes to knock out a transporter whereas one good gene copy will maintain function.
Of course dRTA causes massive CaP stones and kidney disease. But heterozygotes – meaning one good and one defective gene – from families with dRTA if studied in detail, may not lower urine pH normally. These might be diagnosed as ‘incomplete dRTA, because in fact that is what they are.
CaP Stone Formers are Not Like Incomplete dRTA
Unlike our CaP stone formers, urine ammonia is low in dRTA and heterozygotes from families of dRTA, when compared to their acid load – urine sulfate. Urine ammonia is never high. I suspect that some CaP stone formers have high urine pH because they are indeed heterozygotes of dRTA. Low ammonia may be a way to separate them from the high ammonia of routine CaP stone formers.
Risk of Conversion From CaOx to CaP Stones
Some patients gradually increase their stone CaP percent, often enough to alter their classification to CaP stone former. The opposite, conversion from CaP to CaOx stones must be very uncommon, as we have no cases to report. We wanted to know how to detect risk of conversion.
Who We Studied
From 4767 patients in our program, we collected all CaOx stone formers who had two or more stone analyses and clinical follow up data (445 patients). From these we selected all who had a last stone CaP% at least 20% higher than that of the first stone (62 patients). Men and women were combined because we had so few cases.
Of these 62 cases, 26 had had three initial (pre-treatment) 24 hour urine studies before they passed the stone whose CaP percent was at least 20% higher than their first stone. We labeled these transformers with prior laboratory work – labs before they transformed – as ‘TP’.
For controls we chose 181 patients whose first stones were >90% CaOx and who increased their stone CaP percent <20% between the first and last stone.
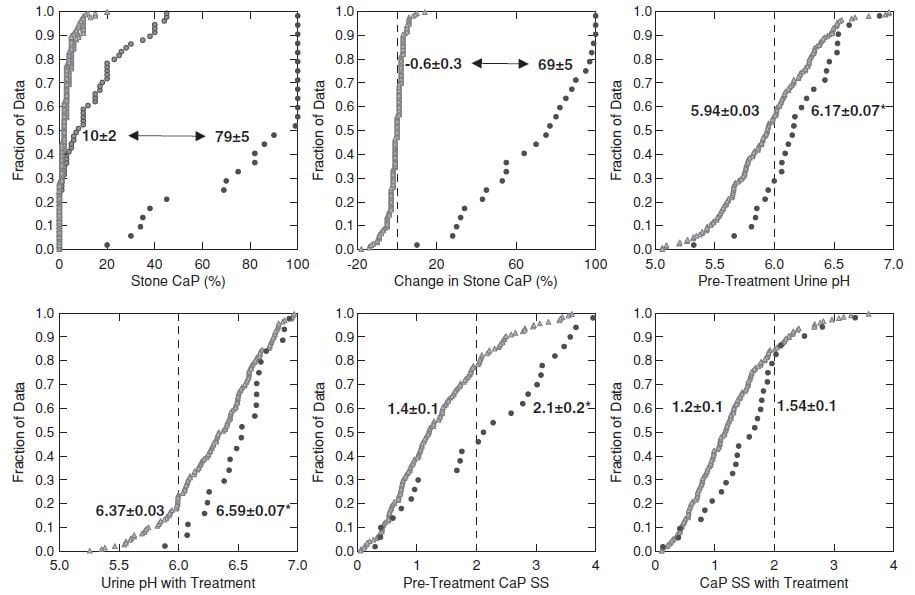
This figure shows the 26 TP cases and the 181 controls.
CaP% Was High at the beginning
Even though their initial stone CaOx percent was >50%, the 26 TP cases (black circles, upper left panel) had an average stone CaP of 10% before treatment, whereas it was much lower in the controls – who never added significant CaP.
During follow-up (upper left and middle panels) the 26 TP (black circles) increased their stone CaP markedly (average 10% to 79%, top left). The controls (gray triangles) hardly changed (-0.6% for controls, 69% change, for TP, upper middle panel).
Higher Urine pH Increased CaP SS
Urine pH and CaP SS before treatment and before conversion (upper right panel and lower left panels) and during treatment (lower middle and lower right panels) were higher in TP (black circles) than controls. CaP SS rose because we used potassium citrate as part of our treatment program.
SWL May Have Played a Role
ESWL associated with conversion: 112 of the 136 total cases with no ESWL procedures were controls, whereas only 21/41 cases with >2 ESWL were controls (X2=17, p<0.001). Furthermore, a predominance of ESWL procedures preceded the final stone (not shown here but shown in the paper), meaning ESWL could have been a causal factor.
Who is at Risk?
When stone CaP is above 10%, average 24 hour pH is as high as 6.3, or CaP supersaturation is above 2 before treatment risk of increasing stone CaP may be high. More than 2 ESWL procedures likewise. Given these risk factors in a CaOx SF perhaps one is prudent to treat as if CaP stones were already forming, so as to possibly prevent further stone CaP accumulation.
Prevention of Calcium Phosphate Stones
The objective is to lower CaP SS – reported with respect to brushite – below 1.
The main modifiable factors are urine volume, and calcium and citrate excretion. Because we cannot lower urine pH, the most crucial factor, we have to use what is left to achieve our goal. Likewise, because citrate regulation is abnormal in CaP stone formers, use of potassium citrate may not raise urine citrate so much as it raises urine pH, and therefore this otherwise valuable treatment can be ineffective.
Fluids
Relative calcium stone risk falls to 1 (no excess risk) at about 2.3 l/d of urine volume. Given the limitations of our treatments, I usually strive for 2.5 l/d spread out over the waking hours. This is an achievable goal if patients understand why it is important for their stone prevention.
Reduced Calcium Excretion
Genetic hypercalciuria is very common among calcium stone formers. If we understand that relative risk of stones rises above 1 at a urine calcium of 200 mg/d, both sexes, our goal is to reduce urine calcium to or below that point.
Reduced Diet Sodium
Multiple articles on this site detail the power of diet sodium to control urine calcium and bone calcium balance. The US diet recommendations for sodium are 100 mEq (2300 mg)/day as a tolerable upper limit, and 65 mEq (1500 mg)/day as ideal. These values concern blood pressure and bone rather than kidney stones. But if we achieve an ideal diet sodium it will lower urine calcium as well as defend blood pressure and bone mineral. So I have no reservations about promoting the ideal diet sodium, but also am prepared for compromise in this fast food dominated world.
Reduced Diet Sugar
As for diet sodium, I have written extensively about sugar as a factor that raises urine calcium, abruptly after the sugar load and with proven increase in supersaturations. Once again, US guidelines call for reducing sugar intake, and there is no benefit to anyone from eating refined sugar in any form. So I am shameless in my zeal to encourage patients to eat as little of it as possible.
Thiazide
Drugs of this class lower urine calcium about 80 to 100 mg/d below the level predicted by sodium intake. They act in part to increase proximal tubule calcium reabsorption. They are trial proven agents to reduce calcium stone recurrence. We have shown thiazide drugs lower urine pH, a possible benefit.
I have often argued to use diet as much as possible before adding thiazide to avoid drug side effects. But phosphate stones are not easy to prevent, so far as I have observed, and they damage kidney tissue. Moreover, we have no trials – none. These patients may have been in trials but are doomed to perpetual minority status unless specifically a focus.
So I am not shy about adding thiazide after perhaps only one to two efforts at diet control, should CaP supersaturation remain above 1.
Why NIH has yet to fund a calcium phosphate stone prevention trial escapes me. I cannot imagine how this has not been a priority.
Potassium Citrate
This drug will lower urine calcium below the level predicted by diet sodium intake. It may raise urine citrate excretion. But It may also raise urine pH.
Being as it is therefore able to raise or lower CaP supersaturation, I do not so much avoid using it as view it with a cold eye.
If thiazide is not attractive to a given patient I will try citrate and watch the effect on CaP supersaturation. CaP supersaturation is the final resultant of whatever changes it induces in urine calcium, pH, and citrate. If it indeed lowers CaP supersaturation, I am prone to use it but with appropriate 24 hour urine followup and an inextinguishable skepticism.
Reduced Diet Oxalate
I am aware that calcium oxalate in stones matters, and that even high phosphate stones often contain that crystal. If urine oxalate is high enough to confer risk – above 25 mg/d in both sexes – I make appropriate diet recommendations.
But patients cannot do everything all at once, so I generally put most emphasis on the calcium phosphate side. The exception is when urine oxalate is quite high – above 40 mg/d, for me – whereupon I do what I can with diet.
Monitoring Treatment
The objective is to lower CaP supersaturation below 1 in the 24 hour urine, and that is what I aim to achieve.
If fluids are enough, so be it. If not I add more treatments more or less as in the paragraphs above. Lacking trials, this is the best we can do. I watch supersaturation for calcium oxalate as a secondary endpoint, and if it is high enough to promote risk – above 3 – I attempt to lower it by reducing diet oxalate.
Monitoring is crucial. What we try to do may not be done because patients cannot or will not do it, so we have to know when to try another approach.
Put another way, for stone prevention, especially calcium phosphate stones, deliberation is reality.
I wish to thank Dr John Asplin for his careful reading of this article and suggestions for improvement.



Thank you Dr. Coe for the informative article. I just had my stones analyzed and shows calcium phosphate (hrdroxyl) 80% calcium phosphate carbonate form 16% and 4% protein and blood. I have Osteoporosis and kidney stones 3 times in 6 years with 2 stents and ESWL. My age is 63. My Urologist put me on sodium citrate solution. What else can I do to prevent these stones. Thank You Sir, respectfully Brad
Hi Brad, Calcium phosphate stones in an older man are not the rule, and leads me to suspect primary hyperparathyroidism – would also cause bone disease. Are your serum calcium values all below 10 mg/dl?? Another possibility is medications – you do not mention any but a few cause calcium phosphate stones. ANother is a primary bone problem with high urine calcium. You need a very complete evaluation, and the cause should turn up. Let me know, Regards, Fred Coe
Dr. Coe, Thank You for your response! My serum Calcium levels are 7.8 and 8.1. I take Hydroxychloroquine and Leflunomide for RA, Alendronate for Osteoporosis, Albuterol and sodium chloride 3.5 nebulizer meds for Bronchiectasis and Advair inhaler, and eye drop meds for Glaucoma. My urine ph is 6.0 to 7.0 from 2016 to 2021. Regards, Brad
Hi Brad, I suspect the eye drops. Fred
Hello Dr. Coe, It’s me again! Could Dorzolamide/Timol eye drops be the cause of my calcium phosphate stones as it has a topical carbonic anhydrase in it just like Acetazolamide. I started this eye drop 2 times a day in each eye in 2013 and had my first kidney stone in 2015. In 2015 I only had 1. In 2018 I had them again, this time 3 of them and obstructing my kidney so stent and ESWL. In May 2021 I was hospitalized with 10 stones in right kidney and 3 small ones in left. Stent placed and ESWL again. I have been on this eye drop and Travatan Z since 2013. Thank You for all you do for us! We appreciate it! Very Respectfully, Brad
Hi Brad, I am certain it is the eye drops. Would you consider sharing your 24 hour urine and serum data with me? A colleague and I have studied another patient whose like eyedrops clearly lowered urine citrate and raised urine pH. Your data would be private. If we should want to publish it I would solicit your consent, and it would be entirely unidentifiable. My email is on the site. Of course work with your ophthalmologist to get away from CA inhibitor eyedrops. Your stone disease is of an appreciable magnitude. Regards, Fred Coe
Dr Coe, I have a appointment on the 15th of Feb with my Urologist. I will have the results of my ct scan and 1st 24 hour urine test. I have some blood tests in the past I can send also. So I will let you know the results 3rd week of February. I couldn’t find your email on my smart phone. Respectfully. Brad
Hi Brad, fredcoe@gmail.com will do. Fred
Dr Coe, you mentioned Glaucoma eye drops can cause calcium phosphate stones, what about over the counter lubricant eye drops for dry eye prescribed by my eye doctor? They contain Carboxymethylcellulose sodium, glycerin, polysorbate, and some of the inactive ingredients are Calcium chloride, magnesium chloride, potassium chloride, sodium chloride and lactate, hydrochloric acid and sodium hydroxide and varies with drops some containing boric acid among other ingredients.
Hi CF, No. The drops that can cause these kinds of stones contain a chemical that inhibits carbonic anhydrase. They work well, but have this potential. None you mention do this. Regards, Fred Coe
A ct-scan incidentally revealed stones in both kidneys, which are growing fairly rapidly. I am not taking any meds, including no eye drops. Healthy weight, regular exercise. 24-hour urine ph was 7.08, oxalate 50, calcium 159, SS CaP 1.76. Uric acid .817, SS CaOx 6.28, citrate 763. Serum calcium lives in the 9.6-9.8 range. Intermittent high PTH and 1,25 di-OH levels; multiple doctors have ruled out hyperparathyroidism since serum calcium is normal. Significant osteoporosis. My urologist thinks supplementing with citrate is the answer but in reading your article, it seems this will just increase urinary ph, which was the biggest risk factor identified on my lab report. Any suggestions about where I should look for answers on what to do?
Hi Elise, indeed the high pH goes with CaP stones and indeed potassium citrate is not likely to stop them. Of interest your urine citrate is quite high, so K citrate would have little place altogether. We do not know your stone type – you do not say – and your urine oxalate is quite high and SS CaOx 6, so maybe things need rethinking. Low calcium diet can create your picture, with the high oxalate, and perhaps urine volume is not so good. The goal is to bring the SS CaP below 1 and I would consider a trial of very low diet sodium to reduce the (normal) urine calcium, or even thiazide to do the same. I would try to get the urine pH down, which means figure out why it is high – I suspect diet given the high citrate. But you provide little enough information and I can’t say more. Regards, Fred Coe
Hi,
I have got stones in every 3 years since I turned 20 years old. Now I am 40.
Before the stones passed themself with pain but in 2020 I was on surgery for 3mm Calcium phosphate/oxalate stone in left ureter. Biggest stone until 2020. I got Acalca 1080mg potassium citrate 6 times a day in 03/2021 to prevent stones. A week ago (01/2022) a 7mm Calcium phosphate stone was removed from me in a surgery and CT showed 4mm stone waiting in other kidney cortex. Surgery was demanding as there were not enough diameter in ureter and scarring in ureter even though this was first surgery of right ureter. As stone turned from mixed calcium phosphate/oxalate to calcium phosphate and was biggest I ever had should I stop with potassium citrate? 2 other in my family have had phosphate/oxalate stones.
Hi Thomas, complete evaluation is crucial and you do not give any information so I assume it has not been done. Certainly, in the absence of proper evaluation use of any medication is uncertain, and the role of alkali for calcium phosphate stones has never been tested. Regards, Fred Coe
Hi! Thank you for your work in this area. My husband and I have been combing through all of your articles! I’ve been forming what I now know are CaP stones for 13 months (I’ve passed 7 – none needed to be removed, but two required ER visits). All stones dissolve before exiting my system so we haven’t been able to analyze any. These began while I was weaning from breastfeeding my son and it took me two urologists and a year for anyone to be willing to order a 24 hour test for me. I just received results and they are as follows:
Vol-1.85 (but I stated on the form 2 liters, which was actual output)
SSCaOX – 3.11
Ca24 – 134
Ox24 – 19
Cit24 – 366
SS CaP – 1.10
Ph – 6.627
SS UA – .15
UA 24 – .493
K is on the low end of normal at 23 as is Mg 46. Sodium is high end of normal, 149. PCR – 1.1. All bloodwork and parathyroid levels are normal. Most recently the sodium in my blood was at the low end of normal (135). My urologist’s suggestion was to do urocit-k which I’m not comfortable with as I know it will further raise ph or to switch to drinking crystal light for every drink every day and to raise fluid intake. I’ll be raising my citric acid intake in my fluids, raising fluid intake and plan to lower sodium a little more as well. Does this sound like the right course of action? I’m pregnant again and am more determined than ever to decrease the frequency of these stones now that I have the 24 hour information and I know kidney stones can increase in pregnancy.
Hi Corrie, You say your stones are calcium phosphate but also say that none could be analyzed. The key urine findings are the low citrate and high pH. The urine volume is calculated at LL from the concentration of a molecule added to the container in a known amount and is more accurate than a home volume estimate. Urine K of 23 is indeed low, and that could have lowered your urine citrate. You are not pregnant forestalling any further treatments or evaluation. For the time, I would rely on high fluids, and make all effort to gather what crystals pass, even using a filter to catch them. The low urine oxalate and calcium make me wonder why you made stones – very modest values – and suspect things were a lot different when you did make stones. Pregnancy is a very high urine calcium state. The low serum sodium is expected during pregnancy. No, I would not add alkali based on the little bit of information thus far. Regards, Fred Coe
Hi Dr Coe, I’ve read some mixed info on phytates/phytic acid that is in food and the relation to kidney stones. I eat a lot of wheat/bran, oats, peanut butter and walnuts. Some articles I have read state that phytic acid in foods can prevent absorption of calcium, magnesium, and zinc. Does this mean that it would cause more Calcium in the urine from these foods and are phytates bad for people with Calcium Phosphate stones like myself? Other articles state they are good for Calcium Oxalate stones.
Hi Carol, If you are forming calcium phosphate stones, your problems have little to do with oxalate or phytate but with too alkaline a urine and usually too high a urine calcium loss. The high urine calcium loss is not due to food calcium but to genetics and worsened by excess diet sodium. The article you wrote on has a good review of these matters. If you cannot find what you need in it, please write back and I can try to help more. Regards, Fred Coe
Hi Dr. Fred thanks so much for this article. For some reason I form 80% hydroxyapitite and 20% uric acid stones while pregnant. Currently have 3 new ones in my left and one in my right all 5mm or less. Any tips for pregnant women?
Hi Alyson, Stones during pregnancy are indeed a special problem. Uric acid and HA are incompatible as one forms at low urine pH the other at much higher urine pH. Is it uric acid or urate salts? The linked article concerns pregnancy itself and stone effects. When not pregnant nor lactating, I would suggest a complete evaluation to find out the causes of stones.During pregnancy urine pH and calcium both increase. Regards, Fred Coe
Dear Dr Coe, I hope this reaches you. Feel quite desperate here! My son, now 8, has been forming stones since he was 2.5 years old on his left side only. He is assymptomatic. I spotted blood in his urine as a toddler hence the assessments. He was put on potassium citrate 5ml twice daily and bendroflumizide 2.5mg once a day as he has a high calcium output in his urine. His calcium:creatinine ratio is up and down all over the place. His first lithotripsy was at 3 years old on a stone around 6mm. That went. For a few years he developed 2 stones that did not get any bigger than around 6mm and 8mm but then had them removed when one moved in an awkward position so removed with internal lithrotripsy. We have a wonderful consultant who is at a loss as to why treatment isn’t working. Other consultants been involved & was decided Jan-June 2023 to take off all meds as could be making worse. In that time he formed 4 stones. In nov 2023 had surgery again. Stone size approx 11mm, 10mm, 8 & 4 mm. All 100% calcium phosphate confirmed. Back on 2.5 poatassium citrate and bendroflumazide. Drinks min 2 litres lost days. PH level usually around 7 but is something tricking the number? 24 hour urine collections in past show nothing unusual. Eats healthy balanced diet: no high salt or sugar. Lots fruit and veg like Brocolli etc. absolutely baffled. Even the stent after last surgery calcified within 6 weeks. Any advice!??? Can email me directly if like. From a very worried parent.
Hi Sarah, Such high urine calcium in a young child must be considered genetic until proved otherwise so I suggest gene testing for all possible causes of high urine calcium be done as soon as possible. Given a cause one might be better at treatment. Given the calcium phosphate stones I presume his urine calcium is high, perhaps his urine is alkaline from the treatments or because of genetic disease. Regards, Fred Coe
Hi Dr Coe,
In September 2021 (age 37) I discovered I had kidney stones. A “small” 5mm stone in my left ureter (ultimately passed) composed calcium oxalate, and numerous other smaller stones in my left kidney still there. My right kidney was diagnosed as having a staghorn calculus stone taking up nearly all of the collection space in my kidney, and removed via PCNL in November with follow up cystoscopy in December. Stone make up for my right kidney was 70% calcium phosphate (apatite) 20% struvite and 10% calcium carbonate. I had two 24 urine tests, Dr said they were pretty good. One day citrate was borderline low and one day sodium was a little high. PH was nearly 7 on both days. Dr Suggested trying to increase citrate and reduce sodium, which I have been doing. And was not concerned about PH. My dr is not sure what caused stone to form in my right kidney. Due to right flank pain i had a CT scan and I have decent sized (7mm & 5mm) stones already in my right kidney, 4 months after surgery.
I have had a history of UTIs in the past (without typical symptoms) and a bad kidney infection in 2015. Given no scans at this time and small amount of struvite make up in stone, Dr doesn’t know that this is cause for the stone formation. I am feeling frustrated with lack of answers as to why stones are forming and what all needs to be done to prevent them.
Hi Laura, given the fast recurrence of stones (I presume there was a CT after surgery so we know the count then) you may have struvite as a main component of your stones. It arises from infection with bacteria that contain the enzyme urease that hydrolyses urea – a normal urine chemical- to ammonia and thus produces struvite magnesium ammonium phosphate. Common villains are Proteus, Klebsiella, enterococcus, and pseudomonas species. Alternatively, you may just be forming calcium phosphate stones which also can grow rapidly. Stone analysis for struvite is rather mediocre – not rarely over diagnosed by labs. One clue is urine ammonia. If the pH is 7 ammonia should be no higher than or below urine sulfate. Take a look. If ammonia is higher by a lot then infection is a higher probability. Of course cultures help and need to be repeated to be sure about what is growing in your urine. Perhaps the stone material was cultured – a good idea. As for citrate, usually phosphate stones arise from high urine pH and high urine calcium, and frankly I like to use thiazide and avoid alkali – as in the article you are writing on. Regards, Fred Coe
Hello:
32yo Female
Multiple bi-lateral 3-4mm stones on CT one week ago. (I have already passed 4 over the past few months). I am about to complete my first ever 24hr urine study.
Stone analysis: 90% Carbonate Apatite
10% Weddellite
PTH: 78 pg/ml
Ca: 9.6 mg/dl
I am concerned about PHPT but was told my results were normal. I am at a healthy weight and I exercise regularly and eat well. I am so discouraged because of all the stone agony and symptoms as well as fatigue, etc.
Hi Britt, I presume your urine calcium will be high and urine pH also – above 6.3. The normal serum calcium is much against PHPT. If you want surety, get several more, always in the morning always fasting. Assuming all are normal, the high PTH is secondary to something – eg. low 25 vitamin D, low calcium diet, reduced kidney function (increased serum creatinine or reduced eGFR). These stones can be prevented as the article mentions, and prevention is what you should expect. Regards, Fred Coe
Dr. Coe,
You were right on for urine calcium and PH. My LithoLink results said my doctor should consider dRTA.
My 24 hour urine results were as follows –
SS CaOx: 3.21
Ca 24: 246
Ox 24: 22
Clt 24: 518
SS CaP: 2.08
pH: 6.806
SS UA: 0.13
UA 24: 0.789
Na 24: 247
K 24: 96
Mg 24: 82
P 24: 0.958
Nh4 24: 23
Cl 24: 252
Sul 24: 39
UUN 24: 9.89
PCR: 1.0
Cr 24: 1223
Cr 24/Kg: 15.9
Ca 24/Kg: 3.2
Ca 24/Cr 24: 201
Hi Brittany, I do not think it is RTA, the citrate is too normal. I would lower the remarkably high diet sodium from 246 mEq/d to 65 mEq/d (1500 mg/d) and I bet the urine calcium and stone risk come way down. REgards, Fred Coe
Hi Dr. Coe,
Is there anything else that could cause the sodium in urine besides diet?? I eat an average amount of salt (of course I can and will reduce my current amount) but my blood pressure is never above 106/64 and I have no fluid retention or headaches. I am just curious if anything else could cause the excess urinary Na/Cl besides salt intake?
Thank you so much for all of your help!
Increasing diet sodium increases urine calcium loss and the reverse. The only way to know your diet sodium is 24 hour urine testing. Being an atom, all urine sodium came in from food. Regards, Fred Coe
Hi Dr. Coe
I have a read a few of your articles and find them very informative. Although I have to admit, that not being from the medical field, some of them are too “technical” for me.
I am 51 and a recent stone sufferer. My first stone showed up in September 2020 as very painful spasms. Took me to the ER once and finally came out naturally after exactly one year in Sept 2021, about 3 days before my surgery was scheduled to take it out. It was a round 6mm Calcium Oxalate Di stone (very hard). The Urologist constantly monitored kidney function and other vitals, which is why the surgery was not scheduled earlier. I was glad that I was able to pass it without surgery.
Since passing the stone about 7 months ago, I have had mild – moderate flank pain multiple times. The urologist suggested a CT in Feb 2022, which showed up nothing. Since then, I noticed what looked like skin colored flakes passing in my urine (with minor burning when they passed). I thought maybe it was some damage from the stone and some skin peeling off. The second time it happened, I passed, what looked like a much larger “flake” with some obstruction and burning. I picked it up and it was hard (not skin tissue). The size was about 5-6mm, but it was flat like a sesame seed with a raised center. When I held it tight, it was brittle enough to just crumble. I saved it and sent it in for analysis. It turned out to be Hydroxyapatite. Since passing this about 2 weeks ago, I have had a few instances of small flakes appear in my urine, including 3 yesterday. When I picked an analyzed one, it was similar (brittle) in texture and color. I also passed a fourth, larger one (about 4-5mm) flake yesterday with similar characteristics. What is unique about them is that I don’t get very prominent flank pain – mostly undistinguishable from gastric pain. I get some burning when passing the stone and some momentary obstruction. Also unique is that the first (CaOx) came out of the left kidney, but most of the recent discomfort is in the right one.
The urologist ran a few other tests for UTI and everything came back negative. My urine pH was 6. Citrate and Calcium was not measured.
I have increased my fluid intake to more than 3L a day. I have also started drinking Crystal Light about two days ago (after which many of the “fragments” came out). In total, I have excreted about 12 small (0.5 – 1mm) flakes and two larger ones.
I am wondering if these stones are plaque which is peeling off (since they appear with a raised center, like a stone forming on plaque).
What do you recommend in terms of dietary changes (I did eat a lot of nuts over the last few months – as I normally do). I am of a healthy weight although I could lose about 5lbs. Would you recommend any specific tests?
Is it normal for certain stones to not show up in CT scans as I had one in Feb when I has a moderate flank pain?
Thanks
Hi Gaurav, You are passing crystals, not uncommon, and because they are hydroxyapatite they are forming in a urine that is overly alkaline. More, perhaps they are residual from your surgery or perhaps you are actively forming them. You need a proper evaluation with 24 hour urines and matching blood samples, and given stones began a bit late you may have underlying disease as a cause. The calcium oxalate dihydrate stone suggests your urine calcium is high. Regards, Fred Coe
Thanks for your response Dr. Coe.
I had a HPTH test done and the result was normal – 46.7 pg/mL
BTW – I did not have surgery. I have passed all stones naturally.
All blood tests were normal as well – Calcium, Phosphorus, Magnesium, Creatinine, Uric Acid and Electrolytes.
I had a renal ultrasound done, and it came back Normal as well – No Hydronephrosis in either Kidney and no visible Calculi. However, even a day after the Ultrasound, I passed several crystals.
Finally, I completed a 24 hr Urine Test. Here are the results:
SPECIMEN VOLUME 24 HR URINE 3.37 L/day
PH, 24HR URINE 6.6
CALCIUM RATE, 24 HR URINE 417 mg/day
OXALATE RATE, 24 HR URINE 28 mg/day
URIC ACID RATE, 24 HR URINE 509 mg/day
CITRATE RATE, 24 HR URINE 481 mg/day
SODIUM RATE, 24 HR URINE 109 mEq/day
SULFATE RATE, 24 HR URINE 11
PHOSPHATE RATE, 24 HR URINE 890 mg/day
MAGNESIUM, RATE, 24H UR,QN 162 mg/day
24HR URINE AMMONIA RATE (UMOL/24HR) 30 mEq/day
POTASSIUM, 24 HR URINE 46 mEq/day
CREATININE RATE, 24 HR URINE 1059 mg/day
CALCIUM OXALATE INDEX, 24HR URINE 1.30
BRUSHITE RELATIVE SUPERSATURATION 3.04
SODIUM URATE RELATIVE SUPERSATURATION 0.45
STRUVITE RELATIVE SUPERSATURATION 1.68
URIC ACID RELATIVE SUPERSATURATION 0.19
THE PATIENT HAS See Below
Hypercalciuria
SUSPECTED PROBLEM IS See Below
Hypercalciuric Nephrolithiasis
Interestingly the Magnesium levels are also high. Would that be because I take high dose Magnesium (400mg) everyday?
I was also taking Vitamin D3 supplements >6000 IU per day, for the last 2 years. Admittedly, I started taking them after COVID without a doctor’s recommendation. Could the Hypercalciuria be explained by the high Vitamin D intake? I stopped taking them about 2 weeks ago, and was not taking them when I did the 24 hour urine collection.
Hi Gaurav, The high urine pH and obvious high calcium have raised SS with respect to calcium phosphate as brushite (CaHPO4) which will cause crystal attacks, stones, and the latter may be all hydroxyapatite or admixed with calcium oxalate. How To Count Kidney Stones. You already have reasonable urine sodium so the only workable options are an even lower diet sodium or thiazide. Frankly I would ask your physicians if they are in favor of the latter as these drugs lower urine pH and well as calcium and because CaP stones can grow rather large and rapidly. Regards, Fred Coe
Hi Dr. Coe,
I recently passed a 4 mm stone (went to the ER before passing on my own) and have been diagnosed with 8-9 other ones. All are small but one, it is about 7 mm and there is question as to whether to do Shockwave or another surgery for the 7 mm one before it grows. Question: is there anyway to naturally dissolve or would I need surgery?
After reading your article and comparing it against my results, I have decided not to move forward with Shockwave at the moment. I passed the 4mm stone but it must have evaporated / turned to sand on the way out; therefore I was unable to process in the lab. Prior to an unexpected hospitalization 3 months after giving birth, I am not sure if I had stones; they were captured during one of my man CT scans. Genetically speaking, my parents do not carry stones, although my father had Gallstones and removal. I have not been genetically tested however. Question #2: Someone mentioned to test for the MTHFR gene- do you recommend this?
Parathyroid is normal. Numbers from my recent Litholink are as follows:
Vol. 2.94
SS CaOx 1.68
Ca 24 146
Ox 24 18
Cit24 232*
SS CaP 1.05*
PH 7.16
SS UA .03
UA .448
Na 107
K 72
Mg 85
P .630* (seems right on the cusp)
Nh4 35
Cl 115
Sulfate 25
UUN 7.10
PCR .8 *also on the end of the scale
Question #3: In your opinion, do these #’s look tell-tale for CaP stones? Additionally- My stones seem to be the same over the course of the last 4 years. Not increasing rapidly, which I believe you said also is a tell-tale sign of a CaP stone?
A little background:
I am a 42 y/o woman. I work out 3-5 days per week light to moderately. My diet was higher in proteins, and I still ate foods that led to GI inflammation (sugar, gluten, wheat, and other intolerances) more frequently than I’d like to admit. I had for a long time been living with what I thought was IBS up until I worked with a functional doctor to change some dietary habits. I was dairy-free prior to passing the stone. I had been drinking a fair amount of coffee up until 6 months ago. Additionally, I was drinking 4-6 units of alcohol per week. Sleep was often interrupted. I’ve since changed to a lower sodium, no processed sugar, low to no dairy and 1-2 units of alcohol. I had not been measuring my L’s of H20 but now I am.
Final questions: Could mineral imbalance effect the enzymatic processes? In addition to perimenopausal hormonal disruptions? And what, if any, recommendations may you have for natural or more homeopathic solutions to resolve and prevent these going forward?
Thank you in advance. I truly value your article, expertise and time.
Hi Kristina, Your urine values are exactly like those of a calcium phosphate stone formers: Low citrate high pH ammonia higher than sulfate. SWL or other surgery is when stones are causing obstruction, infection, pain, or bleeding, or – in special cases – where they are very numerous and increasing in numbers or size. You already have a low urine calcium and reasonable sodium and volume, but CaP SS is above 1 on a 24 hour basis because of the high pH and low citrate. Figuring out a non medication way for you is threading a fine needle, and I hesitate to try at this distance – it is all compromises in diet lifestyle etc and very individual. I would be afraid of causing trouble with almost anything I said. But the CaP SS needs to be lower, about 0.5-0.7 if one wants to avoid peaks during the day. Regards, Fred Coe
I recently had a 7mm stone and the test results were 50% Calcium Oxalate – 50% Calcium Phosphate.
I am a 40 years old Male and I am not overweight. I don’t eat much meat, but I eat yogurt and eggs routinely. I also eat a lot of vegetables and fruits, What can I change in my diet?
Hi Nima, You are reading the right article. You need a full evaluation with 24 hour urines and serum measurements to determine what is causing the stones, and treatment aimed at what is found. I am sorry to be so late in answering this, I think I missed it on my last round. Regards, Fred
Thanku for giving such wonderful knowledge.
Hi Dr. Coe,
I am a 47-yr old healthy male, and want to be a kidney donor. I don’t drink alcohol (never did), work out 3-5 times a week. The various tests that I have undergone i.e., urine, blood, imaging were perfect. Except for a CT scan which showed a single 4mm non-obstructing stone in one of the kidneys. The 4mm stone finding was accidental, I never had a stone before. No one in my family, or extended family have history of kidney stone. It’s interesting to note that ultrasound didn’t identify the stone, but CT scan (which was supposed to my last test did).
The surgeon ordered a Litholink test, and here are the results:
Urine Volume (liters/day): 4.19
SS CaOx: 0.96
Urine Calcium (mg/day): 77
Urine Oxalate (mg/day): 30
Urine Citrate (mg/day): 751
SS CaP: 0.3
24 Hour Urine pH: 6.677
SS Uric Acid: 0.07
Urine Uric Acid (g/day): 0.573
Vol: 4.19
SS CaOx: 0.96
Ca 24: 77
Ox 24: 30
Cit 24: 751
SS caP: 0.3
pH: 6.677
SS UA: 0.07
UA 24: 0.573
Na 24: 109
K 24: 84
Mg 24: 132
P 24: 0.878
Nh4 24: 45
Cl 24: 121
Sul 24: 26
UUN 24: 7.58
PCR: 0.7
Cr 24: 1707
Cr 24/Kg: 19.7
Ca 24/Kg: 0.9
Ca 24/Cr 24: 45
I have couple of questions. Will be grateful for a response:
1. How do my Litholink results look? If I keep my diet and physical activity the same, are the stones likely to recur?
2. The SS CaOx is 0.96. 1/10th of the allowed range. What does this mean?
3. Do you have any concerns that would prevent me from donating my kidney?
Thank you in advance!
Hi Kamil, from what you tell me and from the results you have no stone risk, yet you formed a stone meaning that these results do not reflect your actual life – nor can a single sample. Acceptance for donation is program dependent, and your program will have its personal say. Assuming your blood studies are normal and you have no medical issues you seem a reasonable donor, but the program will no doubt base its decision on a much larger array of information. If you donate, I would suggest you obtain other 24 hour urine samples – I suspect this one was with an unusual volume of fluids, and that there is something about your life – sports, business responsibilities, flying, diet variations, commutes that caused stone forming. You will want to track that down for long term prevention. Regards, Fred Coe
Dear Dr. Coe,
Thanks a lot for the response. I will work with the donor committee and look for their guidance.
My typical day is similar to the day when I took the 24-hr Litholink. However, there is one key difference: oxalates in my diet. After the CT scan, few weeks ago, I adopted the low-oxalate diet (without any consultation from the doctor). The majority of oxalates in my diet came from Dates, Black tea, Sweet Potato, Almonds and Oranges. I was probably consuming 400-500mg/day of oxalates in my diet. I eliminated the first 3 items in the above food items to cut out calories in my diet (want to get best shape before the surgery).
Would you please take a guess, how my LL results would have looked if I had the above mentioned high-oxalate diet? Does the diet likely explain the reason why I formed the 4mm stone?
Also, One of the test parameters, SS CaOx is extremely low (0.96); is this a good thing? I can’t make sense of this. Please clarify.
When would you have time for a telehealth meeting? I am willing to wait, and if you’ve time before my surgery, it’ll be great.
Thanks so much!
Hi Kamel, The value of 0.96 means the urine could not produce calcium oxalate crystals, so when you did indeed do that urine would have been considerably different. I cannot assess urine oxalate from your diet change, but if it doubled SS would still have been too low to make stones. As for telehealth, I believe you have my secretary’s phone number. If not: 773 702 1475 – and she can make arrangements for you. Regards, Fred Coe
Dear Dr. Coe,
I posted a question couple of days ago. I am anxiously waiting for your response; if at all possible, please reply soon.
Would you be available for a tele appointment after you look at my Litholink report? The Donor review committee at the hospital (Virginia Mason, Seattle) is going to review my case soon. I want to learn as much as possible about kidney stones, and be ready to talk to my Nephro and Surgeon.
(Kindly do not post this comment on the website).
Thanks,
Kamil
Hi Kamil, I am afraid you posted the comment. I doubt a telehealth meeting would be helpful as the program will make its own decisions and I am very booked so I cannot offer a time on short notice. However I am rather encouraged that they will take you within the limitation of my information as in my prior note. Regards, Fred Coe
Hello Dr Coe, little background, my name is mutarr I’m from The Gambia west Africa,I’m 29 years old, I was diagnosed with 1 cm kidney stones and was causing blockage in my ureter, I did an operation last year Ureteroscopy and I had 16 fragmentation and I was able to past most of them except four and they’re growing larger one is 7 and 9 mm, my options are limited as I don’t have the possibility to have my stone analysis, I have consulted a laboratory they don’t do 24 hour urine collection but after reading your article I’ve realized the importance of doing one so I talked to the man at the lab to help analyze my urine which I’ll store in a container in a fridge for 24 hours through your explanation on how, My question is what can the clinician look for in my urine my doctor doesn’t know how to help me with the type of stones I’ve, I feel lost but after seeing your reply’s to this other comments gave me hope, I’ve the test this week I hope I can get an answer from you before my test, thank you and God bless you.
Hi Mutarr, the kind of stone is so important. If you had a CT scan, one can guess. In the urine, amount of calcium, oxalate, citrate, pH and volume all are very crucial. If you can get those, I can try to help. Regards, Fred Coe
Hello Dr. Coe,
I know Calcium intake is necessary for bone health for one and to help people with oxalate stones by eating their oxalates with the calcium but how does Calcium work with phosphate stone formers? I eat a very low sodium/sugar diet with my calcium all coming from dairy and food sources with around 800-900 mg intake a day. My question is if I were to increase to the 1000-1200 mg a day keeping everything else the same would my urine calcium increase? Thank you!
Hi CF, Indeed calcium is important so the usual approach is what you are doing: low diet sodium (1500 mg or so) and 1000 mg calcium from food and – given phosphate stones – perhaps also thiazide to lower urine calcium as much as possible. 24 hour urine testing can reveal if more diet calcium really raises your urine calcium, and also if your treatment is adequate (calcium phosphate saturation is presented by all national kidney stone testing vendors). Regards, Fred Coe
What number do you like to see for phosphorus on a 24 hour urine for someone with phosphate stones? I eat large amounts of fruits and vegs a day, does the natural fructose raise urine calcium?
Hi J, Urine phosphate is diet phosphate absorption – balance is absolute. Fruits and veggies have modest amounts of phosphate compared to meats because by volume and weight are higher in water content. The urine phosphate per se is not viewed as a stone risk factor unless so high as to suggest excessive cola drinks (the largest contributor). The amounts of fructose in fruits is very small – fruit juices are something else altogether: one uses 8 oranges to make a large glass of orange juice, but who can eat 8 oranges?. The main issue in your stones is supersaturation with respect to calcium phosphate, which is on your 24 hour urine report – it needs to be below 1. Common factors that matter are urine calcium, pH, and volume. Best, Fred Coe
Hi Dr Coe,
I am a 31 year old female and I suffer from hypothyroidism, sjogrens, and lupus as well as infertility. Recent bloodwork and urine analysis showed elevated calcium and reduced egfr. Upon further analysis i completed a 24 hour urine study which returned
Slightly low urine volume (1.89 L)
pH of 6.8
High Calcium at 298
High sodium at 232
High Brushite at 5.1
High Sodium Urate at 2.59
All other results of the stone risk analysis are within normal ranges.
What is the recommended course of action for already at risk kidney patients due to underlying autoimmune disorders? Watch and monitor? Removal surgery?
Secondarily I’m an avid runner and struggling to understand how to monitor my sodium and sugar intake given marathon training requires those exact things during the run. Any advice is very appreciated!
Hi Brooke, The low volume, high pH and high calcium are the main drivers of calcium phosphate stones. Sjogren and lupus both can reduce kidney ability to lower urine pH, and high pH is commonly present in women for other reasons, in their absence. As a runner you lose sodium from rapid breathing and sweating but your urine sodium is very high so your sodium intake over balances your athletic losses. I would think high sodium sports drinks need to be used when actually running but not otherwise. Likewise for high sugar energy beverages, they help during peak exertion and are worse than useless between running episodes. More water altogether is an obvious suggestion. The 5.1 for brushite (calcium phosphate) is not good – it needs to be below 1, so you can use this in assessing your strategy with your physician. As for stone surgery, it is important if there is pain, bleeding, obstruction, or infection, and optional otherwise. Many suggest removal of stones that a too large to pass, a surgical issue I prefer to not comment on. You mention reduced gfr, and my concern is whether there is some obstructing stone; that must be excluded by your urologist as obstruction can injure kidneys. If blood calcium is high, you may have primary hyperparathyroidism, a curable condition your physicians need to pursue as a possibility. Regards, Fred Coe
Hi Dr. Coe,
Phosphate stone former here doing all the right things for prevention, following with urologist, and doing 24 hour urines. Urologist doesn’t favor CT scans or putting me on thiazide even with having 3 stones total in the kidneys and recently one of those is newer per ultrasound. I know women have more alkaline urine in general and just wondering from the fruits and vegs I eat a day which ones make the urine the most alkaline. I eat a serving of banana, apple, pear, clementine, sweet bell pepper, broccoli, cucumber, campari tomato daily. Others I eat in place of some of these would be grapes, strawberries, and blueberries. I’m not a vegetarian but it sure looks like it with all these I do eat! Just wanted your opinion on which ones would be the most alkalizing and I could swap them out for others. I’m trying to do other little things since my urologist doesn’t think I need to do anything else or even see a nephrologist. Have very low sodium and sugar diet, right amount of protein, and drink 12-14 cups of water a day. Thank you!
Hi Jen, Phosphate stone formers have something amiss in their regulation of urine pH vs. citrate. IN general the stones arise because of high urine calcium and high pH. Thiazide lowers urine calcium and urine pH and I favor it as opposed to letting more stones form. Perhaps your physician might want to consider this, or involve your PCP or a nephrologist. Regards, Fred Coe
Greetings,
I just passed a handful of stones, two being 8mm and 10mm in size. All were 100% hydroxyapatite. I had a CT at the hospital that shows I have 30 more to go distributed among both kidneys. I have been under care of a nephrologist and urologist with quarterly 24 hour urine collections, blood tests and urine tests. They showed pretty normal levels. I take medication daily. I have sponge kidneys. I had a clear CT a year ago. I need help. I think my doctors are missing the mark. I had less before the medication. I am an otherwise healthy 42 year old woman. Any help is appreciated. Thank you
Hi Jessica, It is most likely that you do not have MSK but rather calcium phosphate stones that when very numerous can give that impression. Usually these stones arise because of an alkaline urine pH (above 6.5) with a higher than normal urine calcium excretion (over 200 mg/d) or low urine volume (below 1.5 liters/d) or citrate (below 400 mg/d) or some combination of these. Given regrowth of stones, I would suggest your physicians might want to obtain consultation for you at a convenient stone center. I would endorse that idea. Regards, Fred Coe
Hello Fred,
I am a 33yr old woman, who has developed numerous CaP stones since I was 20 (2010). I am otherwise healthy with no additional illnesses/diseases with a BMI of 21. I have 3 children. 1st pregnancy was fine (2017), 2nd pregnancy(2019) I developed numerous CaP stones in the last trimester and had ureteroscopy to remove them after birth. During my last pregnancy (2021), I developed numerous Brushite stones (95%) and 5% HA stones. I was hospitalized 8 times during the 2nd and 3rd trimesters due to a 1.2cm stone being stuck in EACH ureter, causing chronic kidney infections. I was induced at 38weeks because my fevers were affecting the baby’s heart rate and I promptly had 2 ureteroscopies to remove numerous large stones. Now, 1yr later, I am starting to pass stones again. I drink approximately 100oz of water/day because i’m still breastfeeding. I am taking no medications, other than a post-natal vitamin. I eat a primal-type diet that’s already low in sugar. Do you have any more suggestions? Any thoughts on Vitamin K2? Thanks!
Hi Kelli, Brushite stones are critical to treat, usually with low dose thiazide + low diet sodium. They recur, grow all to rapidly, and cause trouble. I do not manage them with just diet. Low diet (24 hour urine) sodium of about 1500 mg/d is the base to which I add chlorthalidone 12.5 mg daily with pre treatment and treatment 24 hour monitoring. The article you write on tells about it and links to others that expand on my little comment here. Please do not let these stone go unchecked. Regards, Fred Coe
Hello-
I am a 56 year old female with a history of passing stones for 31 years. The analysis of stones is 100 % CaP. Litholink results (like many others here), High urine pH (6.84), low citrate (115), high calcium (259). My doc only reported that “labs were stable”, which is frustrating. I take a very small amount of hydorcholorthiazide because it makes my potassium and blood pressure drop so much. In fact I am on midodrine to counteract the blood pressure drop. I also just got the results of my bone scan, and I have osteoporosis in the femur. I am afraid that any additional medicine that may be suggested for me will further add to an increase in more urinary calcium. Don’t know what to do. I am a high school teacher and do not want to retire yet. Please help. Thank you
Hi Laura, Your problem is complex and even more now than when I wrote this article. There is something wrong with how your kidneys manage pH and citrate, and also calcium. You might have gene abnormalities (heterozygous for one of the genes causing renal tubular acidosis) causing this, and your physicians might want to consider getting your DNA analysed for them. It can help in adjusting treatment. However, this is rather a new approach and best done if you have a kidney stone center within reasonable distance. Your physicians might want to suggest telehealth consultation if that is not the case. I say this because your citrate is very low. Regards, Fred Coe
Hi, my daughter is 14, almost 15. She just had her first kidney stone and is still in aching pain from them. They have tested her blood and will soon get back to us. Her mother (45) has had calcium phosphate stones since having her 2nd child. My wife’s mother has had these same stones most of her life and now is dealing with kidney failure. So, 3 generations of these types of stones.. what can I do to help my 14 year old daughter? She is very healthy, active and slim. Sounds like cutting sodium and sugars from diet is a starter? My daughter is very down and disappointed. I will also look for stone centers in the Salt Lake area.
Regards, Jay
Hi Jay, Given three generations of stones one suspects a genetic cause and gene disorders are not being diagnosed with some important specific treatments as a result. I would prevail on the physicians you use to seek out gene variants in the family and perhaps aim treatment more accurately. Regards, Fred Coe
Hello Doctor,
I am 58 Years old, and have producing Calcium Phosphate Stones for 30 years. The older I get the more stones. In the last 5 years I have had 38 Lithotripsy and more recent Laser procedures. The stones also are getting larger. In 2019 I had 7 different procedures to remove a 16mm, and a 22mm stones. From 09/10/22 to 12/3/22 I had 9 procedures to remove 6 stones from the left kidney, and a 52mm stone from the right that required Percutaneous Nephrolithotomy. I was diagnosed with Stage 3b Kidney Disease and High Blood Pressure in 2021. Also take medication for severe heartburn, and digestive problems, but Doctors cant figure out what is causing it. I have 3 sons (2 different Marriages) 2 were diagnosed with Pancreatitis when they were 5 years old. My first son (38 years old) had his Pancreas removed in 2010 by the pioneer Dr. David Sutherland of the University of Minnesota. He told me then that my stone production, and the Pancreatitis I gave to my boys could be related. I have had the same Urologist for 26 years, but he has never been able to prevent the stones. Thank You for any advise you could offer.
Hi Bill, I do not know how to relate the familial pancreatitis with your kidney stones. But given so many and for so long you need seemingly more diagnostic work to find the cause and more effective treatment. A medical school stone program would seem reasonable if your physicians agreed. I would suspect to high a urine calcium and pH, but these can have have their own causes, some genetic. WIthout more information I cannot say much more. Regards, Fred Coe
I am a 48 year old male. Diagnosed with Sjogren Syndrome and limited Wegener (vasculitis), interstitial nephritis, DRTA. I have had several stone removals of smaller ca/phosphate stones but they have recently become staghorn brushite requiring going through my back to remove. They have put me on potassium citrate 4x a day and in a years time the large staghorn stone have reformed. Is there anything other than the potassium I should be doing to keep these stones from forming? Thank you
Hi Steven, I presume you have acquired distal renal tubular acidosis from Sjogren Syndrome. If so your urine pH is high (>6.5) and citrate low (<200 mg). At issue is calcium phosphate supersaturation. If the Dx is correct one aims to bring that SS below 1. More and more citrate may raise urine citrate, and urine pH cannot go above 7.9, so sometimes this works – of course along with as high a urine volume as you can tolerate. Sometimes urine calcium is above normal, and thiazide or low diet sodium helps. There are a lot of ‘sometimes’ here, and all I can do here is mention a few. Ultimately one can usually succeed in lowering CaP SS but it may take multiple tries. Regards, Fred Coe
I am a 64yo female, BMI 22, no chronic medical issues or medications except that I have suddenly (in the last year) developed fast-growing kidney stones that are seemingly unrelated to any change in diet. Urine ph is 7.08; citrate is .763; SS CaOx 6.28; SS CaP 1.76; Calcium 159; Daily uric acid .817. My gfr has been steadily decreasing, suggesting that the stones are affecting kidney function. I have yet to pass one so there is no stone analysis available, but bilateral stones nearly tripled in size in ~6 months. I’ve seen 2 different urologists, who seem content to treat the stones surgically without looking for underlying causes. I eat a whole food, plant-based diet, drink lots of water. Not sure where to turn and appreciate any guidance you can provide.
Hi Noelle, I am suspicious that your diet may be causing stones via high oxalate – you do not mention urine oxalate and your SS CaOx is high at 6 as though oxalate is a main problem. Is that true? Regards, Fred Coe
Hi Dr. Coe. Thank you for the excellent content. I’m a 36 year old male and have been having large CaP stones requiring multiple interventions since 2018. I’ve been on Pot Citrate 10meq BID and chlorthalidone 25 mg daily for > 1 year. Just received 24 hr urine results and appear to be getting worse. Volume >4L, SS CaOx 4.27, Urine Ca 371, Urine oxalate 70, Urine citrate 548 – improved!, SSCaP 2.22, pH 7.095, SS Uric Acid 0.06, Urine uric acid 1.471, urine Creatinine (2975) and Urine Phos (2.132) also significantly increased from last measurement. The only improvement (maybe?) since starting Pot Citrate and chlorthalidone is higher urine citrate, but urine calcium (worse), pH (stable), oxalate (worse), SS CaP (worse). Unsure if these are helping, or what next step is. Dietary sodium had improved, but still above 1500mg (~2500mg/day which was a substantial decrease from before). Any thoughts are so greatly appreciated! Thank you.
Hi Tyler, you have very high urine pH and calcium but the creatinine value looks very high unless you are a muscle builder or very large. This suggests over collection error. The urine phosphate is also too high. The calcium/creatinine is 124 mg/gram, within normal limits. This would make your urine citrate very low once we adjust for the collection presumed error. Low citrate and high pH suggest you may have an underlying gene abnormality in the dRTA family. Your physicians might wish to look at this possibility given the common use of gene testing today. If you have this problem treatment might be affected. Regards, Fred Coe
Hello Dr. Coe,
I appreciate you so much. I am a 58(f) and started making large stones 2019, I have never had Lithotripsy and my para thyroid is normal. I recently whet on Hydrochlorothiadine (25mg) for the past three months. My urine did change with the thiadine showing only a high PH and my blood calcium went up(10.4, )I had two more CAP stones removed three weeks ago to make my count eight in the past three years with laser and basket. . I came home with a UTI staph and ecoli infection only to make the recovery sweeter. Three weeks later I am still having kidney pain and another CT scan next week. Any advice?
Maria Johnson
I should also mention I do 80 ounces of water a day and have had my diet evaluated and changed over the years.
Hi Maria, Your stones began rather late in middle age and I suspect you may have primary hyperparathyroidism. The latter is cured by surgery. I would rule out the latter very carefully. The other possibility is postmenopausal bone loss, so bone mineral density is very important to measure. Regards, Fred Coe
HI, Dr Coe,
I have read your articles with great interest over and over and been trying to figure out what to do to help my son
I have a 21 year old son who was injured in an accident in early 2020 and now has a traumatic brain injury and is now severely disabled. He started passing CaP stones (HA) in Oct 2021. In Nov 2022, the stones got so large that they blocked both of his ureters and required emergency surgery to put in nephrostomy bags.
Since he is on a feeding tube, we have absolute control over his diet and nutrition. We purchase food at the store and cook and blend for him so he is on a very healthy diet.
We did a Litholink 24 hour urine in Oct 2022 and again in Apr 2023. Despite making significant dietary changes to lower CaP saturation to reduce stone risk, it had minimal impact. Urine pH averages 7-7.3. CaP SS is 2.2. Urine Calcium is around 300, Citrate in the upper 300s, Creatinine around 1000. Oxalate went from 45 to 25 mostly with removal of Vit C supplementation we think. We reduced Sodium intake to around 1500 mg per day and saw his serum Sodium drop to 134 but his Urine sodium went from 147 to 117 with no change in Urine calcium.
I’m kind of at a loss of what to do. We’ve considered Potassium Citrate but concerned about raising Urine pH. Does Potassium Chloride help with Urine Calcium and would that be worth trying (and how much)?
Any other suggestions or ideas you have to help would be appreciated. My son can’t communicate so we just have to guess and read his body to determine if stones are bother him or not.
Thanks for your help!
Dan
Hi Dan, What an unfortunate problem! I am so sorry it happened. I think bone mineral loss from immobilization may be a main factor raising both urine calcium and pH. Perhaps his physicians might want to consider means to stabilize bone. Of course upright posture – if workable – and perhaps other means known to physical medicine. Likewise perhaps a bisphosphonate might stabilize bone and reduce urine losses. All this is, of course, the business of his physicians as I do not know his actual clinical state. Regards, Fred Coe
Thank you for your reply! It is a complex situation that has gotten quite a bit of physician attention.
The bisphosphonate idea is an interesting one we have not explored yet. I will bring that up with his GP
Any thoughts on using potassium phosphate (like K-phos) to help lower urine pH and calcium? Is that a potential option to explore?
Thanks!
Hi Dan, Use of acid phosphates to lower urine pH would be highly experimental as the imposed acid load may have untoward effects on bone. Acid will raise urine calcium, in general, as well. Regards, Fred Coe
Hello Dr. Coe,
My son is 17yrs old and has spastic diplegia cerebral palsy. He is active at school and plays sled hockey. He has neurogenic bladder and needs to self catheterize every 4-5 hours. He has stage two kidney disease. He has suffered from many UTI’s and kidney infections on and off since he was a baby. He was septic for two of them. But in the last year since he has been drinking more water and cathing more often he has not had as many infections.
In January he developed a stone. He had a 7x5mm , 52mg stone: 70% calcium phosphate (carbonate form includes carbonate apatite and carbonated amorphous calcium phosphate), 10% CaHPO4 (Brushite), and MgNH4PO4 (Struvite). He had an ultrasound which still showed a 1.3cm stone in lower lobe of left kidney. He had a 24 urine litholink test.
urine volume 1.75
SSCaOx 3.56
Urine Calcium 154
Urine oxalate 19
Urine citrate 295
SSCaP 1.81
24 hour urine pH 6.629
SS uric acid 0.19
Urine Uric Acid 0.603
Recent blood work that seemed a little off WBC 12.7, Neutrophils 9.2, Monocytes 1.0, Albumin 5.5, Cystatic C 1.02
Because he has to self- cath, the stone is not going to pass through the catheter.
What is the best way to take care of this stone? Are there any diet adjustments he can make? Is it likely that this type of stone will come back?
Thank you for any help you can provide,
Heather
Hi Heather, The MgNH4PO4 – struvite – suggests infection with urease possessing bacteria. The urine studies show the high pH and low citrate that are compatible with infection but no ammonia – Is is high? If high that confirms infection. If not, perhaps his kidneys no longer produce a properly acid urine. As for the stone, if it is indeed infected surgery is especially crucial so cultures and the urine ammonia are very important. I always favor ureteroscopy via a sheath with removal of fragments. Regards, Fred Coe
Hello, I’m a 25 year old female with 3 children. I’ve always suffered from UTI’s my childhood into my adult life. My 2nd pregnancy I began forming stones in third trimester, we were unaware at the time what this was and a couple months later I delivered. Because of this, this was left unattended into my 3rd pregnancy. I had all of my children back to back to back. I had 4 surgeries to remove stones out of both kidneys as I ended up in AKF. (Halfway through my 3rd pregnancy).Even after the last surgery, in which involved removing a nephrostomy tube that was placed in my right kidney for the time being, I had passed a 8mm stone consisting of
90% Calcium Phosphate(Brushite)
10% Calcium Phosphate (Apatite). I’m assuming I passed it with little pain from having stents placed twice on both sides and removed of course, during this period.(ureters we’re stretched). It’s been 2 years since 3rd pregnancy, and stayed pregnancy free, where the surgeries took place and I’ve ended up with yet another stone. It’s excruciatingly painful and a size 5mm. At this point, I’m having them outside of pregnancy, so I’m dumbfounded on what this underlying cause for these stones are, as we suspected it was from a condition during pregnancy. I want to do what I can to prevent myself from having such terrible stones.
Hi Alivia, Brushite stones are a perfect horror and hard to prevent. Here is a special article about the crystal. I advise finding expertise in a university stone program convenient to where you live. Do it sooner than later. Regards, Fred Coe
Hello again Dr Coe, I want to thank for referring me to the UCM for in detail diagnosis and prevention. I’ve read your articles multiple times and tried to learn as much as possible. You mentioned in a previous post, I may have RTA even with my serum results which are normal.
I have taken potassium citrate for 2 years now and all I have saw is increase in renal calcification in both kidneys. I have passed maybe 30 calcium phosphate stones measuring 1-3mm. For some strange reason I don’t have renal colic.
I cannot proceed with with UCM until I have my genetic testing results which will take around 2 months to obtain. As you can see the potassium citrate is hardly increasing my citrate levels. I have seen where you explain that taking POTC may actually increase phosphate supersaturation if it fails to increase citrate levels.
Due to my high urine PH and no citrate I’m making stones at an alarming rate. I’ve increased my urine output to 3lTR and also lowered sodium and increased dietary calcium. I have decided to stop taking the POTC. What are your thoughts on this?.
Im having a dexa scan soon to identify if my condition is lowering my bone chemistry. I’m really into fitness and this stone burden is really bringing me down and I feel a bit hopeless.
My only suggestion that may somewhat help my condition it appears is thiazides. The nephrologists in the UK are very reluctant to prescribe as they don’t have any knowledge in this field. My question to you is. As I have normal serum and normal calcium excretion, do you think indapamide or any other will be effective at reducing my stone burden. I have only really saw you recommend it to people with hypercalciuria which I don’t have. I have possible familial RTA, no urine citrate and high urine PH of 7.
I have done tests with and without potassium citrate (5G per day split between 3 doses) and nothing changes. PH and citrate remain the same.
Sample BB940686N (URINE) Collected 08 May 2021 09:00 Received 13 May 2023 14:25
Urine Stone Former Profile
Urine PH 7.00
Creatinine Concentration 6.7 mmol/L
Creatinine Excretion * 20.2 mmol/d 7 – 13
Urine Calcium Concentration 2.46 mmol/L
Calcium Excretion 7.4 mmol/d 2.5 – 7.5
Urine Phosphate Concentration 5.89 mmol/L
Phosphate Excretion 17.79 mmol/d 15 – 50
Urine Stone Former Profile
Miscellaneous Sendaway :
Assayed at HSL, London.
Collection Period 23 h
Volume 3.020 L
Oxalate Concentration 89 umol/L
Oxalate Excretion 269 umol/d 100 – 460
Urine Oxalate:Creatinine ratio 14 umol/mmol 0 – 34
Urine Citrate concentration 0.10 mmol/L
Citrate excretion * 0.30 mmol/d 0.6 – 4.8
Urine Citrate:Creatinine ratio * 0.02 mmol/mmol 0.04 – 0.33
Sample BB752751C (SERUM) Collected 26 May 2021 09:36 Received 26 May 2023 14:14
Bicarbonate
Bicarbonate 24.7 mmol/L 22 – 29
Estimated GFR (CKD-EPI)
Estimated GFR (CKD-EPI) 82 mL/min
Profile
Sodium 141 mmol/L 133 – 146
Potassium 4.3 mmol/L 3.5 – 5.3
Creatinine * 105 umol/L 59 – 104
Total Protein 71 g/L 60 – 80
Albumin 46 g/L 35 – 50
Calcium 2.34 mmol/L 2.25 – 2.55
Corrected Calcium 2.26 mmol/L 2.20 – 2.60
Inorganic Phosphate 1.11 mmol/L 0.8 – 1.5
Total Bilirubin 12 umol/L 0 – 21
Alkaline Phosphatase 53 IU/L 30 – 130
Alanine Transaminase 36 IU/L 5 – 41
Urate
Urate * 163 umol/L 200 – 430
Stone Analysis (Calculus)
Biomnis N-Z :
Stone type : Unknown
Composition :
Component 1 : Carbapatite (CA) 95%
Component 2 : Whewellite(calcium oxalate monohydrate)(C1) 5%
Comments : Phosphate lithiasis
Any advice or information would be greatly appreciated Dr Coe.
Hi Jonathan, It does seem like genetic distal RTA and I am not so sure that the k citrate is a good idea. One really wants to know which genes are involved. Your urine calcium though not dramatic is not entirely benign and I would suggest very low diet sodium as a way to lower it. One might consider stopping the alkali as doing more harm than good. These are just ideas, as I do not really know your medical situation. Regards, Fred Coe
Hello again Dr Coe,
I’m currently in communication with UCM. I’ve decided to take your advice wait until I have gene testing result and have attempted to see my urine calcium on a very low sodium diet. I will then seek your guidance if I cannot lower my urine calcium under 200mg/d
In the meantime I just have some general questions if I may.
Would someone like myself be able to stop future stones by bringing my urine calcium below 200mg/d & 3 litres of fluids even though I have no citrate and PH 7 urine. I’m unable to locate anywhere to test for capss here in the uk.
Could low potassium levels cause me to have no citrate. My serum potassium was in range but my radiometer bloods came back only 3.0 potassium and acidic PH of 7.329.
Much appreciated looking forward to speaking to you in future.
Hi Jonathan, If you have RTA from one of the gene defects the low citrate will generally be fixed but I suggest waiting until you know before making any attempts at stone preventions apart from low diet sodium and high fluids. Potassium chloride will help correct the low serum potassium and does not contain alkali. Best, Fred
Thanks so much for your guidance. I’ve now taken the following steps.
1: Lowered sodium to under 1500mg
2: increased water intake to 4ltres spread through waking hours.
3: Stopped taking alkali foods/supplements.
4: Added potassium chloride to my water at 4grams spread out.
5: Reduced protein intake to 1.2g per kg.
I’m having a lot of difficulty trying to get my urine labs recently.
My question is do you believe with these steps I’m stopping stone formation at PH 6-7-6.9. My average calcium excretion at 143 mmol of sodium was 300mg. I also have a low phosphate excretion and last test showed no urine citrate.
I’m hoping my citrate was gone because of hypokalemia but It’s likely I have because of rta.
No one in the UK provided SS CAP values.
Sorry for all the questions it’s just my stone burden is causing me a lot of stress sleepless nights to having more than 20 stones in my kidneys at present. thanks in advance.
Hi Jonathan, I do not mean evasiveness, but you ask for my beliefs about your present situation but I do not really know your situation to say much beyond generalities. Urine with no citrate usually means an underlying disease, such as renal tubular acidosis and that should be sought via genetic analysis. I do not believe I know the gene results in your case. Regards, Fred Coe
Hello again Dr Coe,
Do you think this study below brings a strong argument for the increase in calcium phosphate stone formation and also strong case for use of Chlorthalidone for phosphate stone prevention. Thank you in advance.
https://journals.lww.com/jasn/Fulltext/2019/07000/Chlorthalidone_Is_Superior_to_Potassium_Citrate_in.8.aspx
Hi, NO. I know the work well as the authors have been colleagues for many years. These animals did not have renal tubular acidosis. Fred
Hello Dr. Coe
i am 35 years old and produce stones once every year since 2017. Always in the left Kidney and always Calcium Phosphate (last one was Brushit 70%, Sodium hydrogenurate 30%). starting to get tired of it. maybe you have an idea or an approach to therapy?
Best Regards
Oliver
Hi Oliver, Brushite stones are a major problem and difficult to stop. Commonly they reflect to alkaline a urine pH and also high urine calcium, and given only one kidney is doing this you may have some damage or some urological drainage problem on that side. Ideally you might be best served in a university based stone program if there is something near enough. Brushite stones are not very common so physicians in general do not have much experience with them. Regards, Fred Coe
Hello Dr. Coe,
I am 52 years old and have CaP stones. I was originally diagnosed with Calcium Oxalate stones and recently found that my urologist didn’t relay that they were CaP stones. I had a 24 hour urine study in Sept 2022 with the following results.
PH Urine – 7.4
Calcium – 317
Oxalate – 31
Uric Acid – 588
Citric Acid – 572
Sodium – 128
Sulfate – 14
Phosphorus – 762
Magnesium – 148
Ammonium – 28
Potassium – 79
Creatinine – 1570
Calcium Oxalate – 1.10
Brushite – 3.13
Sodium Urate – .64
Struvite – 12.03
Uric Acid – .04
The urologist prescribed potassium citrate but after reading your article that may be leading to my increase in Urine PH. I recently starting seeing a kidney specialist and he mentioned that it could be having an adverse effect. As of today, I will not be taking the potassium citrate.
What should I be pushing my kidney specialist to test? I have already asked that we get an updated 24 urine test. I know you mentioned hyperparathyroidism as a possible contributor, is there a test for this? Is there a test for ammonia production and what can I do to decrease the ammonia production? How do you test for the CaP supersaturation?
Is it accurate to say that anyone with CaP stone formation has an abnormality in their proximal tubules?
Hi Scott, You have a high urine calcium which probably is the original cause of your stones. One might imagine the K citrate raised your urine pH and helped convert the stones to calcium phosphate, and I cannot be sure that is incorrect. But conversion can arise from shock wave lithotripsy and perhaps from repeated stone passage with kidney injury from repeated obstruction. I presume you have been fully evaluated and have no systemic cause of stones.In our studies of CaP stone formers we have shown higher urine ammonia and lower citrate than in other stone formers. On citrate you have normal urine citrate and ammonia well above your urine sulfate (which is rather low) – presumably off citrate urine ammonia will rise and citrate fall, as noted above. CaP SS is ‘Brushite’ SS on the report. Brushite is the initial crystal phase in urine. Regards, Fred Coe
Hi Dr. Coe,
If urine calcium is WNL (~135) via thiazide, am I at risk of calcium phosphate kidney stones if urine PH is high (6.85), SSCaP > 2? (no idea about urine ammonia) My nephrologist thinks I’m ok on Kcitrate (for lower end of potassium limits) with that urine calcium, but I’m inclined to switch to Kchloride as you suggest since I’m not sure this answer is even known.
Thanks,
Mary P.
Hi Mary, if your CaP SS is above 2 I would be concerned about calcium phosphate stones. Do you know your stone type? Possibly your physicians might want to use potassium chloride for repletion, but they would have access to more information about you than do I. Regards, Fred Coe
Dear Dr Coe,
I had a stone last April which was 4 mm by CT. I believe it came out (or dissolved) after one month. I was getting flank pain till than but stopped getting pain afterwards. In June no stone was found on X-ray.
My urine pH was 5.4 . No other test was done.
The I started taking potassium/magnesium citrate. First I was taking 15 ml daily but it failed to raise pH. Then from 3 months ago I have been taking 30 ml daily. The latest report has pH 6.5.
Now, am I at risk of calcium phosphate stone? Should I lower the pH by reducing the citrate supplement a bit?
Hi Mactoul, pH alone is not a sufficient guide to answer your question. Was your stone uric acid? What else is in your 24 hour urine that might cause stones? You really should consider a proper evaluation for causes such as this one, to be sure things are indeed on track. Regards, Fred Coe
Hello Dr. Coe,
I recently passed a 2nd known stone after 4 years and I have a high ph–6.7. I am 49 years old woman who was also recently diagnosed with osteopenia. The 24-hour urine test leaned towards CaP stones. My urologist said that I should add more citrus to my diet after my 24 hr test due to the lower citrate with the following results:
Urine Volume – 3.4
PH Urine – 6.7
Calcium – 170
SS Ca Ox- 2.49
SS Cap – 0.56
Oxalate – 23
Citrate – 479
SS Uric Acid – .04
Urine UA – 0.355
Sodium – 82
Potassium – 29
Magnesium – 63
Ammonium – 28
Phosphorus – 0.404
Nh4 -28
Cl 24 – 97
Sul <11
UUN 24 – 3.27
PCR 0.5
Reading your article, I am not sure if I should. It seems that I should just be doing lots more water every day to help push off these stones. I have an appt next week with the urologist and I have a lot of questions for her. Plus, my GP had me do a US after the CT at the ER in November, that said I may have a "possible, non-shadowing 5mm" that my urologist finds unlikely. But now I dont know until I get more imaging.
In the meantime, what can I do? Neither stones were caught so I dont know the true makeup of them. All I know is that I want them to be gone, as I am a very busy woman with a demanding job and family. I appreciate any insight. Thank you.
Hi Natalie, At the moment, with your current high urine volume, stones should not form, so I presume the conditions of this collection may not represent your overall situation and urine volume as you pursue your busy work and personal lives. That said, there are odd features that may be worth considering. Your urine sulfate is too low to measure (sul), meaning you have a very low diet acid load from protein sources, and in fact your measured diet protein (PCR) is below WHO standards for normal protein intake to maintain good health. Possibly the urine was not a full 24 hours – there is no creatinine in your record. But urine ammonia, which is to remove diet acid is normal, at 28, so there is a great disproportion. We have described this in CaP stone formers and indeed your urine pH is high. But – this is a long answer – your urine potassium is stunningly low at 29 which points to either laxatives, diuretics, some bowel disturbance, or a less than 24 hour urine collection. Put another way your results cannot be interpreted except in relation to a detailed review of your diet and life management which is to say except by your personal physicians. They would need an appropriate amount of time to really understand things. However the good part is that numbers are what they are and always have rational explanations. So I suggest you and your physicians take the time to figure out the ammonia, sulfate, potassium issues and resolve on a plan. I cannot, being a mere outsider. I know this kind of answer is not satisfactory but I cannot do better from a long way off. Regards, Fred Coe
Dear Dr Coe,
I am a 33 year old healthy male with no known health issues. I once got a calcium phosphate stone 10 years ago that past naturally 80% CaPh. I recently had a laser lithotripsy to to remove 2-6 mm stones in my lower ureter and one in 5 mm stone in my kidney. It was a difficult experience with a 2 month blockage, The stones were 80% Calcium Phosphate, 20% Calcium Oxalate.
Post operation I had a 24 hour urine test and the results were following:
Vol 2.3 L
Ca 620 mg/24 hr
Oxalate 34
Citrate 609
CaP Sat 3.42
PH 6.23
Na 174
Mg 190
Ph 1314
Ammonium 73
Creatinine 1877
Protein Carbonic Rate 1.2
Uric Acid Sat 0.51
The results indicate extreme hypercalciura (620). What can make it that high of a value? Could they be wrong? I though maybe it was Primary Hyperparathyroidism (PTH) and got a blood test:
Parathyroid Hormone 98 pg/ml
Calcium 8.9 mg/dl
Vitamin D 22.3 ng/ml
The results would appear to throw out PHT because of my average/low Ca levels. Could it be Normocalcemic PHT? I have a hard time believing that would make my urine calcium that high? What could the other causes be: Sarcoidosis, malgligant neoplasm? How does a healthy person be such an outlier? I have my serious doubt it’s kidney disease and have no family history of kidney stones. I have concerns about my bone density and osteoporosis over time. That’s a massive Ca leakage from my body. Can dietary changes even make a impact with such a large number?
Any recommendations I would appreciate. Your video and article were the best source of information on CaPh stones out there. Thank you for provided so much great detail.
Hi George, About why you make stones I see little problems, it is mainly the high urine calcium. This latter is unusual – very high, low normal serum calcium and very high serum PTH. A serum 1,25Vitamin D (special test, not the regular 25D) might help and I predict it will be high indeed. But at the same time PTH is very high and you do not report a low calcium diet. Possibly you may have a gene defect involving calcium handling. Let us say you need extremely expert help here. The usual gene defects, NaPi 2c and CYP24A1 do not fit well, although you should be sure about your serum phosphate. I notice a very high urine NH4 (no sulfate) which is not explained by very high protein intake (PCR 1.2, which may be a clue. Nothing else will do here except you get someone with great expertise – usually found in a university stone program. Regards, Fred Coe
Dear Dr. Coe.
Thank you for this comprehensive article. I was alarmed to hear that CaP stones can be damaging, but think my situation might be different and would appreciate your thoughts.
I am male, aged 32 and otherwise in good health. I noticed small, chalky, off-white stones in my urine with some mild stinging last year. This happened infrequently, perhaps only 2-3 times with each episode lasting a few days. I decided to see a Urologist on it happening again for and took a sample of stones with me. They turned out to be 100% CaP. A urine test was normal, showing a pH of 6 with no infection, blood, cells, casts, protein, glucose or crystals. Blood tests showed normal ranges including Calcium (2.38 mmol/L), Albumin (43 mmol/L), Adjusted Calcium (2.43 mmol/L), Potassium (4.1 mmol/L), Urea (4.6 mmol/L), Uric Acid (292 umol/L), Creatinine (85 umol/L) and eGFR (CKD-EPI) at >90 ml/min. However I did personally note that Sodium (144 mmol/L) and Chloride (106 mmol/L) were at the top end of the normal range given.
A flexible cystoscopy then found 20+ small white stones and sediment in my bladder, along with a high bladder neck that may be causing urine stasis. I then had CT and US imaging with urine flow testing. My prostate is not enlarged at 14cc. My peak flow rate was 7.5ml/sec, significantly low for my age, despite voiding 512 ml of urine. I also had a post-micturition residual of 72ml which was abnormal. Other than this, US and CT images came back normal, though I noted the report stated “small simple cysts within both kidneys”.
Further urodynamic testing is apparently needed to confirm that it is the high bladder neck causing urine stasis (rather than weak bladder tone) but my urologist believes it probably is this enabling the CaP stones to form.
I am now taking Tamsulosin for 3 months and increasing my daily liquid intake to 2L+ to see if I can pass the stones myself (I am very poor with my liquid intake so hoping this will make a real change!). Otherwise, a rigid cystoscopy would be needed to remove the stones and potentially a transurethral incision of the prostate to remove the high bladder neck obstruction.
At 32, I am hesitant to have surgery, especially with risk of retrograde ejaculation, so I am hoping this can be avoided. Do you think if I am unable to pass the stones myself, surgery to both remove them and the obstruction are necessary, given I don’t think I have other symptoms apart from the rare passing of the stones and associated mild discomfort? Can I live a healthy life with CaP stones still present in my bladder?
Thanks in advance.
Hi Don, I would think your physician might want to obtain a 24 hour urine to see if there are major risk factors for crystallization which mild voiding irregularities would amplify. The most likely one would be high urine calcium. If surgery is planned to prevent crystals, 24 hour urine testing for causes of crystals cannot be a bad idea as it is easier to reduce urine crystallization potential than it is to do surgery. On the other hand of your urologist believes that the crystals are not the main problem and that you need surgery to protect urinary drainage of your kidneys that is a surgical decision in which crystals are not consequential. But even then one might make the measurements in case high urine stone risk eventuates, some day, in kidney stones. Regards, Fred Coe
Hi Dr. Coe,
I am 60 year old female who just had my first ordeal with kidney stones. I am a sufferer of OAB, incomplete bladder emptying requiring self cathing 4-6 times a day with bladder spasms. When I wX diagnosed with kidney stones, I had 4 kidney stones with the largest 12mm and all were obstructing stones with pyelonephritis. I had an emergent ureteroscopy and it was discovered that I had severe hydronephrosis with purulent urine coming from the kidney via the ureterel stent. A month later, I had the laser lithotripsy and I did pass a stone. It was analyzed as being Calcium phosphate ( hydroxy carbonate apatite.
Should I have any work up based on the analysis?
I have recurrent UTI’s and once concern I have is my serum creatinine is 2.1 with a stent present but prior to the stone treatment. Any advice is appreciated. Thanks
Hi LCR, The need for catheterization raises risk for infection which I fear you have. My main concern is that you have infection stones, not calcium phosphate but struvite and the fragments analysed did not reveal the complete story. Of course your physicians need to establish drainage, save as much of the infected kidney as possible and all, but as for cause I am sure they will pursue infection itself as a main issue. If indeed the stones are new onset and really are from metabolic causes – not bacteria producing stone crystals – here is an article detailing causes of such in later life.Primary hyperparathyroidism seems the most common probable cause, but that is for your physicians to figure out. Regards, Fred Coe
Thanks! My urologist haven’t offeted any advice on what to do going forward. I reviewed my first note and I mistakenly said my creatinine was 2.1 but it was 1.1(my mistake). That is a little elevated from my baseline however but I suppose that’s just something to watch. I also found out I have staphylococcus in my urine and it’s been treated with cipro and then changed to Bactrim. So far no symptoms of UTI but still having to catheterize 4-6 times a day with very little voiding on my own( an ounce maybe once a day and sometimes its just overflow and very weak bladder. Thanks for your. response. It helps me
Hi LCR, I am concerned for you given the need for frequent catheterizations and infections. Perhaps it is time your physicians considered referral to a university based program. The reason for the voiding issue and remedy both seem very important here. Regards Fred Coe
Thank you for your concern. I had urodynamics last week and I have an under active bladder and OAB . The OAB requires botox and I have incomplete emptying requiring tve self caths. Luckily no uti in over a mo th. I am having recurrent flank pain again. My dr scheduled me for 24 hour urine metabolic stone analysis through litholink and a bilateral renal ultrasound complete in 2 weeks. I just pray the flank pain doesn’t mean anything… hope we are getting somewhere
Thanks for your concern. I saw my regular urologist this past Friday and the cathing is needed due to underactive bladder and poor emptying. I have significant OAB requiring botox. My UTI’s are vlear gor now but my flank pain has returned. My doc ordered the 24 hour metabolic analysis via litholink. He also scheduled me for bilateral renal ultrasound to make sure the flank pain is due to a stone since no imaging has been done since the lithotripsy in February
Hi I have had two kidney stone surgeries after pregnancy due to 80% caphos, 20% caox stones= calcium phosphate stones. I was put on Potassium citrate 15meq 2x a day. Did another 24 hour test and now my oxalates rose from 30 to 58 in my 24 hour urine. Any recommendations that would benefit me. My Dr is more reactive vs proactive so I figured id reach out and see if I could get additional advise.
Hi Hanna, The changes in oxalate are from diet, and you can try to track them down. I would watch the urine calcium and citrate and pH given calcium phosphate stones do not have much oxalate in them. Regards, Fred Coe
I submitted a question on March 12th2024. Could someone please review and answer if possible?
Thanks
Hi LCR, with apologies! I believe I answered you today. Fred
Hi Dr. Coe,
Can you explain to me about catheterization dependence ( self cath) in females fuev to an underactive bladder and bacteria colonizing in bladder leading to positive nitrites when it is not a utI nut just colonization. I have been told that nitrites are present in urine if you’re colonized. I know infections can lead to struvite stones so isn’t it risky to not treat bacteria in your urine since these stones form fast. I have recurrent infections, had 4 stones with infected stones and pyelonephritis in January. The stone I had analyzed revealed it was calcium phosphate
The analysis states
:
“ tan calculi. Hydroxy carbonate apatite. 33 mg weight
What should i do to prevent stones if it is calcium phosphate and how to know if I am at risk for Struvite stones?
Hi Leanne, Your stone is calcium phosphate meaning it formed in high pH urine. Possibly bacteria have played a role but more likely it is high urine calcium and pH. Stasis will accentuate crystallizations from whatever cause so the catheterization can play a role. I would think 24 hour urine studies are a best direction to look for causes one can directly treat. Regards, Fred Coe